Abstract
Objective: MiRNAs play crucial roles in progression of cancer. However, the underlying mechanisms of miRNAs in non small cell lung cancer are still poorly understood. The aim of this study was to investigate the expression level of microRNA-126 (miR-126) and microRNA-133b (miR-133b) and also their association with clinicopathological features in patients with non small cell lung cancer (NSCLC). Methods: Total RNA was purified from NSCLC tissues and adjacent non-tumor tissues and then quantitative real-time PCR (qRT-PCR) was used to evaluate the expression rate of microRNAs. Furthermore, the association of miR-126 and miR-133b level with clinicopathological features and prognosis were evaluated. Results: Our findings showed that expression of miR-126 was decreased in NSCLC tissues compared with adjacent non-tumor tissues. On the other hand, a lower expression of miR-133b was seen in NSCLC tissues when compared with adjacent non-tumor tissues. In term of miR-126, our results showed that miR-126 was associated with tumor stage and lymph nodes metastasis (P<0.05). In term of miR-133b, our finding indicated that decreased expression of miR-133b was correlated with advanced tumor stage and lymph nodes metastasis (P<0.05). Kaplan-Meier analysis and log-rank test indicated that patients with low expression of miR-126 and miR-133b had a shorter overall survival (log-rank test; P<0.05). Multivariate Cox proportional hazards model revealed that low expression of miR-126 and miR-133b, advanced tumor stage and lymph nodes metastasis were independent prognostic factors for overall survival of NSCLC patients. Conclusions: These findings suggested that miR-126 and miR-133b might play a key role in the progression and metastasis of NSCLC and would be applied as a novel therapeutic agent.
Keywords: Non small cell lung cancer, microRNA-126, microRNA-133b, prognosis
Introduction
Lung cancer is currently the most common malignant disease and the leading cause of mortality in the world, and non small cell lung cancer (NSCLC) accounts for 75-80% of lung cancer cases [1]. Despite great advances in chemotherapy and surgical techniques, the 5-year survival rate of NSCLC patients is still around 15% [2]. Thus, it is necessary to elucidate the molecular mechanisms involved in lung carcinogenesis, and to identify diagnostic and prognostic markers for early detection and therapeutic target treatment of NSCLC.
MicroRNAs (miRNAs) are small, non-coding RNA molecules involved in inhibition of gene expression through binding to the 3’-untranslated region of target mRNA, leading to mRNA cleavage or translational repression [3]. MiRNAs can function as oncogenes or tumor suppressors depending on their specific target genes and different cancers [4,5]. MiRNAs are involved in a variety of biological processes, including development, cell proliferation, differentiation, metastasis and chemotherapy resistance [6,7]. Emerging evidence showed that dysregulation of miRNAs occurs in a variety of cancers. For example, Zhang et al. reported that miR-144 was up-regulated in nasopharyngeal carcinoma and promoted cell proliferation, migration and invasion through repression of PTEN [8]. Yang et al. showed that miR-506 was down-regulated in clear cell renal cell carcinoma and inhibits cell growth and metastasis via targeting FLOT1 [9]. Song et al. showed that miR-630 was down-regulated in NSCLC and suppressed the proliferation, migration, and invasion of NSCLC cells by down-regulating LMO3 expression [10]. However, the roles of miR-126 and miR-133b in NSCLC remain poorly understand.
Therefore, in the current study, we investigated the miR-126 and miR-133b expression levels in human NSCLC and the association of these miRNAs expression levels with clinicopathological features and prognosis.
Materials and methods
Patients and tissue samples
Non-small cell lung cancer (NSCLC) tissues and adjacent non-tumor tissues from patients who underwent resection of the primary NSCLC at Luoyang Center Hospital Affiliated to Zhengzhou University between 2003 and 2007. This study was approved by the Research Ethics Committee of Zhengzhou University, and written informed consent was obtained from all patients. All patients did not receive chemotherapy or radiotherapy prior to surgery. After collection, all tissue samples were immediately frozen in liquid nitrogen and stored at -80°C until use. Moreover, the diagnosis and the histological grading were approved by pathologists. The clinicopathologic features of NSCLC patients were summarized in Table 1.
Table 1.
Correlation of miRNAs expression with clinicopathological features of NSCLC
| Clinicopathological features | Number | miR-126 expression | miR-133b expression | P value (miR-126) | P value (miR-133b) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low | High | Low | High | ||||
| Age (years) | 0.349 | 0.790 | |||||
| <60 | 37 | 16 | 21 | 19 | 18 | ||
| ≥60 | 76 | 40 | 36 | 37 | 39 | ||
| Gender | 0.182 | 0.426 | |||||
| Male | 83 | 38 | 45 | 43 | 40 | ||
| Female | 30 | 18 | 12 | 13 | 17 | ||
| Tumor size (cm) | 0.173 | 0.501 | |||||
| <3 | 78 | 42 | 36 | 37 | 41 | ||
| ≥3 | 35 | 14 | 21 | 19 | 16 | ||
| Histological type | 0.641 | 0.157 | |||||
| Squamous | 59 | 28 | 31 | 33 | 26 | ||
| Adenoma | 54 | 28 | 26 | 23 | 31 | ||
| Differentiation | 0.606 | 0.150 | |||||
| Mod-well | 72 | 37 | 35 | 32 | 40 | ||
| Poor | 41 | 19 | 22 | 24 | 17 | ||
| Tumor stage | 0.003 | 0.000 | |||||
| I-II | 64 | 24 | 40 | 20 | 44 | ||
| III | 49 | 32 | 17 | 36 | 13 | ||
| Lymph nodes metastasis | 0.011 | 0.001 | |||||
| No | 88 | 38 | 50 | 36 | 52 | ||
| Yes | 25 | 18 | 7 | 20 | 5 | ||
Quantitative real-time PCR
The total RNA isolated from tissues using TRIzol (Invitrogen) reagent based on the constructor’s instructions. Gene specific primers were used to synthesize cDNA from the TaqMan MicroRNA Assays and reagents from the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems). Real-time PCR was carried out to detect the expression level of microRNAs using an Invitrogen kit by system of Rotor-gene 6000 (Qiagen). The primers were used from the TaqMan miRNA Assays. The relative amount of microRNAs was normalized with U6 gene as internal reference. The ΔΔCt (ΔΔCt = ΔCt tumor samples -ΔCt control sample) to qualify the expression rate of miR-126 and miR133b.
Statistical analysis
All data were presented as the mean ± SD and were analyzed using SPSS 18.0 software (SPSS Inc., USA). Differences between groups were evaluated using Student’s t-test or χ2 test. Survival analysis was done by using the log-rank test and Kaplan-Meier method. Cox proportional hazards model was performed to evaluate prognostic values of clinicopathological features. Differences were considered statistically significant when P was less than 0.05.
Results
We evaluated miR-126 and miR-133b expression levels between patients with NSCLC and adjacent non-tumor tissues. Our findings showed that miR-126 expression level was significantly lower in NSCLC tissues compared with adjacent non-tumor tissues (P<0.05; Figure 1A). Furthermore, we found that the expression level of miR-133b was decreased in NSCLC tissues than those adjacent non-tumor tissues (P<0.05; Figure 1B).
Figure 1.
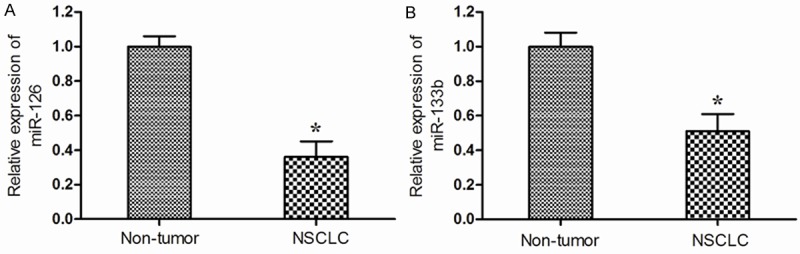
Relative expression levels of miR-126 (A) and miR-133b (B) in non small cell lung cancer (NSCLC) and adjacent non-tumor tissues. Both the expression levels of miR-126 and miR-133b were decreased in NSCLC tissues than in adjacent non-tumor tissues. *P<0.05.
According to the median expression level of miR-126 and miR-133b, we categorized the patients into low and high expression groups. The correlation between clinicopathological features and tow microRNAs expression in high and low expression groups were summarized in Table 1.
Our results showed that down-regulated expression of miR-126 was clearly correlated with tumor stage and lymph nodes metastasis (P<0.05; Table 1). There was no significant association of miR-126 expression with age, gender, tumor size, histological type and differentiation (P>0.05; Table 1). In addition, low expression of miR-133b was associated with tumor stage and lymph nodes metastasis (P<0.05; Table 1). There was no significant correlation of miR-133b with other clinicopathological features (P>0.05; Table 1).
Kaplan-Meier survival and log-rank analysis were performed to evaluate association of miR-126 and miR-133b expression with overall survival of NSCLC patients. The results indicated that the decreased expression of miR-126 and miR-133b was strongly correlated with shorter overall survival (log-rank test, P<0.05; Figure 2A, 2B).
Figure 2.
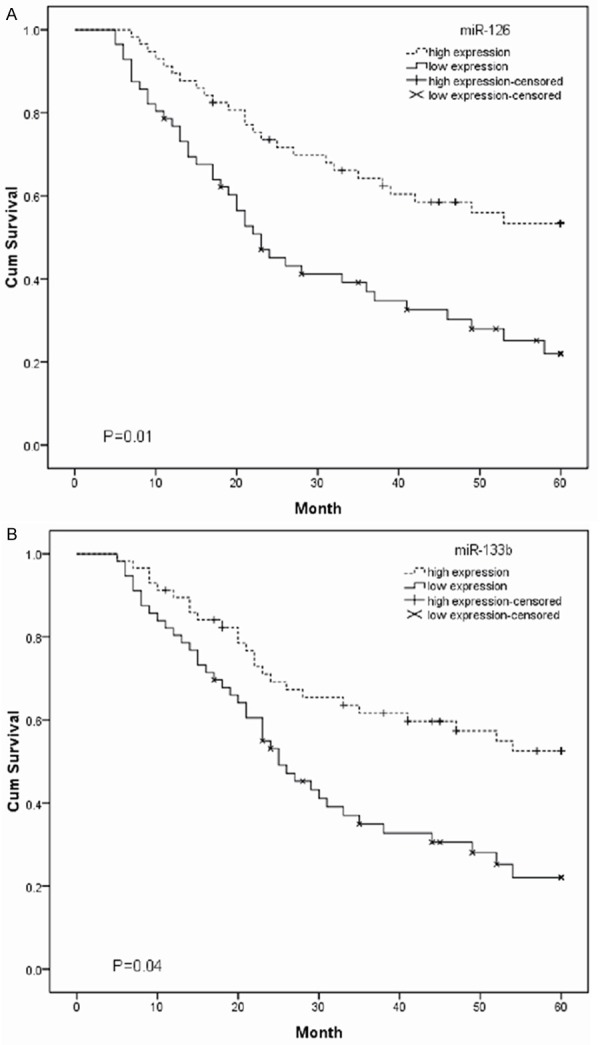
The association between miR-126 (A) and miR-133b (B) expression with overall survival of NSCLC patients was analyzed using Kaplan-Meier survival curves. Low expression levels was associated with shorter overall survival compared with high expression levels (log-rank test; P<0.05).
Multivariate Cox proportional hazards model revealed that low expression of miR-126 and miR-133b, tumor stage and lymph nodes metastasis were independent prognostic factors for overall survival of patients with NSCLC (P<0.05; Tables 2 and 3).
Table 2.
Multivariate analysis with a Cox proportional hazards model between miR-126 and clinicopathological features
| Clinicopathological features | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.287 | 0.724-2.781 | 0.614 |
| Gender | 1.065 | 0.538-2.137 | 0.375 |
| Tumor size (cm) | 1.836 | 0.417-3.925 | 0.174 |
| Histological type | 1.281 | 0.794-3.175 | 0.286 |
| Differentiation | 2.125 | 0.82-6.792 | 0.078 |
| Tumor stage | 2.276 | 1.453-5.687 | 0.008 |
| Lymph nodes metastasis | 3.117 | 1.682-9.213 | 0.003 |
| miR-126 | 2.853 | 1.416-7.762 | 0.011 |
Abbreviations: HR hazard ratio, 95% CI, 95% confidence interval.
Table 3.
Multivariate analysis with a Cox proportional hazards model between miR-133b and clinicopathological features
| Clinicopathological features | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.412 | 0.851-3.043 | 0.379 |
| Gender | 0.872 | 0.549-1.967 | 0.284 |
| Tumor size (cm) | 2.377 | 0.635-4.283 | 0.104 |
| Histological type | 1.416 | 0.639-3.358 | 0.146 |
| Differentiation | 1.893 | 0.682-4.962 | 0.084 |
| Tumor stage | 2.692 | 1.375-6.849 | 0.013 |
| Lymph nodes metastasis | 4.016 | 1.882-11.358 | 0.008 |
| miR-126 | 2.418 | 1.305-8.159 | 0.009 |
Abbreviations: HR hazard ratio, 95% CI, 95% confidence interval.
Discussion
Dysregulation of miRNAs was reported to be associated with the development and progression of human malignancies [11]. Aberrant expression of miRNAs has been suggested to be as potential sensitive and accurate biomarkers for cancer diagnosis and prognosis of NSCLC. For example, Xu et al. suggested that miR-9 was up-regulated in NSCLC and correlated with adverse clinical features and unfavorable survival [12]. Bai et al. revealed that miR-32 was decreased in NSCLC and associated with tumor progression and patient survival [13]. Yang et al. found that down-regulation of miR-181b was correlated with aggressive disease progression and poor prognosis of NSCLC patient [14]. Therefore, determination of functional and clinical importance of a specific miRNA may provide effective management of the disease. In current study, we evaluated the expression pattern of miR-126 and miR-133b in term of NSCLC.
Dysregulation of miR-126 has been reported in a number of cancers and it has various expression patterns in diverse human carcinomas. For example, Chen et al. demonstrated that decreased expression of miR-126 correlated with metastatic recurrence of hepatocellular carcinoma [15]. Yang et al. revealed that miR-126 was decreased in cervical cancer and down-regulated expression of miR-126 was associated with poor prognosis [16]. Khella et al. suggested that low expression of miR-126 was a prognostic marker for metastatic clear cell renal cell carcinoma [17]. However, the clinical significance of miR-126 in NSCLC is still unclear. In the present study, we found that miR-126 was down-regulated in NSCLC tissues when compared with adjacent non-tumor tissues. Furthermore, low expression of miR-126 was associated with advanced tumor stage and lymph nodes metastasis. Kaplan-Meier survival and log-rank test analysis showed that the low expression of miR-126 was correlated with shorter overall survival. Multivariate Cox proportional hazards model revealed that miR-126 expression, tumor stage and lymph nodes metastasis were detected to be as independent prognostic biomarkers of overall survival in NSCLC patients.
Aberrant regulation of miR-133b has been reported in many kinds of malignancies. For example, Karatas et al. showed that miR-133b was significantly down-regulated in recurrent prostate cancer, indicating that miR-133b could serve as novel biomarkers for prediction of prostate cancer progression [18]. Zhao et al. indicated that miR-133b was decreased in gastric cancer and its overexpression reduced the metastatic potential of gastric cancer cells [19]. Xiang et al. showed that miR-133b was down-regulated and inhibited cell proliferation, migration and invasion by targeting TBPL1 in colorectal cancer [20]. However, the clinical significance of miR-133b in NSCLC has not yet been elucidated. In the present study, our findings revealed that the expression level of miR-133b was lower in NSCLC tissues than those adjacent non-tumor tissues. In addition, low expression of miR-133b was correlated with tumor stage and lymph nodes metastasis. Moreover, kaplan-Meier survival and log-rank test analysis showed that the low expression of miR-133b was correlated with shorter overall survival. Multivariate Cox proportional hazards model suggested that the down-regulated expression of miR-133b, tumor stage and lymph nodes metastasis were detected to be as independent prognostic biomarkers of overall survival in NSCLC patients. Our study expanded the function of miR-126 and miR-133b to the development and progression of NSCLC.
In conclusion, our result showed that down-regulation of miR-126 and miR-133b was associated with progression of NSCLC. miR-126 and miR-133b might play a key role in suppression of tumor in NSCLC and would be applied as novel therapeutic agents.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X. MicroRNA: function, detection, and bioanalysis. Chem Rev. 2013;113:6207–6233. doi: 10.1021/cr300362f. [DOI] [PubMed] [Google Scholar]
- 7.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and function. Thromb Haemost. 2012;107:605–610. doi: 10.1160/TH11-12-0836. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL, Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY, Fu L. MicroRNA-144 promotes cell proliferation, migration and invasion in nasopharyngeal carcinoma through repression of PTEN. Carcinogenesis. 2013;34:454–63. doi: 10.1093/carcin/bgs346. [DOI] [PubMed] [Google Scholar]
- 9.Yang FQ, Zhang HM, Chen SJ, Yan Y, Zheng JH. MiR-506 Is Down-Regulated in Clear Cell Renal Cell Carcinoma and Inhibits Cell Growth and Metastasis via Targeting FLOT1. PLoS One. 2015;10:e0120258. doi: 10.1371/journal.pone.0120258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song YF, Hong JF, Liu DL, Lin QA, Lan XP, Lai GX. miR-630 targets LMO3 to regulate cell growth and metastasis in lung cancer. Am J Transl Res. 2015;7:1271–1279. [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu T, Liu X, Han L, Shen H, Liu L, Shu Y. Upregulation of miR-9 expression as a poor prognostic biomarker in patients with non-small cell lung cancer. Clin Transl Oncol. 2014;16:469–475. doi: 10.1007/s12094-013-1106-1. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Wang YL, Yao WJ, Guo L, Xi HF, Li SY, Zhao BS. Expression of miR-32 in human nonsmall cell lung cancer and its correlation with tumor progression and patient survival. Int J Clin Exp Pathol. 2015;8:824–829. [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Liu H, Wang H, Sun Y. Down-regulation of microRNA-181b is a potential prognostic marker of non-small cell lung cancer. Pathol Res Pract. 2013;209:490–494. doi: 10.1016/j.prp.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Miao R, Fan J, Han Z, Wu J, Qiu G, Tang H, Peng Z. Decreased expression of miR-126 correlates with metastatic recurrence of hepatocellular carcinoma. Clin Exp Metastasis. 2013;30:651–658. doi: 10.1007/s10585-013-9569-6. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Song Kl, Chang H, Chen L. Decreased expression of microRNA-126 is associated with poor prognosis in patients with cervical cancer. Diagn Pathol. 2014;9:1001. doi: 10.1186/s13000-014-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khella HW, Scorilas A, Mozes R, Mirham L, Lianidou E, Krylov SN, Lee JY, Ordon M, Stewart R, Jewett MA. Low Expression of miR-126 is a prognostic marker for metastatic clear cell renal cell carcinoma. Am J Pathol. 2015;185:693–703. doi: 10.1016/j.ajpath.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Karatas OF, Guzel E, Suer I, Ekici ID, Caskurlu T, Creighton CJ, Ittmann M, Ozen M. miR-1 and miR-133b are differentially expressed in patients with recurrent prostate cancer. PLoS One. 2014;9:e98675. doi: 10.1371/journal.pone.0098675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu B, Yan M, Yu Y, Liu B, Zhu Z. MiR-133b is frequently decreased in gastric cancer and its overexpression reduces the metastatic potential of gastric cancer cells. BMC Cancer. 2014;14:34. doi: 10.1186/1471-2407-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang KM, Li XR. MiR-133b acts as a tumor suppressor and negatively regulates TBPL1 in colorectal cancer cells. Asian Pac J Cancer Prev. 2013;15:3767–3772. doi: 10.7314/apjcp.2014.15.8.3767. [DOI] [PubMed] [Google Scholar]


