Abstract
CIP2A is highly expressed in a variety of malignancies. We determined the expression and clinical significance of CIP2A in patients with advanced gastric cancer. CIP2A protein was expressed in 25 of 37 cancer tissue specimens. There was no correlation between CIP2A and PGP, GST-π, Topo-II, and LRP expression. Survival analysis showed significant differences between the survival rate of the CIP2A protein-positive and -negative groups (χ2=4.509, P=0.034), but the degree of positive expression was unrelated to survival time (χ2=4.639, P=0.098). CIP2A expression may have no prospective value for optimizing chemotherapy regimens, but it can be an indicator for patient prognosis.
Keywords: Gastric cancer, CIP2A, p53, Helicobacter pylori, MRD, survival time
Introduction
Uncontrolled cellular proliferation is a hallmark of cancer cells, and tumorigenesis is related to the disordered expression of some key factors which take part in regulating cell cycle progression, differentiation, senescence, and apoptosis [1]. Aberrant expression of some key proteins can result in the formation of cancers in humans. Cancerous inhibitor of protein phosphatase 2A (CIP2A) is a recently identified human oncoprotein that inhibits c-MYC protein degradation in cancer cells, which has been found to be overexpressed in different human cancers [2]. MYC is a multifunctional transcription factor which has been linked to a diverse range of cellular functions, such as cell cycle regulation, proliferation, growth, differentiation, and metabolism. Abnormal MYC signaling has been observed in human cancers and it has been demonstrated that MYC promotes cell transformation and tumor progression [3]. Many studies have reported that PP2A prompts the proteolytic degradation of the oncoprotein, Myc, and prevents malignant cells from growing [4,5]. Nevertheless, CIP2A can stabilize the MYC protein by inhibiting PP2A activity and promoting tumor formation in vivo [2].
We determined the expression of CIP2A in patients with advanced gastric cancer to better understand the role of CIP2A as a prognostic marker and the relationship between Helicobacter pylori infection and differentiation of gastric cancer cells. Moreover, the relationship between CIP2A expression, and p53 and drug resistance-related factors, including PGP, GST-π, Topo-II, and LRP, were studied.
Materials and methods
Patients
Thirty-seven resected specimens from patients who were tested pre-operatively for H. pylori and histopathologically-confirmed to have advanced gastric cancer in a university-affiliated hospital between January and December 2009 were retrospectively reviewed. None of the patients received chemotherapy pre-operatively. All of the patients had undergone distal or total gastrectomy with D2 lymph node dissection and removal of metastases with margins free of tumor cells. Patients considered having gastric H. pylori infections were confirmed by a 13C-urea breath test before surgery. Advanced cancer was defined as >T2 tumor, regardless of nodal involvement.
Our study was approved by the Research Ethics Committee of the institution, and written informed consent was obtained from each subject. The patients included 20 men and 17 women, ranging in age from 29-76 years (mean, 60.65 years). Thirty-two patients had lymphatic metastases. Moreover, three patients had hepatic metastases. All patients were confirmed to have adenocarcinomas based on the WHO histologic classification of tumors of the stomach, with 18 well- or moderately-differentiated and 19 poorly-differentiated adenocarcinomas.
Clinical data and pathologic findings, including gender, age at diagnosis, tumor location, tumor diameter, lymphatic and distant metastases, and histologic differentiation, were collected.
Patient follow-up included blood count, computer tomography (CT) scanning of the abdomen and pelvis, and physical examination once every 3 months for the first year, then once every 6 months for the remaining 2 years. Those patients who were not able to attend the clinic regularly were contacted by telephone.
Immunohistochemistry
Paraffin-embedded tissues were cut into 4-µm-thick sections and fixed on glass slides. After drying for 12-24 hours at 37°C, sections were deparaffinized in xylene and rehydrated in a descending ethanol series. Endogenous peroxidase activity was blocked by soaking in hydrogen peroxide. Antigen retrieval was performed by microwave heating to a boil in 10 mmol/L sodium citrate buffer (pH 6.0) for two cycles of 5 min each. After rinsing in PBS (pH 7.2), 10% goat serum was applied for 20 min at room temperature to block non-specific reactions. The sections were then incubated overnight at 4°C with rabbit anti-human antibodies against CIP2A (diluted 1:150; Bluegene, Shanghai, China). After rinsing in PBS, the biotinylated secondary goat anti-rabbit antibody was applied for 30 min at 37°C. Diaminobenzidine (DAB) was used as a chromogen. The sections were then counterstained with hematoxylin. For the negative controls, the primary antibodies were replaced with PBS. For p53, PGP, GST-π, Topo-II, and LRP staining, the slides were stained as above. All samples were scored independently by two pathologists without any knowledge of clinical status and outcome of data. For each section, we examined 20 high-power fields at random, and in each field we checked the immunoreactivity of 50 cells. Cells with brown-colored staining were considered as positive. The intensity of expression of proteins was stratified into three categories, which were scored as follows: 1) negative, no appreciable cytomembrane, nuclear, or cytoplasmic staining or staining in ≤5% of target cells; 2) 1+, appreciable staining in 6%-50% of target cells; and 3) 2+, appreciable stains in 51%-100% of target cells.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. Statistical analyses were performed with a chi-square or Fisher’s exact test. The correlation between CIP2A, p53, PGP, GST-π, Topo-II, and LRP were tested by Spearman rank correlation. The life tables of overall survival were performed using the Kaplan-Meier method, and the survival curves were compared with the log-rank or t-test. P values <0.05 were considered as statistically significant.
Results
CIP2A expression in gastric cancer
Twenty-five of 37 gastric cancer specimens were CIP2A-positive with various levels of expression, and 12 specimens were negative. Moreover, 10 positive specimens had strong expression of CIP2A (≥50%), and 15 had weak expression (6%-50%). Most tumor cells predominantly expressed CIP2A in the cytoplasm and nucleus (Figure 1).
Figure 1.
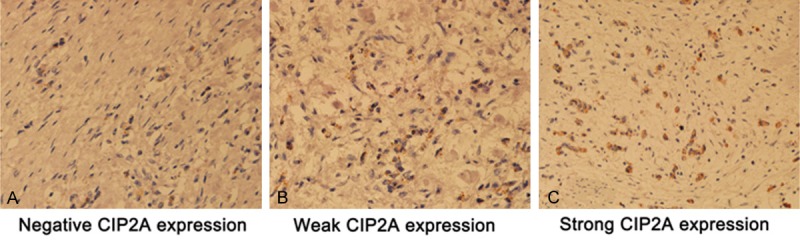
Different expression of CIP2A in immunostained specimens (1:400).
CIP2A expression in Hp infection tissues
Positive CIP2A expression was much more prominent in the Hp-positive group than the negative group (χ2=9.375, P=0.009). No significant differences existed between CIP2A expression and patient gender, age, tumor location, tumor diameter, Lauren classification, histopathologic grade, and lymph node and distant metastases (P>0.05, Table 1).
Table 1.
Clinical characteristics (n = 37)
| Total | Negative | Weak | Strong | Fisher X2 | P | |
|---|---|---|---|---|---|---|
| Age | ||||||
| <60 | 10 | 4 | 4 | 2 | 0.564 | 0.897 |
| ≥60 | 27 | 8 | 11 | 8 | ||
| Gender | ||||||
| Male | 20 | 4 | 9 | 7 | 3.185 | 0.263 |
| Female | 17 | 8 | 6 | 3 | ||
| Tumor Location | ||||||
| Upper | 13 | 3 | 7 | 3 | 2.796 | 0.650 |
| Middle | 5 | 3 | 1 | 1 | ||
| Lower | 19 | 6 | 7 | 6 | ||
| Tumor Diameter | ||||||
| <5 CM | 22 | 9 | 8 | 5 | 1.820 | 0.481 |
| ≥5 CM | 15 | 3 | 7 | 5 | ||
| Nodal Status | ||||||
| No | 5 | 3 | 2 | 0 | 2.608 | 0.339 |
| ≥N1 | 32 | 9 | 13 | 10 | ||
| Metastatic Status | ||||||
| Mo | 34 | 12 | 13 | 9 | 1.636 | 0.606 |
| M1 | 3 | 0 | 2 | 1 | ||
| HP Infection | ||||||
| Negative | 18 | 10 | 6 | 2 | 9.375 | 0.009 |
| Positive | 19 | 2 | 9 | 8 | ||
| Lauren Classification | ||||||
| Intestinal | 22 | 9 | 8 | 5 | 1.820 | 0.481 |
| Diffused | 15 | 3 | 7 | 5 | ||
| Mixed | ||||||
| Histopathologic Grade | ||||||
| G1-G2 | 18 | 8 | 7 | 3 | 2.881 | 0.268 |
| G3 | 19 | 4 | 8 | 7 | ||
Correlation between CIP2A, p53, and drug resistance-related proteins
A correlation existed between CIP2A and p53 expression in 37 gastric cancer specimens (rs=0.345, P=0.036), but the correlation was not strong. No correlation existed between CIP2A, and PGP, GST-π, Topo-II, and LRP (P>0.05, Table 2).
Table 2.
Correlation between CIP2A expression and the expression of MDR-related proteins
| Total | Negative | Weak | Strong | rs | P | |
|---|---|---|---|---|---|---|
| P53 | ||||||
| Negative | 21 | 10 | 7 | 4 | 0.345 | 0.036 |
| Positive | 16 | 2 | 8 | 6 | ||
| PGP | ||||||
| Negative | 15 | 5 | 5 | 5 | -0.058 | 0.733 |
| Positive | 22 | 7 | 10 | 5 | ||
| GST-π | ||||||
| Negative | 19 | 6 | 7 | 6 | 0.002 | 0.991 |
| Positive | 18 | 6 | 8 | 4 | ||
| TOP-II | ||||||
| Negative | 14 | 2 | 9 | 3 | -0.127 | 0.453 |
| Positive | 23 | 10 | 6 | 7 | ||
| LRP | ||||||
| Negative | 20 | 8 | 8 | 4 | 0.206 | 0.222 |
| Positive | 17 | 4 | 7 | 6 | ||
Prognostic role of CIP2A in advanced gastric cancer
The course of follow-up lasted for 3 years. We maintained contact with the all 37 patients via telephone and face-to-face visits. The overall survival rate for the 37 patients during the 3 years of follow-up was 32.43% (12 of 37). The Kaplan-Meier method was used to draw the survival curves for the CIP2A-positive and -negative groups. Thus, the presence of CIP2A expression was associated with a reduced survival rate, and the two survival curves exhibited a gradually increasing trend of separation after 2 years of follow-up (Figure 2A). The log-rank test was used to analyze the outcomes in the two groups, and indicated a difference between the CIP2A-positive and -negative groups (χ2=4.509, P=0.034). Taken together, the 3-year survival rate in the CIP2A-positive group was significantly lower than the CIP2A-negative group. In addition, the Kaplan-Meier method was used to draw the survival curves for the negative, weak (level of expression ≤50% [+]), and strong CIP2A expression groups (level of expression >50% [2+]). The weak and strong expression group curves did not exhibit separation (χ2=4.639, P=0.098; Figure 2B). To summarize, the survival rate of CIP2A-positive patients was significantly different from the CIP2A-negative patients. Moreover, the level of positive expression was unrelated to survival time.
Figure 2.
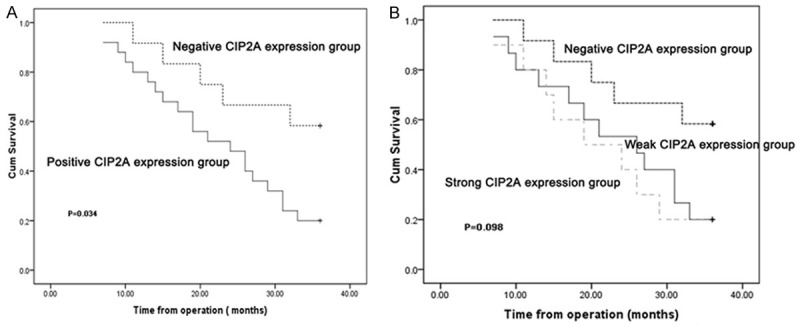
Correlation of CIP2A expression with OS.
Discussion
Gastric cancer is one of the most common malignancies worldwide, ranking second in terms of global cancer-related mortality; the high mortality rate of gastric cancers is mostly attributed to late diagnosis [6,7]. In China, gastric cancer was the most common cancer amongst the entire population during the 1970s and early 1990s. Currently, gastric cancer is still the most common major cancer and a big burden for health resources and facilities [8]. Host genes, bacterial virulence, and environmental factors have been implicated in affecting the gastric oncogenic process. The development of cancer is a complex process that involves many genes and steps, including the expression and regulation of multiple oncogenes and cancer suppressor genes [9,10].
Since the development of molecular biology and tumor genetics, many genes have been reported to be relevant to the diagnosis, treatment, and prognosis of gastric cancer, such as Ras, c-myc, Rb, and E-cadherin. The position of the CIP2A gene is in the long chain of the 3rd chromosome, but the biological function of the CIP2A gene is still poorly understood. Until et al [2,3] reported that CIP2A is expressed at low levels in most non-malignant tissues, but the expression of CIP2A is increased in malignant cells, and CIP2A stabilizes Myc protein through inhibition of PP2A activity and promotes tumor formation in vivo. Moreover, Li [11] showed that CIP2A protein is present in both cytoplasm and nuclear compartments of tumor cells, but a much stronger signal exists in the cytoplasm. Our study also showed that the CIP2A protein is mainly expressed in the cytoplasm and nucleus of gastric cancer cells. A quantitative RT-PCR analysis showed that CIP2A mRNA is expressed at very low levels (<1% of b-acting mRNA expression) in the majority of 21 non-malignant samples, with the exception of bone marrow, prostate, testis, cerebellum, and brain [2]. Our results showed CIP2A protein to be expressed in 25 of 37 samples (68%), which approximated the results of Khanna et al (145/223 [65%]), but were higher than the results of Soo Hoo et al (6/11 [55%]) and lower than the results of Li et al (8/10 [80%]) [11-13].
A clinical study reported that the expression of CIP2A mRNA in human breast cancer increased as the SRB histologic grade increased, indicating that CIP2A may be associated with histologic grade and play a role in the differentiation process of cancer cells [14]. CIP2A depletion leads to the partial differentiation of leukemic HL60 cells, although the effect is not robust [15]. Hence, it is thought that high expression of CIP2A disturbs the process of differentiation and promotes the transformation of malignant cells. In the current study, we found the positive rate of CIP2A expression was unrelated to the stage of differentiation of advanced gastric cancer.
H. pylori infection is considered a risk factor for gastric tumorgenesis. The persistent colonization of H. pylori in the stomach leads to an increased risk of peptic ulcers and gastric adenocarcinoma [16,17]. Few studies have reported on the relationship between Hp infection and CIP2A expression. We found that the CIP2A-positive rate was much more prominent in the Hp-positive group compared to the Hp-negative group (P=0.009), suggesting that Hp infection has some correlation with c-MYC over-expression, and Hp induces gastric mucosa tumorigenesis by activating c-myc. Thus, the CIP2A gene may play a role in Hp infection related to gastric carcinogenesis.
A previous study reported that CIP2A mRNA expression was positively correlated with lymph node positivity of patients and p53 mutations in breast cancer samples [14]. Our study also showed that expression of CIP2A protein was associated with p53 (r=0.345, P=0.036); however, our results suggested that the level of CIP2A expression in specimens was not significantly different with respect to nodal and metastatic status.
Khanna [12] reported that CIP2A positivity was related to overall survival among gastric cancer patients. In that study, 223 patients were included for tissue microarrays, showing that CIP2A immunostaining in tumors was associated with reduced survival, and the 10-year overall survival in the CIP2A-positive group was lower than the CIP2A-negative group. We showed that the 3-year survival rate of the immunopositive group was lower than the immunonegative group (χ2=4.509, P=0.034); however, we did not find any correlation between CIP2A expression and survival time (χ2=0.170, P=0.680). Because of the limited number of cases, the conclusion should be confirmed by evaluating CIP2A expression in larger cohorts of patients with advanced gastric cancer.
The multidrug resistance (MDR) phenotype plays a potentially significant role in successful cancer chemotherapy [18]. Therefore, it is critical to know the MDR expression in cancer patients in order to choose new individually tailored chemotherapeutic options. Many mechanisms are involved in MDR, including increasing drug efflux (e.g., P-glycoprotein [PGP]), activation of detoxifying systems (e.g., glutathione/glutathione-S transferases [GST]), alterations in drug targets (e.g., topoisomerase-II [Topo-II]), or sequestration of drugs (e.g., lung resistance protein [LRP]). Moreover, increasing repair of drug-induced DNA damage, blocked apoptosis, disruptions in signaling pathways, and alterations of factors are also responsible for MDR [19].
In the present study, we determined whether or not CIP2A is a valuable new biomarker for choosing an optimal individualized chemotherapeutic protocol. Hence, the correlation between the expression of MDR-related proteins (PGP, GST-π, Topo-II, and LRP) and CIP2A in patients was investigated using immunohistochemical methods. The study showed no correlation between CIP2A protein, and PGP, GST-π, Topo-II, and LRP expression.
In conclusion, our study showed that patients with a stronger expression of CIP2A in tumor tissues tended to have higher Hp infection rates, stronger p53 expression, and poor long-term survival. CIP2A, however, had no significant relationship with these genes that are related to gastric cancer chemotherapy drug resistance.
Acknowledgements
This study was sponsored by the Zhejiang Provincial Top Key Discipline in Surgery and Wenzhou Key Laboratory Project in Surgery.
Disclosure of conflict of interest
None.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, Lu SL, Lin S, Chan EK, Wang XJ, Grènman R, Kast J, Kallunki T, Sears R, Kähäri VM, Westermarck J. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Junttila MR, Westermarck J. Mechanisms of MYC stabilization in human malignancies. Cell Cycle. 2008;7:592–596. doi: 10.4161/cc.7.5.5492. [DOI] [PubMed] [Google Scholar]
- 4.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol. 2006;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, Counter CM, Nevins JR, Means AR, Sears R. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 6.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 8.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson GB, van Heerden JA, Sarr MG. Adenocarcinoma of the stomach: are we making progress? Lancet. 1993;342:713–718. doi: 10.1016/0140-6736(93)91711-t. [DOI] [PubMed] [Google Scholar]
- 10.Wright PA, Williams GT. Molecular biology and gastric carcinoma. Gut. 1993;34:145–147. doi: 10.1136/gut.34.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia J, Xu D. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14:3722–3728. doi: 10.1158/1078-0432.CCR-07-4137. [DOI] [PubMed] [Google Scholar]
- 12.Khanna A, Böckelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, Junnila S, Murphy DJ, Evan GI, Haglund C, Westermarck J, Ristimäki A. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst. 2009;101:793–805. doi: 10.1093/jnci/djp103. [DOI] [PubMed] [Google Scholar]
- 13.Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21:5006–5015. doi: 10.1038/sj.onc.1205625. [DOI] [PubMed] [Google Scholar]
- 14.Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, Kallioniemi O, Thézenas S, Westermarck J. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15:5092–5100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 15.Eckhardt SG, Dai A, Davidson KK, Forseth BJ, Wahl GM, Von Hoff DD. Induction of differentiation in HL60 cells by the reduction of extrachromosomally amplified c-myc. Proc Natl Acad Sci U S A. 1994;91:6674–6678. doi: 10.1073/pnas.91.14.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 18.Peer D, Margalit R. Fluoxetine and reversal of multidrug resistance. Cancer Lett. 2006;237:180–187. doi: 10.1016/j.canlet.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Shi H, Lu D, Shu Y, Shi W, Lu S, Wang K. Expression of multidrug-resistance-related proteins P-glycoprotein, glutathione-S-transferases, topoisomerase-II and lung resistance protein in primary gastric cardiac adenocarcinoma. Cancer Invest. 2008;26:344–351. doi: 10.1080/07357900701788072. [DOI] [PubMed] [Google Scholar]


