Abstract
Background: The mixed lineage kinase domain-like protein (MLKL) has recently been identified as a key RIP3 (receptor interacting protein 3) downstream component of tumor necrosis factor (TNF)-induced necroptosis. Objective: To evaluate the expression and clinical significance of MLKL in cervical squamous cell carcinoma.Methods: The expression of MLKL in 54 cervical squamous carcinoma samples was detected by immuneohistochemical method. Chi-square, correlation analysis and kaplan-Meier method were used to analyze the data. Results: The MLKL expression in cervical squamous cell carcinoma was higher than that in normal cervical tissues (P = 0.004). The MLKL expression was negatively correlated with histological grade, lymphatic metastasis (P<0.05). Survival analysis showed the low expression of MLKL indicated poor prognosis. Conclusion: MLKL was a prognostic biomarker for cervical squamous cell carcinoma.
Keywords: Cervical squamous cell carcinoma, MLKL, phosphorylate, immunohistochemistry
Introduction
The mixed lineage kinase domain-like protein (MLKL) has been recently identified as a key RIP3 downstream component of TNF-induced necrosis [1,2]. Although the mechanism how MLKL mediates the necrosis induced by TNF is still not very clear, some researchers have suggested MLKL can serve as a potential prognostic biomarker for patients with early-stage resected pancreatic cancer and ovarian cancer [3,4]. So we want to detect whether the expression of MLKL has relation with the development of cervical squamous cell cancer and the prognosis of the patients.
Materials and methods
Materials
This study was approved by the Research Ethics Committee of West China Second Hospital, Sichuan University, People’s Republic of China. Informed consent was obtained from all of the patients. The cervical cancer tissue samples were collected from 54 patients diagnosed with cervical squamous cell carcinoma after operation at West China Second Hospital from January 2006 to December 2007 (Table 1). Surgical staging was established according to the International Federation of Gynecology and Obstetrics (FIGO) system. Histopathological classification, including the stage, grade, and tumor type, was performed by an experienced pathologist. Overall survival (OS) was calculated from the date of histological diagnosis to the date of cancer-caused death or to the date of the last follow-up examination.
Table 1.
The clinical information of 54 patients
| Clinical feature | |||
|---|---|---|---|
| Histological grade | Invasion depth | ||
| Low | 42 | ≤1/2 | 30 |
| Moderate | 10 | >1/2 | 24 |
| High | 2 | ||
| FIGO stage | Vaginal invasion | ||
| I | 41 | Yes | 12 |
| II | 13 | No | 42 |
| Lymphatic metastasis | Parametrial invasion | ||
| Yes | 9 | Yes | 3 |
| No | 45 | No | 51 |
| Vascular invasion | Age | ||
| Yes | 30 | ≤50 years | 40 |
| No | 24 | >50 years | 14 |
Immunohistochemistry
Formalin-fixed paraffin-embedded slides were used to identify representative sections of tumor and normal tissues. The tissue section was stained using anti-MLKL (phosphor S358) antibody (EPR9514, Abcam) at a concentration of 1:150. Antigen was retrieved by EDTA (PH9.0) microwave antigen retrieval. PBS was used to instead of primary antibody in the negative control.
Judgment of immunohistochemical results
Histological images were captured under the microscope (Carl Zeiss AX10; Carl Zeiss Meditec AG, Jena, Germany) with an objective magnification of X40. The proportion of positive tumor cells was scored as: 0 = less than 10%; 1+ = 10%-30%; 2+ = 31%-50%; 3+ = 51-80%; and 4+ >80%. The intensity was arbitrarily scored as 0 = weak (no color or light blue), 1 = moderate (light yellow), 2 = strong (yellow brown), and 3 = very strong (brown). The overall score was calculated by multiplying the two scores obtained from each sample. A score of ≥4 was defined as high MLKL expression and a score of <4 was defined low MLKL expression.
Statistical method
Data was analyzed by SPSS16.0. The relationships between the expression of MLKL and clinical pathological features were detected by chi-square and correlation analyses. Prognosis was determined by Kaplan-Meier method.
Results
MLKL expressed in the cytoplasm (Figure 1A). The expression of MLKL in cervical squamous cell carcinoma was obviously higher than that in normal cervical tissues (P = 0.004) (Figure 1C, 1D). The expression in cervical squamous cell carcinoma had correlation with histological grade, lymphatic metastasis (P<0.05) (Table 2). The MLKL expression in poorly differentiated cervical squamous cell carcinoma was lower than that in moderately- and well-differentiated cervical squamous cell carcinoma. Samples with lymphatic metastasis had lower MLKL expression than that without lymphatic metastasis (Table 3). Survival analysis showed the low MLKL expression levels were associated with the poor prognosis of patients with cervical squamous cell carcinoma (P = 0.036) (Figure 2).
Figure 1.
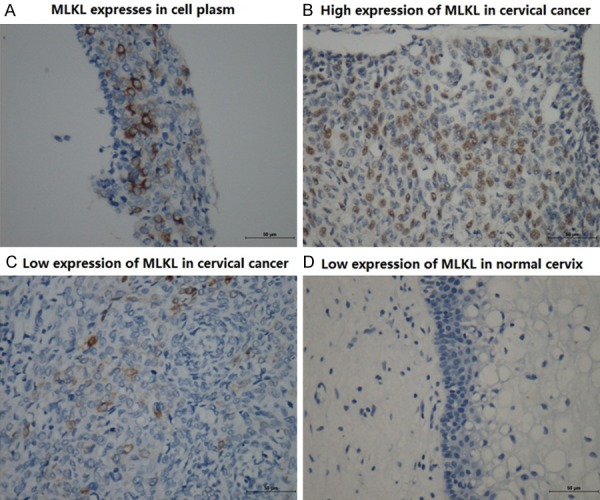
Expression of MLKL in cervical squamous cell cancer and inflammatory cervix.
Table 2.
Chi-square test of MLKL expression
| Chi-square test | |||
|---|---|---|---|
|
| |||
| Positive | Negative | P | |
| Normal cervix | 1 | 15 | 0.004 |
| Squamous cancer | 25 | 29 | |
| Histological grade | |||
| Low | 15 | 27 | 0.008 |
| Moderate | 9 | 1 | |
| High | 1 | 1 | |
| FIGO stage | 0.172 | ||
| I | 17 | 24 | |
| II | 8 | 5 | |
| Lymphatic metastasis | 0.022 | ||
| Yes | 1 | 8 | |
| No | 24 | 21 | |
| Invasion depth | 0.156 | ||
| >1/2 | 11 | 19 | |
| ≤1/2 | 14 | 10 | |
| Vaginal invasion | 0.101 | ||
| Yes | 8 | 4 | |
| No | 17 | 25 | |
| Parametrial invasion | 0.443 | ||
| Yes | 2 | 1 | |
| No | 23 | 28 | |
| Vascular invasion | 0.415 | ||
| Yes | 13 | 17 | |
| No | 12 | 12 | |
| Age | 0.494 | ||
| >50 years | 7 | 7 | |
| ≤50 years | 18 | 22 | |
Table 3.
Correlation analysis of MLKL expression
| Correlation analysis | ||
|---|---|---|
|
| ||
| Correlation index | P | |
| Histological grade | -0.382 | 0.002 |
| Lymphatic metastasis | -0.216 | 0.048 |
Figure 2.
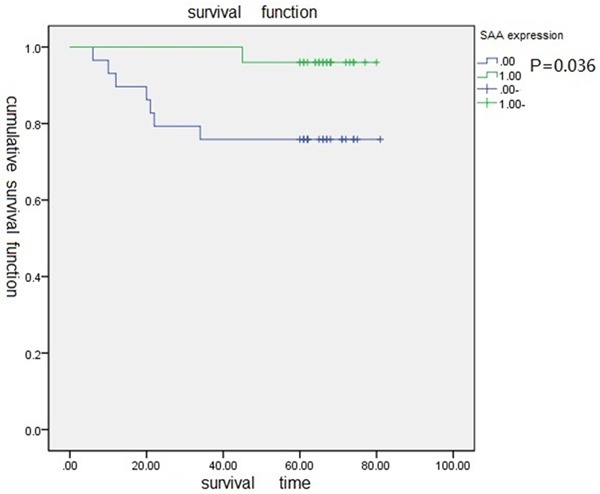
Survival curve of high expression of MLKL and low expression of MLKL.
Discussion
Necrosis is a type of cell death and is morphologically characterized by a gain in cell volume, swelling of organelles, plasma membrane rupture, and subsequent loss of intracellular contents [5]. Necroptosis is a caspase-independent form of cell death that contributes to the pathogenesis of several human diseases, including ischemia-reperfusion injury, sepsis, and viral infection [6-8]. Necroptosis plays an important role in health and disease [9]. MLKL is initially identified as a key mediator in TNF-induced necroptosis. Tumor necrosis factor (TNF) plays a critical role in diverse cellular events including apoptosis and necroptosis [10,11]. The mechanism of TNF-induced apoptosis is well elucidated. The signaling events that lead to TNF-initiated necroptosis are still largely unknown. Programmed necrotic cell death induced by the tumor necrosis factor alpha (TNF-α) family of cytokines is dependent on a kinase cascade consisting of receptor-interacting kinases RIP1 and RIP3. The mixed lineage kinase domain-like protein MLKL is a functional RIP3 substrate that binds to RIP3 through its kinase-like domain but lacks kinase activity of its own. Wang et al reported RIP3 phosphorylated MLKL at the T357 and S358 sites. The phosphorylated-MLKL formed an oligomer that binds to phosphatidylinositol lipids and cardiolipin, which allowed MLKL to move from the cytosol to the plasma and intracellular membrane. Then MLKL directly disrupted membrane integrity, resulting in necrotic death [12]. Cai et al also reported that MLKL formed a homotrimer through its amino-terminal coiled-coil domain and located to the cell plasma membrane during TNF-induced necroptosis [13]. The plasma membrane localization of trimerized MLKL was critical for mediating necroptosis and the membrane localization of MLKL was essential for Ca2+ influx, which was an early event of TNF-induced necroptosis [13].
It has been reported that MLKL expression can serve as a potential prognostic biomarker for patients with early-stage resected pancreatic cancer [4]. Ling He et al reported that low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients [3]. Our research found the expression of MLKL had relation with histological grade, lymphatic metastasis of cervical squamous cell cancer. Interestingly, low expression of MLKL was also associated with poor prognosis in cervical squamous cell cancer patients. Low expression MLKL may lead to decreased necrosis, which may be the reason of poor prognosis. The MLKL expression in cervical squamous cell cancer of high malignancy is lower than that of low malignancy, which indicates cervical squamous cell cancer of high malignancy may have lower necrosis rate and be more likely to metastasis. The MLKL expression in patients with lymphatic metastasis is lower than that in patients without lymphatic metastasis, which also support the viewpoint that MLKL mediates the necrosis and inhibits the development of cervical squamous cell cancer.
In conclusion, our study suggested that MLKL might serve as a potential therapeutic target in cervical squamous cell cancer patients. MLKL may be used to estimate the prognosis of cervical squamous cell cancer patients. Furthermore, it may be used as a target of chemotherapy or radiotherapy effect, which needs further study.
Disclosure of conflict of interest
None.
References
- 1.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–7. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He L, Peng K, Liu Y, Xiong J, Zhu FF. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Oncol Targets Ther. 2013;6:1539–43. doi: 10.2147/OTT.S52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colbert LE, Fisher SB, Hardy CW, Hall WA, Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K, Kowalski J, Kooby DA, El-Rayes BF, Staley CA 3rd, Adsay NV, Curran WJ Jr, Landry JC, Maithel SK, Yu DS. Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer. 2013;119:3148–55. doi: 10.1002/cncr.28144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zong WX. TC: necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 6.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–18. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemiareperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–9. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2012;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT, Declercq W, Vandenabeele P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010;17:922–30. doi: 10.1038/cdd.2009.184. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–5. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]


