Abstract
To see the possible relationship between COMT Val158Met polymorphism and blood pressure (BP) and serum lipid levels and its putative role in human longevity, we genotyped COMT Val158Met (rs4680) by PCR-RFLP for members from Bama long-lived families (BLF, n = 1538), Bama non-long-lived families (BNLF, n = 600), Pingguo (a county outside Bama region) long-lived families (PLF, n = 538) and Pingguo non-long-lived families (PNLF, n = 403) after anthropometric measures were collected and serum lipid levels were detected. The distribution of genotypes and alleles among four family groups was significantly different (all P < 0.01), with GA/AA genotype and minor allele A presenting more frequently in Bama population than Pingguo Population (P < 0.01). The systolic blood pressure (SBP), pulse pressure (PP), total cholesterol (TC), triglyceride (TG) and low density lipoprotein-cholesterol (LDL-C) levels of GG genotype carriers were dramatically higher than non-GG carriers in BNLF (P < 0.05); the SBP and PP levels of GG carriers were lower (P < 0.05) while TC, LDL-C level were higher (P < 0.01) than that of non-GG carriers in PLF; no difference in blood pressure and lipids were observed between genotypes in BLF and PNLF (P > 0.05). Correlation analyses revealed that COMT Val158Met was mainly correlated negatively with SBP, diastolic blood pressure (DBP) and LDL-C in BNLF and negatively with TC level in BLF, BNLF and PLF. These data suggest that COMT Val158Met polymorphism may have more impact on the modulation of BP and lipid profiles in the average families than in the long-lived families in Bama region. The association between this SNP and other phenotypes (e.g. cognition) and its roles in the longevity in Bama area thus warrant further investigation.
Keywords: COMT, blood pressure, blood lipid, longevity
Introduction
Catechol-O-methyl-transferase (COMT) is an enzyme that catalyses the degradation of catecholamine neurotransmitters including dopamine (DA), norepinephrine, epinephrine, L-dopa and their metabolites and thus inactivate them [1]. It is well established that DA plays essential roles in executive processing, especially in the function of pre-frontal cortex (PFC) [2,3], and age-related loss of dopaminergic function correlates with cognitive impairment [4].
The gene encoding human COMT is mapped to chromosome 22q11 which spans 27 kb with 6 exons and is the most widely studied member of the neurotransmission-related gene classes [5]. A common functional variant at nucleotide 472 (G > A, rs4680) on exon 4 of human COMT gene results in a valine to methionine substitution at the 158 amino acid (Val158Met) and reduces its thermostability and activity by ~40% [6]. Higher enzymatic activity of COMT may accelerate the degradation of DA and attenuate cognitive performance while lowered COMT activity may perturb DA metabolism and enhance cognitive functionality. For instance, individuals carrying the ancestral Val allele present higher enzymatic activity but perform less well on working memory tests and executive cognition than those with the low activity Met allele [3,7]. Patients with Parkinson’s disease (PD) and Alzheimer’s disease (AD) who exhibit poor cognition are more prevalent of COMT Val allele than average controls [8]. The COMT Val allele is associated with a younger age at onset in men with idiopathic PD [9]. Preclinical trials showed that COMT inhibitors improved working memory and attention in model animals and humans [2,10]. More intriguingly, this polymorphism has so far only been found in humans, the only species that can develop cognition, a behavioral domain in which humans differ from non-human primates [3]. Together, these observations demonstrate that COMT Val allele correlates strongly with poorer while Met allele with better cognitive performance. In this context, it is therefore reasonable to hypothesize that long-lived populations who preserve good cognition may be endowed a higher COMT Met frequency.
Nevertheless, it is worth noting that COMT has both central and peripheral effect, elevated circulatory COMT may increase peripheral vascular resistance and predisposes individuals to hypertension. In addition, cognition is a considerably complex trait which is not only determined by cerebral structure and volume but also influenced by other factors such as blood and nutrition supply. Hypertension and unfavorable lipid profile may impair cerebral vascular functionality and be associated with poorer cognitive status.
Bama long-lived individuals residingalong the midstream of Hongshuihe River in Guangxi Pro-vince, P. R. China have emerged as an optimal cohort for human aging/longevity study over the past decades [11]. They had been found to preserve better cognition and daily living activity as compared to age-matched populations from other regions in China [12]. In the current investigation, we genotyped the COMT Val158Met polymorphism and evaluate the potential correlation with blood pressure and lipid levels for Bama long-lived families with normal cognition to see whether this variant overrepresents in these families and account for the longevity in Bama area by interplaying with common cognitive influencing factors.
Materials and methods
Subjects studied
The study design and the participating families had been described elsewhere [11]. Briefly, 1538 family members from Bama long-lived families residing Bama area along the midstream of Hongshuihe River Basin (Bama, Fengshan, Donglan and Du’an County), Guangxi Zhuang Autonomous Region, P. R. China were enrolled as our target study group (BLF, 870 males and 668 females, aged 61. 8 ± 25. 8 ranging 30-104 years). Family members from three other families living in Bama and out-of-Bama area were recruited as comparison groups: (1) Bama non-long-lived families (BNLF, n = 600, 394 males and 206 females, age 53. 1 ± 23. 5 ranging 30-77 years), who live in the same area as BLF (environment-matched) but without a history of exceptional longevity (no past or current nonagenarian/centenarian in the first, second and third degree relatives); (2) Pingguo long-lived families (PLF, n = 538, 342 males and 196 females, aged 58. 5 ± 25. 5 ranging 30-95 years) from Pingguo, a county which belongs to Youjiang River system and is 200 km away from Bama area (environment-unmatched); (3) Pingguo non-long-lived families (PNLF, n = 403, 258 males and 145 females, aged 48. 5 ± 19. 6 ranging 30-72 years), average families from Pingguo County which have no history of exceptional longevity. These control groups were set to attenuate the potential confounding effect of environmental factors such as dietary habit and lifestyle or ethnic background on our observational variables. All subjects were ethnically Zhuang, apparently healthy and had no evidence of any chronic illness. Participants with a history of myocardial infarction, stroke, hypertension and diabetes were excluded. The current study was approved by the Ethics Committee of Guangxi Medical University. All participants gave their written informed consents after an extensive description of aims of the study.
Epidemiological survey
Information on socio-demographic and lifestyle factors was collected with standardized questionnaires. Anthropometric variables including blood pressure, body height, body weight, waist circumference and body mass index (BMI) were measured or calculated in all groups as described previously [11]. Hypertension was defined as systolic blood pressure (SBP) > 140 mmHg and/or diastolic blood pressure (DBP) > 90 mmHg, and/or self-reported anti-hypertensive treatment within 2 weeks prior to the survey [13]. Normal weight, overweight, and obesity were defined as BMI < 24, 24 to 28, and > 28 kg/m2, respectively [14].
Biochemical measurements
An overnight fasting venous blood sample of 8 mL was drawn by venipuncture from each subject, 4 mL of which was collected in a glass tube for serum separation and subsequent lipid determination while the remaining was transferred to an anticoagulant tube (4.80 g/L citric acid, 14.70 g/L glucose, and 13.20 g/L trisodium citrate) for DNA extraction. Total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) levels were measured by enzymatic methods with commercially available kits as also previously described [11]. Individuals with TC > 5.17 mmol/L and/or TG > 1.70 mmol/L were defined as dyslipidemia [15].
Genotyping
Genomic DNA was extracted from peripheral blood leukocytes by using standard methods. The COMT Val158Met polymorphism was genotyped by PCR-RFLP method using endonuclease Nla III for digestion as described by Lachman et al [16]. To ensure the reliability of genotyping, six randomly selected DNA samples (two for each genotype) were sequenced and the sequencing results were all in line with that of genotyping. Laboratory technicians who performed genotyping were blinded to clinical and biochemical data.
Statistical analyses
Levels of the quantitative variables are presented as mean ± SD (TG levels are presented as medians and interquartile due to skewed distribution). Allelic and genotypic frequencies were calculated directly. Hardy-Weinberg equilibrium was computed for the expected genotype distribution using the standard goodness-of-fit test. Difference in genotype and allele distribution between the groups was estimated by using the chi-square test. The statistical evaluation for the categorical variables was based on the calculation of the Student t-test. The association of COMT genotypes with blood pressure and serum lipid variables was evaluated using analysis of covariance (ANCOVA). Multiple logistic analyses with stepwise modelling were used to evaluate the association of blood pressure and serum lipid levels with genotypes (GG = 1, GA = 2, AA = 3) and several environment factors. In all hypothesis tests, two-tailed values of P < 0.05 were considered statistically significant. All data were analyzed using the statistical software package SPSS 16.0 (SPSS Inc, Chicago, IL).
Results
General clinical characteristics
The comparison of basic demographic and clinical data between BLF and other referent groups was summarized in Table 1. The SBP, DBP, BMI, FPG, TC, TG, LDL-C levels of BLF were similar with that of local BNLF controls but were significantly higher than that of non-local counterparts. This might be ascribed to the higher mean age of BLF. No difference was found on pulse pressure and HDL-C levels among the four groups.
Table 1.
Comparison of clinical characteristics among groups (x̅±s)
| Parameters | BLF (n = 1538) | BNLF (n = 600) | PLF (n = 538) | PNLF (n = 403) | F (χ2) | P |
|---|---|---|---|---|---|---|
| Male/female (n) | 870/668 | 394/206 | 342/196 | 258/145 | 20.872 | 0.001 |
| Age (years) | 61.75±25.80 | 53.12±23.51▲ | 58.54±25.45▲ | 48.53±19.60▲,# | 77.477 | 0.000 |
| BMI (kg/m2) | 21.37±3.41 | 21.41±3.20 | 21.00±3.14▲ | 21.63±3.68 | 3.162 | 0.024 |
| FPG (mmol/L) | 4.75±1.34 | 4.53±1.09▲ | 4.41±1.47▲ | 4.590±1.60 | 6.007 | 0.004 |
| SBP (mmHg) | 143.45±27.69 | 137.47±25.33 | 136.17±27.00▲ | 129.26±21.56▲,# | 11.180 | 0.000 |
| DBP (mmHg) | 86.56±12.10 | 85.30±11.55 | 81.67±11.03▲ | 81.53±11.15▲,# | 27.312 | 0.000 |
| PP (mmHg) | 56.89±23.08 | 52.18±20.74 | 54.59±22.92 | 47.73±17.72 | 1.440 | 0.229 |
| TC (mmoL/L) | 5.16±1.05 | 5.12±0.98 | 4.576±0.93▲ | 4.58±0.98▲,# | 60.096 | 0.000 |
| TG (mmoL/L) | 1.06 (0.77) | 1.08 (0.82) | 0.94 (0.68)▲ | 0.91 (0.79)▲,# | 5.287 | 0.001 |
| HDL-C (mmoL/L) | 1.60±0.43 | 1.56±0.39 | 1.57±0.37 | 1.57±0.40 | 0.380 | 0.768 |
| LDL-C (mmoL/L) | 2.92±0.92 | 2.94±0.87 | 2.42±0.78▲ | 2.42±0.85▲,# | 59.133 | 0.000 |
BLF, Bama long-lived families; BNLF, Bama non-long-lived families; PLF, Pingguo long-lived families; PNLF, Pingguo non-long-lived families; BMI, body mass index; FPG, fasting plasma glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. The quantitative variables were presented as mean ± standard deviation and the values of TG were presented as median (interquartile range). As compared to BLF;
indicates P < 0.05.
Comparison between PNLF and HNLF;
indicates P < 0.05.
Genotype and allele distribution
None of the genotypic and allelic distributions deviated significantly from that predicted by the Hardy-Weinberg equilibrium (P > 0.05). The frequencies of minor allele (A, Met) and its homozygotic (AA) and heterozygotic (GA, Val/Met) genotype of BLF were almost identical to that of BNLF (25.88%, 7.22% and 37.32% vs. 24.67%, 7.00% and 35.33%) but markedly higher than that of Pingguo groups (Figure 1A-C). However, these differences did not maintain through sex stratification (data not shown).
Figure 1.
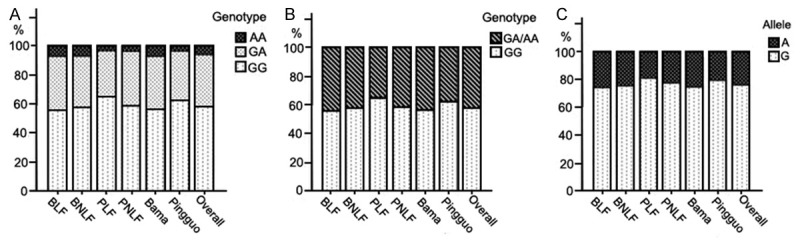
A. Comparison of genotypes among groups, x2 = 24.901, P = 0.000, between regions, x2 = 19.904, P = 0.000; B. Comparison between combined genotypes (GA/AA) and GG genotype among groups, x2 = 15.139, P = 0.002, between region, x2 = 10.288, P = 0.001; C. Comparison of allele among groups, x2 = 21.010, P = 0.000, between regions, x2 = 17.337, P = 0.000.
Genotype and blood pressure and serum lipids
As shown in Table 2, in the overall, Bama and Pingguo population, no impact of COMT Val158Met polymorphism on SBP, DBP and PP was detected (P > 0.05 for all), however, when it came to lipids, the TC and LDL-C levels of A allele carriers (GA/AA) were remarkably lower than non-A carriers (GG) (P < 0.05 for each). When analyzed according to families, no difference was observed on blood pressure and lipid levels between the mutant (GA/AA) and the ancestral genotype (GG) in BLF and PNLF, which persisted after sex stratification. However, GA/AA genotype dramatically lowered SBP, PP, TC, TG and LDL-C levels in BNLF while raised SBP in PLF. When gender was taken into account, the impact of GA/AA genotype on blood pressure in BNLF mainly existed in males while its impact on lipids mainly existed in females. In PLF, GA/AA genotype was associated with elevated DBP and lowered TC and LDL-C in males while with higher PP in females (Table 3). Together, in Bama population, the influence of COMT Val158Met polymorphism on blood pressure and lipid levels predominantly in average families rather than long-lived families.
Table 2.
Impact of COMT Val158Met genotype on BP and lipid levels in different groups
| Group/genotype | n | SBP (mmHg) | DBP (mmHg) | PP (mmHg) | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) |
|---|---|---|---|---|---|---|---|---|
| Overall | ||||||||
| GG | 1785 | 139.48±26.57 | 85.00±12.01 | 54.47±22.15 | 5.02±1.04 | 1.01 (0.69) | 1.59±0.40 | 2.81±0.89 |
| GA+AA | 1294 | 138.72±27.13 | 84.52±11.71 | 54.19±22.22 | 4.91±1.04 | 1.04 (0.74) | 1.57±0.42 | 2.73±0.93 |
| F/Z | 0.196 | 0.215 | 0.798 | 5.722 | -0.231 | 0.931 | 4.563 | |
| p | 0.658 | 0.643 | 0.372 | 0.017 | 0.818 | 0.335 | 0.033 | |
| Bama region | ||||||||
| GG | 1199 | 142.50±27.25 | 86.68±12.15 | 55.82±22.87 | 5.19±1.03 | 1.07 (0.83) | 1.60±0.40 | 2.95±0.89 |
| GA+AA | 939 | 140.77±27.03 | 85.58±11.67 | 55.18±22.10 | 5.09±1.03 | 1.06 (0.71) | 1.58±0.44 | 2.89±0.92 |
| F/Z | 0.744 | 2.593 | 0.000 | 5.171 | -0.906 | 0.989 | 2.116 | |
| p | 0.388 | 0.107 | 0.994 | 0.023 | 0.365 | 0.320 | 0.146 | |
| Pingguo region | ||||||||
| GG | 586 | 133.11±23.89 | 81.48±10.89 | 51.63±20.28 | 4.65±0.96 | 0.91 (0.67) | 1.57±0.39 | 2.51±0.82 |
| GA+AA | 355 | 133.28±26.70 | 81.72±11.37 | 51.56±22.36 | 4.45±0.93 | 0.94 (0.76) | 1.57±0.39 | 2.28±0.79 |
| F/Z | 2.929 | 0.411 | 2.707 | 5.740 | -0.111 | 0.069 | 10.583 | |
| p | 0.087 | 0.522 | 0.100 | 0.017 | 0.912 | 0.792 | 0.001 | |
| BLF | ||||||||
| GG | 853 | 143.57±27.57 | 86.95±12.08 | 56.63±23.17 | 5.19±1.04 | 1.04 (0.80) | 1.61±0.41 | 2.93±0.89 |
| GA+AA | 685 | 143.29±27.85 | 86.07±12.12 | 57.22±22.99 | 5.11±1.07 | 1.08 (0.74) | 1.58±0.45 | 2.91±0.95 |
| F/Z | 0.150 | 1.324 | 1.339 | 1.275 | -0.432 | 1.531 | 0.089 | |
| p | 0.699 | 0.250 | 0.247 | 0.259 | 0.665 | 0.216 | 0.765 | |
| BNLF | ||||||||
| GG | 346 | 139.90±26.31 | 86.02±12.33 | 53.88±22.05 | 5.21±1.02 | 1.15 (0.83) | 1.56±0.38 | 3.00±0.88 |
| GA+AA | 254 | 134.19±23.58 | 84.31±10.33 | 49.87±18.62 | 5.02±0.91 | 1.03 (0.63) | 1.56±0.40 | 2.86±0.86 |
| F/Z | 4.775 | 1.087 | 3.976 | 8.513 | -2.316 | 0.108 | 7.205 | |
| p | 0.029 | 0.298 | 0.049 | 0.004 | 0.021 | 0.743 | 0.007 | |
| PLF | ||||||||
| GG | 350 | 135.29±24.91 | 81.17±10.94 | 54.12±20.90 | 4.69±0.93 | 0.91 (0.67) | 1.58±0.39 | 2.54±0.78 |
| GA+AA | 188 | 137.82±30.37 | 82.40±11.12 | 55.43±26.23 | 4.34±0.89 | 1.03 (0.69) | 1.56±0.35 | 2.22±0.76 |
| F/Z | 4.543 | 0.744 | 4.128 | 11.724 | -0.305 | 1.168 | 11.929 | |
| p | 0.034 | 0.389 | 0.043 | 0.001 | 0.761 | 0.280 | 0.001 | |
| PNLF | ||||||||
| GG | 236 | 129.99±22.03 | 81.93±10.81 | 48.06±18.82 | 4.59±1.00 | 0.92 (0.68) | 1.55±0.39 | 2.47±0.86 |
| GA+AA | 167 | 128.24±20.90 | 80.96±11.63 | 47.27±16.10 | 4.56±0.97 | 0.91 (0.97) | 1.59±0.42 | 2.35±0.82 |
| F/Z | 0.059 | 0.016 | 0.148 | 0.020 | 0.130 | 0.340 | 1.038 | |
| p | 0.809 | 0.900 | 0.701 | 0.888 | 0.896 | 0.560 | 0.309 |
Table 3.
Impact of COMT genotype on BP and lipids in different families stratified by gender
| Group/genotype | n | SBP (mmHg) | DBP (mmHg) | PP (mmHg) | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | |
|---|---|---|---|---|---|---|---|---|---|
| BLF | |||||||||
| Male | GG | 485 | 138.26±22.67 | 87.33±11.46 | 50.93±18.91 | 5.19±1.07 | 1.10 (1.01) | 1.59±0.44 | 2.85±0.93 |
| GA+AA | 385 | 137.99±23.09 | 86.08±11.31 | 51.90±18.08 | 5.05±1.06 | 1.11 (0.90) | 1.58±0.51 | 2.80±0.94 | |
| F/Z | 0.963 | 1.577 | 4.214 | 3.013 | -0.866 | 0.005 | 0.340 | ||
| p | 0.327 | 0.209 | 0.040 | 0.083 | 0.387 | 0.942 | 0.560 | ||
| Female | GG | 368 | 150.77±31.73 | 86.42±12.86 | 64.35±26.03 | 5.19±1.00 | 0.99 (0.61) | 1.64±0.36 | 3.03±0.82 |
| GA+AA | 300 | 150.36±31.85 | 86.05±13.14 | 64.31±26.67 | 5.20±1.08 | 1.04 (0.64) | 1.59±0.35 | 3.05±0.94 | |
| F/Z | 0.143 | 0.352 | 0.010 | 0.000 | -1.862 | 3.153 | 0.015 | ||
| p | 0.705 | 0.553 | 0.922 | 0.982 | 0.063 | 0.076 | 0.904 | ||
| BNLF | |||||||||
| Male | GG | 230 | 137.80±25.22 | 87.27±12.49 | 50.53±20.77 | 5.11±0.94 | 1.22 (0.86) | 1.53±0.38 | 2.88±0.84 |
| GA+AA | 164 | 131.51±18.89 | 84.37±10.25 | 47.15±13.79 | 4.98±0.93 | 1.04 (0.84) | 1.52±0.41 | 2.83±0.90 | |
| F/Z | 5.548 | 6.225 | 1.216 | 2.805 | -1.865 | 0.027 | 0.616 | ||
| p | 0.019 | 0.013 | 0.271 | 0.095 | 0.062 | 0.870 | 0.433 | ||
| Female | GG | 116 | 144.14±28.03 | 83.51±11.67 | 60.63±23.09 | 5.40±1.14 | 1.07 (0.81) | 1.62±0.37 | 3.25±0.92 |
| GA+AA | 90 | 139.07±29.83 | 84.22±10.52 | 54.84±23.47 | 5.08±0.87 | 0.95 (0.52) | 1.64±0.38 | 2.93±0.76 | |
| F/Z | 0.970 | 0.392 | 2.268 | 5.296 | -1.409 | 0.083 | 8.484 | ||
| p | 0.326 | 0.532 | 0.134 | 0.022 | 0.159 | 0.774 | 0.004 | ||
| PLF | |||||||||
| Male | GG | 218 | 134.24±21.20 | 81.96±10.02 | 52.28±18.48 | 4.78±0.93 | 1.01 (0.90) | 1.53±0.39 | 2.58±0.81 |
| GA+AA | 124 | 134.62±24.73 | 84.23±11.35 | 50.39±20.04 | 4.33±0.90 | 1.07 (1.03) | 1.55±0.38 | 2.15±0.74 | |
| F/Z | 0.928 | 4.974 | 0.121 | 19.797 | -0.765 | 0.356 | 22.769 | ||
| p | 0.336 | 0.026 | 0.728 | 1.17E-5 | 0.445 | 0.551 | 2.74E-6 | ||
| Female | GG | 132 | 137.28±30.75 | 79.67±12.42 | 57.61±24.57 | 4.52±0.90 | 0.76 (0.44) | 1.66±0.36 | 2.47±0.74 |
| GA+AA | 64 | 144.33±38.86 | 78.67±9.69 | 65.67±33.59 | 4.36±0.88 | 0.91 (0.41) | 1.57±0.31 | 2.36±0.79 | |
| F/Z | 2.286 | 0.311 | 4.878 | 1.447 | -1.735 | 2.879 | 0.739 | ||
| p | 0.132 | 0.578 | 0.029 | 0.231 | 0.083 | 0.092 | 0.391 | ||
| PNLF | |||||||||
| Male | GG | 157 | 128.09±20.75 | 83.11±11.12 | 44.98±18.34 | 4.59±1.05 | 1.00 (0.94) | 1.48±0.37 | 2.47±0.94 |
| GA+AA | 101 | 126.77±19.23 | 82.23±11.97 | 44.53±14.35 | 4.62±0.99 | 0.95 (1.17) | 1.55±0.43 | 2.33±0.87 | |
| F/Z | 1.014 | 0.002 | 1.335 | 0.511 | -0.278 | 2.781 | 0.776 | ||
| p | 0.315 | 0.962 | 0.249 | 0.475 | 0.774 | 0.097 | 0.379 | ||
| Female | GG | 79 | 133.69±24.038 | 79.63±9.84 | 54.06±18.41 | 4.59±0.89 | 0.84 (0.43) | 1.69±0.40 | 2.49±0.70 |
| GA+AA | 66 | 130.42±23.14 | 79.08±10.91 | 51.35±17.73 | 4.48±0.94 | 0.81 (0.52) | 1.64±0.40 | 2.37±0.75 | |
| F/Z | 0.403 | 0.101 | 0.362 | 0.207 | -0.155 | 0.302 | 0.541 | ||
| p | 0.527 | 0.751 | 0.548 | 0.650 | 0.877 | 0.583 | 0.463 | ||
Correlation analyses
Multiple linear regression analyses showed that COMT Val158Met correlated positively with PP in BLF while negatively with SBP and DBP in BNLF, i.e., A-allele carries tended to have higher PP in BLF but lower SBP and DBP in BNLF. No any correlation was observed between blood pressure and COMT polymorphism in the two Pinguo groups.
On lipids, COMT Val158Met correlated with TC and LDL-C inversely in BNLF and PL, with A allele carriers tending to produce less TC and LDL-C. An absence of association was noted this polymorphism and lipids in BLF and PNLF (Tables 4, 5).
Table 4.
Relationship between blood pressure and relative factors in different families
| BP | Relative factor | B | Std. error | Beta | t | P |
|---|---|---|---|---|---|---|
| BLF | ||||||
| SBP | Age | 0.646 | 0.023 | 0.616 | 28.210 | 0.000 |
| DBP | Age | 0.178 | 0.018 | 0.380 | 9.835 | 0.000 |
| TC | 0.964 | 0.314 | 0.083 | 3.067 | 0.002 | |
| BP | Age | 0.479 | 0.025 | 0.541 | 18.907 | 0.000 |
| Genotype | 2.568 | 1.002 | 0.056 | 2.563 | 0.010 | |
| BNLF | ||||||
| SBP | Age | 0.702 | 0.037 | 0.651 | 18.874 | 0.000 |
| Genotype | -4.953 | 1.738 | -0.095 | -2.849 | 0.005 | |
| DBP | Age | 0.120 | 0.021 | 0.249 | 5.833 | 0.000 |
| Sex | -3.829 | 0.969 | -0.159 | -3.952 | 0.000 | |
| Genotype | -2.436 | 0.921 | -0.104 | -2.645 | 0.008 | |
| LDL-C | 1.490 | 0.569 | 0.110 | 2.617 | 0.009 | |
| PP | Age | 0.540 | 0.029 | 0.614 | 18.634 | 0.000 |
| PLF | ||||||
| SBP | Age | 0.735 | 0.035 | 0.680 | 20.814 | 0.000 |
| TG | 14.344 | 3.736 | 0.133 | 3.840 | 0.000 | |
| DBP | Age | 0.169 | 0.020 | 0.379 | 8.634 | 0.000 |
| PP | Age | 0.535 | 0.032 | 0.577 | 16.536 | 0.000 |
| TG | 13.611 | 3.444 | 0.147 | 3.952 | 0.000 | |
| HDL-C | 4.836 | 2.120 | 0.078 | 2.281 | 0.023 | |
| PNLF | ||||||
| SBP | Age | 0.532 | 0.046 | 0.491 | 11.672 | 0.000 |
| DBP | Age | 0.184 | 0.035 | 0.310 | 5.251 | 0.000 |
| TG | 8.021 | 2.152 | 0.191 | 3.728 | 0.000 | |
| PP | Age | 0.509 | 0.039 | 0.552 | 13.189 | 0.000 |
Table 5.
Relationship between serum lipid parameters and relative factors in different families
| Lipid parameters | Relative factor | B | Std. error | Beta | t | P | |
|---|---|---|---|---|---|---|---|
| BLF | |||||||
| TC | DBP | 0.008 | 0.002 | 0.092 | 3.195 | 0.001 | |
| Age | 0.010 | 0.001 | 0.243 | 7.005 | 0.000 | ||
| TG | None | ||||||
| HDL-C | None | ||||||
| LDL-C | Age | 0.008 | 0.001 | 0.227 | 7.943 | 0.000 | |
| DBP | 0.005 | 0.002 | 0.064 | 2.263 | 0.024 | ||
| BNLF | |||||||
| TC | Age | 0.006 | 0.002 | 0.154 | 3.536 | 0.000 | |
| Genotype | -0.210 | 0.083 | -0.105 | -2.527 | 0.012 | ||
| Sex | 0.214 | 0.088 | 0.104 | 2.446 | 0.015 | ||
| DBP | 0.010 | 0.004 | 0.112 | 2.542 | 0.011 | ||
| TG | Sex | 0.065 | 0.027 | 0.127 | 2.428 | 0.016 | |
| Genotype | -0.039 | 0.020 | -0.078 | -1.975 | 0.049 | ||
| HDL-C | None | ||||||
| LDL-C | Age | 0.010 | 0.002 | 0.271 | 5.131 | 0.000 | |
| Sex | 0.358 | 0.090 | 0.202 | 3.979 | 0.000 | ||
| DBP | 0.008 | 0.003 | 0.108 | 2.475 | 0.014 | ||
| PLF | |||||||
| TC | Genotype | -0.354 | 0.078 | -0.182 | -4.515 | 0.000 | |
| Age | 0.010 | 0.002 | 0.264 | 6.453 | 0.000 | ||
| TG | PP | 0.002 | 0.001 | 0.175 | 3.389 | 0.001 | |
| Age | -0.002 | 0.001 | -0.150 | -2.553 | 0.011 | ||
| HDL-C | Age | 0.004 | 0.001 | 0.239 | 5.528 | 0.000 | |
| LDL-C | Age | 0.008 | 0.001 | 0.242 | 5.748 | 0.000 | |
| Genotype | -0.320 | 0.069 | -0.193 | -4.621 | 0.000 | ||
| PNLF | |||||||
| TC | Age | 0.008 | 0.002 | 0.168 | 3.435 | 0.001 | |
| TG | Sex | -0.094 | 0.025 | -0.164 | -3.720 | 0.000 | |
| DBP | 0.003 | 0.001 | 0.144 | 3.144 | 0.002 | ||
| HDL-C | Age | 0.005 | 0.001 | 0.247 | 5.031 | 0.000 | |
| LDL-C | Age | 0.011 | 0.003 | 0.259 | 4.557 | 0.000 |
Discussion
In the current study, the frequency of the minor A allele of the COMT gene at +158 locus in the overall population studied is around 0. 24, similar to that of other Chinese healthy populations (e.g. Shanghai Hans, 0. 25; Southwestern Hans, 0. 21), Japanese population (0.32) and even American blacks (0.32), but profoundly lower than that of Caucasians (German, 0.52) [17-21], indicating a geographical-specific allelic distribution pattern worldwide. The underlying mechanism for this great discrepancy and its implication has yet to be elucidated. Further stratification analyses by gender and region showed that the frequency of mutant COMT genotypes (GA/AA) of BLF is statistically analogous to that of local general families, but significantly higher than that of out-of-Bama populations. The overrepresentation of an allele in Bama population seems to implicate that if it acts as an advantage factor it may be a genetic contribution to the longevity in the area; by contrary however, if it is a deleterious variant there may be other unknown buffering variants to counteract its unfavorable effect [22].
COMT 158Met has not only been linked to a reduced COMT activity and an increased cerebral DA level which is favorable of the maintenance of better cognition, but also to a higher risk of hypertension due to a spontaneous elevation of peripheral DA concentration. For instance, systolic and diastolic pressure had been found to be raised in Met/Met COMT homogenous individuals in Taiwanese females and Sweden and Japanese middle-aged males as compared to the other two genotypes, which was interpreted by a lowered degradation of DA and catechol estrogen and their resultant peripheral synaptic levels [7,23]. By investigating the impact of COMT rs4680 on blood pressure and its association with daily intake of salt and calories, Htun et al observed that Japanese middle-aged men carrying COMT Met/Met genotype displayed higher systolic and diastolic pressure levels and higher incidence rate of hypertension than who carried Val/Val and Val/Met genotypes [24]. These ob-servations had found their support in animal models, in which hypertensive rats exhibited higher COMT activity than normotensive rats [25], and COMT knockout mice were more resistant to salt-induced hypertension than genetically normal mice [26]. Nevertheless, Hagan et al noted almost opposite results in Norwegian population: enhanced instead of reduced COMT activity correlated with hypertension, Val/Val rather than Met/Met homogenous genotype presented more frequently in SBP-elevated subjects [27]. In the current study, after adjustment for age, we found that although the blood pressure level of BLF was significantly higher than that of geographic- and non-geographic-matched controls, it lacked distinct association with COMT Val158Met polymorphism, while in BNLF, Met allele was found to be related to lowered SBP and DBP, particularly in men. These divergent finding may be attributed to the different genetic background, sample size, sex ratio, age range of the enrolled subjects across studying populations [28]. Assuming that COMT Met is indeed correlated with hypertension, COMT inhibitor entacapone may be a therapeutic strategy, but its elusive anti-hypertensive effect has not been established [29]. Thus, there is no clear consensus on the association between COMT rs4680 and hypertension to date. We have no appropriate explanation for the higher blood pressure in BLF currently; a long-term longitudinal follow-up is thus warranted.
Very little data was available as far as the association between COMT Val158Met polymorphism and lipid profile is concerned, most of which were mainly based on population models of some disorders. For instance, Almeida and colleagues did not reveal any impact of this variant on lipid levels in Brazilian perimenopausal women [30]. Patients with chronic renal disorders who carried COMT 158Met presented lowered LDL-C level [19]. Schizophrenia sufferers complicated with metabolic syndrome (MS) who harbored COMT 158Met documented an elevated TG and homocysteine (Hcy) level in females but not in males [17]. This modulation of TG and Hcy was believed to be the outcome of the interactions between COMT 158Met and MTHFR 677C/T, a common variant on methylene tetrahydrofolate reductase (MTHFR) gene [31]. Works from other research group had also shown that COMT might involve in the metabolism of Hcy in that COMT activity positively correlated with serum Hcy level while COMT inhibitor lowered Hcy level [32]. In addition, COMT might affect human appetite and eating behavior via dopaminergic pathway, entailing preschool children a predilection to palatable oily food and resultant hyperlipidemia and obesity [33]. To our knowledge, this is the first family-based study to evaluate the association of COMT Val158Met and lipid profiles in the general population and long-lived subjects, unraveling overall a reverse correlation between COMT Val158Met and TC and LDL-C. This correlation, however, might be affected by family and gender in that it no longer remained in BLF through family and gender stratification, indicating a limited impact, if any, of COMT Val158Met on lipid modulation in long-lived families. The relatively higher lipid levels in BLF might be attributed to its higher proportion of the oldest olds who had less molar teeth and preferred myofiber-free fatty pork [11].
In sum, although presented more frequently in Bama area, COMT 158Met mainly modulate blood pressure and lipid levels in general families rather than long-lived families. The limited impact of this polymorphism on blood pressure and lipid parameters may imply that there are other unveiled variants which might interplay with each other and shape the age-related traits and the survival status in Bama longevous area. Its possible links with other phenotypes (e.g. cognition) and contributions to the longevity in Bama region deserve further exploration.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31160209, 81360066) and the Natural Science Foundation of Guangxi (2013GXNSFAA019180, 2013GXNSFBB053002). We thank all participants for their sample donation. We specifically appreciate Dr. Jian-Hua Huang (Fengshan People Hospital), Dr. Hong-Bo Liang (Donglan People’s Hospital) and Dr. Wen Shi (Pingguo Bureau of Health) for the organization for sampling and field studies.
Disclosure of conflict of interest
None.
References
- 1.Bertocci B, Miggiano V, Da Prada M, Dembic Z, Lahm HW, Malherbe P. Human catechol-O-methyltransferase: cloning and expression of the membrane-associated form. Proc Natl Acad Sci U S A. 1991;88:1416–20. doi: 10.1073/pnas.88.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D. COMT Val158Met and cognition: main effects and interaction with educational attainment. Genes Brain Behav. 2009;8:36–42. doi: 10.1111/j.1601-183X.2008.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Reinvang I, Deary IJ, Fjell AM, Steen VM, Espeseth T, Parasuraman R. Neurogenetic effects on cognition in aging brains: a window of opportunity for intervention? Front Aging Neurosci. 2010;2:143. doi: 10.3389/fnagi.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez MF, Martín XE, Alcelay LG, Flores JC, Valiente JM, Juanbeltz BI, Beldarraín MA, López JM, Gonzalez-Fernández MC, Salazar AM, Gandarias RB, Borda SI, Marqués NO, Amillano MB, Zabaleta MC, de Pancorbo MM. The COMT Val158 Met polymorphism as an associated risk factor for Alzheimer disease and mild cognitive impairment in APOE 4 carriers. BMC Neurosci. 2009;10:125. doi: 10.1186/1471-2202-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klebe S, Golmard JL, Nalls MA, Saad M, Singleton AB, Bras JM, Hardy J, Simon-Sanchez J, Heutink P, Kuhlenbäumer G, Charfi R, Klein C, Hagenah J, Gasser T, Wurster I, Lesage S, Lorenz D, Deuschl G, Durif F, Pollak P, Damier P, Tison F, Durr A, Amouyel P, Lambert JC, Tzourio C, Maubaret C, Charbonnier-Beaupel F, Tahiri K, Vidailhet M, Martinez M, Brice A, Corvol JC French Parkinson’s Disease Genetics Study Group; International Parkinson’s Disease Genomics Consortium (IPDGC) The Val158Met COMT polymorphism is a modifier of the age at onset in Parkinson’s disease with a sexual dimorphism. J Neurol Neurosurg Psychiatry. 2013;84:666–73. doi: 10.1136/jnnp-2012-304475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32:1011–20. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 11.Pan SL, Luo XQ, Lu ZP, Lu SH, Luo H, Liu CW, Hu CY, Yang M, Du LL, Song Z, Pang GF, Wu HY, Huang JB, Peng JH, Yin RX. Microsomal triglyceride transfer protein gene -493G/T polymorphism and its association with serum lipid levels in Bama Zhuang long-living families in China. Lipids Health Dis. 2012;11:177. doi: 10.1186/1476-511X-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv Z, Zheng C, Yang Z, Liang J, Hu C, Li X, Han B, Yang X, Chen J, Pang G. Cognitive Function and Activity of Daily Living of the Old Elderly of Zhuung Nationality in Bama County. Chinese Mental Health Journal. 2003;17:98–100. [Google Scholar]
- 13.Ruixing Y, Jiaqiang D, Dezhai Y, Weixiong L, Shangling P, Jinzhen W, Jiandong H, Xiuyan L. Effects of demographic characteristics, health related behaviors and lifestyle factors on the prevalence of hypertension for the middleaged and elderly in the Guangxi Hei Yi Zhuang and Han populations. Kidney Blood Press Res. 2006;29:312–20. doi: 10.1159/000097019. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B Cooperative Meta-analysis Group of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:5–10. [PubMed] [Google Scholar]
- 15.Ruixing Y, Yuming C, Shangling P, Fengbing H, Tangwei L, Dezhai Y, Jinzhen W, Limei Y, Weixiong L, Rongshan L, Jiandong H. Effects of demographic, dietary, and other lifestyle factors on the prevalence of hyperlipidemia in Guangxi Hei Yi Zhuang and Han populations. Eur J Cardiovasc Prev Rehabil. 2006;13:977–84. doi: 10.1097/01.hjr.0000239476.79428.25. [DOI] [PubMed] [Google Scholar]
- 16.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Chen M, Chen J, Wu Z, Yu S, Fang Y, Zhang C. Metabolic syndrome in patients taking clozapine: prevalence and influence of catechol-O-methyltransferase genotype. Psychopharmacology (Berl) 2014;231:2211–8. doi: 10.1007/s00213-013-3410-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Li H, Tao P, Wang YP, Yuan P, Yang CX, Li JY, Yang F, Lee H, Huang Y. Soy Isoflavones, CYP1A1, CYP1B1, and COMT Polymorphisms, and Breast Cancer: a case-control study in southwestern China. DNA Cell Biol. 2011;30:585–95. doi: 10.1089/dna.2010.1195. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida T, Kato K, Yokoi K, Oguri M, Watanabe S, Metoki N, Yoshida H, Satoh K, Aoyagi Y, Nishigaki Y, Nozawa Y, Yamada Y. Association of genetic variants with chronic kidney disease in individuals with different lipid profiles. Int J Mol Med. 2009;24:233–46. doi: 10.3892/ijmm_00000226. [DOI] [PubMed] [Google Scholar]
- 20.Ittiwut R, Listman JB, Ittiwut C, Cubells JF, Weiss RD, Brady K, Oslin D, Farrer LA, Kranzler HR, Gelernter J. Association between polymorphisms in catechol-O-methyltransferase (COMT) and cocaine-induced paranoia in European-American and African-American populations. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:651–60. doi: 10.1002/ajmg.b.31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witte AV, Jansen S, Schirmacher A, Young P, Flöel A. COMT Val158Met Polymorphism Modulates Cognitive Effects of Dietary Intervention. Front Aging Neurosci. 2010;2:146. doi: 10.3389/fnagi.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huffman DM, Deelen J, Ye K, Bergman A, Slagboom EP, Barzilai N, Atzmon G. Distinguishing Between Longevity and Buffered-Deleterious Genotypes for Exceptional Human Longevity: the case of the MTP Gene. J Gerontol A Biol Sci Med Sci. 2012;67:1153–60. doi: 10.1093/gerona/gls103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worda C, Sator MO, Schneeberger C, Jantschev T, Ferlitsch K, Huber JC. Influence of the catechol-O-methyltransferase (COMT) codon 158 polymorphism on estrogen levels in women. Hum Reprod. 2003;18:262–6. doi: 10.1093/humrep/deg059. [DOI] [PubMed] [Google Scholar]
- 24.Htun NC, Miyaki K, Song Y, Ikeda S, Shimbo T, Muramatsu M. Association of the catechol-Omethyl transferase gene Val158Met polymorphism with blood pressure and prevalence of hypertension: interaction with dietary energy intake. Am J Hypertens. 2011;24:1022–6. doi: 10.1038/ajh.2011.93. [DOI] [PubMed] [Google Scholar]
- 25.Okuda T, Sumiya T, Iwai N, Miyata T. Pyridoxine 5’-phosphate oxidase is a candidate gene responsible for hypertension in Dahl-S rats. Biochem Biophys Res Commun. 2004;313:647–53. doi: 10.1016/j.bbrc.2003.11.149. [DOI] [PubMed] [Google Scholar]
- 26.Helkamaa T, Männistö PT, Rauhala P, Cheng ZJ, Finckenberg P, Huotari M, Gogos JA, Karayiorgou M, Mervaala EM. Resistance to salt-induced hypertension in catechol-O-methyltransferase-gene-disrupted mice. J Hypertens. 2003;21:2365–74. doi: 10.1097/00004872-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, Zwart JA. High systolic blood pressure is associated with Val/Val genotype in the catechol-o-methyltransferase gene. The Nord-Trondelag Health Study (HUNT) Am J Hypertens. 2007;20:21–6. doi: 10.1016/j.amjhyper.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol O-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism. 2008;57:708–11. doi: 10.1016/j.metabol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Jordan J, Lipp A, Tank J, Schröder C, Stoffels M, Franke G, Diedrich A, Arnold G, Goldstein DS, Sharma AM, Luft FC. Catechol-o-methyltransferase and blood pressure in humans. Circulation. 2002;106:460–5. doi: 10.1161/01.cir.0000022844.50161.3b. [DOI] [PubMed] [Google Scholar]
- 30.Almeida S, Zandoná MR, Franken N, Callegari-Jacques SM, Osório-Wender MC, Hutz MH. Estrogen-metabolizing gene polymorphisms and lipid levels in women with different hormonal status. Pharmacogenomics J. 2005;5:346–51. doi: 10.1038/sj.tpj.6500329. [DOI] [PubMed] [Google Scholar]
- 31.Burghardt KJ, Pilsner JR, Bly MJ, Ellingrod VL. DNA methylation in schizophrenia subjects: gender and MTHFR 677C/T genotype differences. Epigenomics. 2012;4:261–8. doi: 10.2217/epi.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamberti P, Zoccolella S, Iliceto G, Armenise E, Fraddosio A, de Mari M, Livrea P. Effects of levodopa and COMT inhibitors on plasma homocysteine in Parkinson’s disease patients. Mov Disord. 2005;20:69–72. doi: 10.1002/mds.20261. [DOI] [PubMed] [Google Scholar]
- 33.Galvão AC, Krüger RC, Campagnolo PD, Mattevi VS, Vitolo MR, Almeida S. Association of MAOA and COMT gene polymorphisms with palatable food intake in children. J Nutr Biochem. 2012;23:272–7. doi: 10.1016/j.jnutbio.2010.12.004. [DOI] [PubMed] [Google Scholar]


