Abstract
Nemo-like kinase (NLK), as a mitogen activated protein kinase (MAPK)-like kinase, is involved in the development of several human cancers. In this study, we explored the expression of NLK in lung squamous cell carcinoma (SCC) and adenocarcinoma tissues, and investigated the associations among NLK, β-catenin, T-cell factor 4 (TCF4), and the clinicopathological factors of lung cancers. The expressions of NLK, β-catenin, TCF4 were examined in 109 cases of lung cancers using immunohistochemistry method. The expression of NLK was observed in the nuclei of lung cancer tissues, and was significantly higher in lung cancer tissues than that in corresponding normal lung tissues (t = 21.636, n = 109, P < 0.001). The high expression of NLK was found in 45 cases of lung SCCs (45/49, 91.84%), which was much more than that in adenocarcinomas (38/60, 63.33%) (P = 0.001). Furthermore, the high expression of NLK was negatively correlated with TCF4 expression and positively correlated with the membranous expression of β-catenin. In conclusion, the present study demonstrated that the expression of NLK was localized in nucleus and significantly increased in lung cancers. The expression of NLK was negatively correlated with TCF4 expression and positively correlated with β-catenin membranous expression in lung cancers.
Keywords: Nemo-like kinase, T-cell factor 4, β-catenin, wnt signaling pathway, lung neoplasm
Introduction
Nemo-like kinase (NLK) is discovered as an evolutionarily conserved protein kinase from worms to humans. Similar to mitogen activated protein kinases/extracellular-signal regulated kinases (MAPKs/ERKs), NLK belongs to a proline-directed serine/threonine protein kinase superfamily [1,2]. The substrates of NLK include several transcription factors, Such as T-cell factor/lymphoid enhancer factor (TCF/LEF), c-Myb and STAT3 [3-6]. NLK can phosphorylate TCF/LEF transcription factors and inhibit TCF/LEF-mediated transcription. So, NLK is considered a negative regulator of Wnt signaling pathway [2-4]. But, recent study found that NLK also functions as a positive regulator of Wnt signaling pathway by phosphorylating LEF1 in neural progenitor cells [7]. NLK is also considered a negative regulator of Notch signaling pathway, because NLK inhibits Notch1 intracellular domain (Notch1-ICD) mediated gene expression by phosphorylating Notch1-ICD [8]. NLK interacts with p53 and enhances p53 activity and stability by blocking MDM2-mediated p53 ubiquitination and degradation [9]. NLK phosphorylates the C-terminal domain of CREB binding protein (CBP)/p300, and suppresses the transcription activity of NF-κB, AP-1, Smad, and p53, all of which utilize CBP as a co-activator [10].
Because of the pivotal roles in regulating transcription factors and signaling pathways, NLK is involved in embryonic patterning, nervous system development, and even development of several human cancers. Generally, NLK is considered as a cancer inhibitor. Overexpression of NLK induced apoptosis in colon cancers, prostate cancers and gliomas [11-14]. Overexpression of NLK can decrease the expression of androgen receptor as well as inhibited androgen receptor-mediated transcription [12]. The low expression of NLK was correlated with glioma grade and poor patient outcome [13]. Recent study showed that NLK was significantly downregulated in the breast cancer tissues and was negatively correlated with c-Myb expression. NLK suppressed proliferation, induced apoptosis in breast cancer MCF-7 cells [15]. But, on the contrary, some studies revealed that NLK was associated with the progression of some cancers. NLK was overexpression in nasopharyngeal carcinomas [16], hepatocellular carcinomas [17,18] and gallbladder cancers [19,20]. The positive expression of NLK was associated with cancer progression and shorter survival in nasopharyngeal carcinomas [16] and gallbladder cancers [19,20]. Knockdown of NLK can suppress cell growth and arrested cell cycle transition of liver cancer cells [17,18], and enhance the sensitivity to Taxol treatment in laryngeal cancer cells [21]. It is unclear why NLK plays different role in diverse cancers. The mechanism of NLK regulating cancer progression needs to be addressed.
Lung cancer is one of the most common cancers in the world. The expression and function of NLK in lung cancers is unclear. In this study, we explored the expression of NLK in lung squamous cell carcinoma (SCC) and adenocarcinoma tissues, and investigated the associations among NLK, β-catenin, TCF4, and the clinicopathological factors of lung cancers, in order to gain an understanding of the role of NLK in lung cancer progression.
Materials and methods
Patients and tissue specimens
In this study, 109 lung SCC and adenocarcinoma samples and corresponding normal lung tissues were obtained from patients selected randomly from a population that underwent surgery in the First Affiliated Hospital of China Medical University between 2009 and 2013. The patients included in the study were 63 men and 46 women, and aged 34-81 years with a mean age of 61 years. The histological diagnosis and grade of differentiation were assessed by examination of hematoxylin-eosin (H/E)-stained sections according to the classification system of the World Health Organization. The tumors were diagnosed as SCCs (n = 49) or adenocarcinomas (n = 60). These tumors were classified as well-differentiated (n = 27), moderately differentiated (n = 58), or poorly differentiated (n = 24) tumors. Forty-seven cases showed lymphatic metastasis. The tumor stage was classified as stages I-III (n = 52, 45 and 12, respectively) according to the TNM classification system of the International Union Against Cancer. The study was conducted according to the regulations of the institutional review boards at China Medical University.
Immunohistochemistry
All resected specimens were embedded in paraffin blocks after fixed with 10% neutral-buffered formalin for 24 hours. Tissue blocks were cut into 4-μm sections. These sections were deparaffinized, rehydrated. The antigen retrieval was performed by pressure cooking in a PH 6 citrate buffer for 1.5 min. Then, the sections were incubated with polyclonal rabbit anti-NLK antibody (sc-48361; 1:200; Santa Cruz Biotechnology Inc., CA), monoclonal mouse anti-β-catenin antibody (610154; 1:200; BD Transduction Laboratories, KY) or polyclonal rabbit anti-TCF4 antibody (sc-13027; 1:200; Santa Cruz Biotechnology Inc., CA) at 4°C overnight. The detection of antibodies was performed using the streptavidin-peroxidase method. Some slides were stained in the absence of primary antibodies and served as negative controls.
Evaluation of immunostaining
All the immunostained sections were evaluated by 2 investigators who were blinded to the clinical data. Five views per slide were randomly examined, and 100 tumor cells were observed per view at × 400 magnification. The positive rate of every case was obtained by calculating the percentage of positively stained cells in each slide. In this study, NLK was expressed in the nuclei of cancer cells. The tumor with positive rate < 25% or ≥ 25% were classified as have low or high expression of NLK. β-Catenin and TCF4 were primarily expressed in the cytoplasm in lung cancers. Percentage scores of β-catenin and TCF4 were assigned as (1) 1-25%; (2) 26-50%; (3) 51-75%; and (4) 76-100%. The intensity of β-catenin or TCF4 cytoplasmic staining were scored as 0, 1, 2 and 3 if negative, weak, moderate, or marked, respectively. Scores from each tumor sample were multiplied to give a final score of 0 to 12, and the tumors were finally determined, based on scores ≤ 6, and ≥ 8 as having low or high cytoplasmic expression, respectively. When ≥ 10% of the tumor cells per specimen were stained in the nuclear or membranous regions, the sample was scored as having positive nuclear or membranous expression of β-catenin.
Statistical analysis
The paired-samples t-test was used to analyze the differences in expression of NLK between the lung cancer tissues and the corresponding normal lung tissues. The Pearson Chi-Square test or the Likelihood-Ratio test was used to assay whether the expression levels of NLK, β-catenin or TCF4 were related to the clinicopathological factors of lung cancers. The Spearman’s correlation test was used to examine the correlation among NLK, β-catenin and TCF4 levels. P values less than 0.05 were considered statistically significant.
Results
The nuclear expression of NLK was increased in lung cancers than in corresponding normal lung tissues
In this study, the expression of NLK was observed in the nuclei of lung cancer cells or normal lung epithelial cells (Figure 1). The average expression rate of NLK was 63.53% ± 24.50% in lung cancer tissues, which was significantly higher than that in corresponding normal lung tissues (10.90% ± 11.75%) (t = 21.636, n = 109, P < 0.001). Eight-three cases of lung cancers were scored as high expression of NLK (83/109, 76.15%), meanwhile, only 14 cases of corresponding normal lung tissues were scored as high expression of NLK (14/109, 12.84%).
Figure 1.
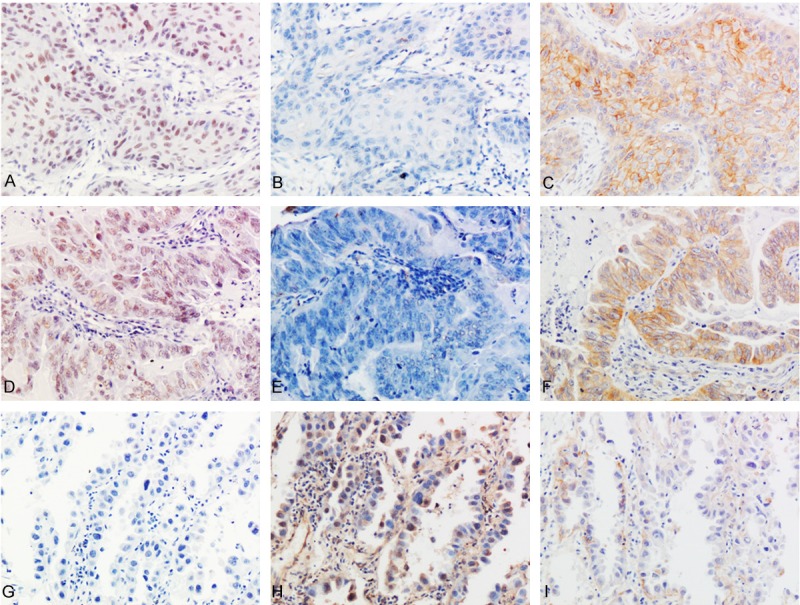
The expressions of NLK, TCF4 and β-catenin in representative lung cancer cases (streptavidin-peroxidase immunohistochemistry method). In a lung squamous cell carcinoma case (A-C) and an adenocarcinoma case (D-F), NLK was highly expressed in the nuclei (A and D), but TCF4 was negatively expressed (B and E). Meanwhile, β-catenin showed positively membranous and cytoplasmic expression (C and F). In another adenocarcinoma case (G-I), the expression of NLK was negative (G), but TCF4 showed positively cytoplasmic and nuclear expression (H). Meanwhile, β-catenin was expressed weakly in the cytoplasm (I) (Original magnification, 200 ×).
The high expression of NLK was negatively correlated with TCF4 expression and positively correlated with the membranous expression of β-catenin
We examined the expression of NLK, TCF4 and β-catenin in lung cancers and investigated the correlations among them. The Spearman’s correlation test revealed that the expression of NLK was negatively correlated with the expression of TCF4 (Correlation coefficient = -0.222, P = 0.020). Furthermore, the expression of NLK was also correlated with the membranous expression of β-catenin (Correlation coefficient = 0.297, P = 0.002), but was not correlated with the abnormal cytoplasmic expression of β-catenin (Correlation coefficient = 0.065, P = 0.501) or nuclear expression of β-catenin (Correlation coefficient = -0.107, P = 0.269) (Table 1).
Table 1.
The correlations between the expressions of NLK, TCF4 and β-catenin in lung cancers
| n | NLK expression | ||||
|---|---|---|---|---|---|
|
| |||||
| Low | High | Correlation coefficient | P value | ||
| TCF4 expression | -0.222 | 0.020 | |||
| Low | 42 | 5 | 37 | ||
| High | 67 | 21 | 46 | ||
| β-catenin membranous expression | 0.297 | 0.002 | |||
| + | 30 | 1 | 29 | ||
| - | 79 | 25 | 54 | ||
| β-catenin cytoplasmic expression | 0.065 | 0.501 | |||
| Low | 28 | 8 | 20 | ||
| High | 81 | 18 | 63 | ||
| β-catenin nuclear expression | -0.107 | 0.269 | |||
| + | 14 | 5 | 9 | ||
| - | 95 | 21 | 74 | ||
The high expression of NLK was more common in lung SCCs than in adenocarcinomas
After immunostaining, we examined the associations between the expression of NLK and clinicopathological factors in lung cancers. The high nuclear expression of NLK was observed in 45 cases of lung SCCs (45/49, 91.84%), which was much more than that in adenocarcinomas (38/60, 63.33%) (P = 0.001). But,the expression of NLK was not associated with the differentiation (P = 0.911), TNM stage (P = 0.705), lymphatic metastasis (P = 0.493), or patients’ sex (P = 0.069) and age (P = 0.924) (Table 2).
Table 2.
The correlations between NLK expression and the clinicopathological parameters of lung cancers
| n | NLK expression | |||
|---|---|---|---|---|
|
| ||||
| Low | High | P value | ||
| Sex | 0.067 | |||
| Male | 63 | 11 | 52 | |
| Female | 46 | 15 | 31 | |
| Age | 0.924 | |||
| < 61 | 47 | 11 | 36 | |
| ≥ 61 | 62 | 15 | 47 | |
| Histological type | 0.001 | |||
| SCC | 49 | 4 | 45 | |
| Adenocarcinoma | 60 | 22 | 38 | |
| Differentiation | 0.911 | |||
| Well | 27 | 7 | 20 | |
| Moderate | 58 | 14 | 44 | |
| Poor | 24 | 5 | 19 | |
| TNM stage | 0.705 | |||
| I | 52 | 14 | 38 | |
| II | 45 | 10 | 35 | |
| III | 12 | 2 | 10 | |
| Lymphatic metastasis | 0.493 | |||
| Yes | 44 | 9 | 35 | |
| No | 65 | 17 | 48 | |
The abnormal expression of β-catenin was associated with the differentiation of lung cancers, but TCF4 expression was associated with none of the clinicopathological factors
The abnormal expression of β-catenin was associated with the poorly differentiation of lung cancers (P = 0.001), but was not associated with the histological type (P = 0.052), TNM stage (P = 0.270), lymphatic metastasis (P = 0.055), or patients’ sex (P = 0.211) and age (P = 0.635). The expression of TCF4 was not associated with any of the clinicopathological factors (data not shown). Furthermore, Spearman’s correlation test revealed that the membranous expression of β-catenin was positively correlated with the cytoplasmic expression of β-catenin (Correlation coefficient = 0.268, P = 0.005), and negatively correlated with the nuclear expression of β-catenin (Correlation coefficient = 0.237, P = -0.013).
Discussion
As a MAPK-like proline-directed serine/threonine protein kinase, NLK can phosphorylate many transcription factors, including TCF/LEF, c-Myb, STAT3 and Notch1-ICD [3-6,8]. So, NLK can regulate the activities of some signaling pathways, such as Wnt signaling pathway and Notch signaling pathway, and take part in the carcinogenesis and development of cancers [4,7,8,22]. NLK is primarily considered as an anti-cancer factor by inhibiting the transcription activity of TCF/LEF-β-catenin complex [2,23]. The expression of NLK was reduced in breast cancers and gliomas [13,15]. Upregulating the expression of NLK induced apoptosis in colon cancers, prostate cancers and gliomas [11-13]. But, surprisingly and interestingly, the overexpression of NLK was also found in nasopharyngeal carcinomas [16] hepatocellular carcinomas [17,18] and gallbladder cancers [19,20]. NLK was associated with the development of nasopharyngeal carcinomas and gallbladder cancers [16,19]. The role of NLK in diverse cancers is contradictory. Resent study revealed that besides negatively regulating the Wnt pathway, NLK also could promote the transcription activation of TCF/LEF [7]. In neural progenitor cells, NLK phosphorylates LEF1 at Thr-155 and Ser-166. The phosphorylation of LEF1 induces itself dissociating from histone deacetylase HDAC1, and activates the transcription of Wnt target genes. So, one possible reason for opposite function of NLK is that the phosphorylation level and phosphorylation sites of TCF/LEF might affect the function of NLK in different cells [2].
In this study, we examined the expression and correlations of NLK in lung cancer tissues. The expression of NLK was localized in nucleus and significantly increased in lung cancers than in corresponding normal lung tissues. The high expression of NLK was more common in SCCs than in adenocarcinomas. But, we failed to find any correlations between the expression of NLK and clinicpathological factors of lung cancers. Furthermore, we found that the expression of NLK was negatively correlated with the expression of TCF4. This result confirmed that NLK could phosphorylates TCF4 and downregulates the level of TCF4, further inhibits the activation of Wnt signaling pathway. Other study also demonstrated that NLK was mainly localized in the nuclei in some cancers [19,24] and the expression of NLK was increased in cancers than normal tissues [16-20]. But, our results were different to the previously study in lung cancers, which indicated that the expression of NLK was low and localized in cytoplasm [23]. We considered this might because of the using of different antibody of NLK, which might detect different localization of NLK. Shaw-Hallgren et al. found that the cytoplasmic localization of NLK induces cell apoptosis in breast cancers. The phosphorylation of NLK at Lys 155 and Thr 286 leads nuclear localization of NLK. The interaction of nuclear NLK and Heat shock protein 27 further protects cancer cells from apoptosis [24]. So, the localization of NLK might be another reason for its opposite function in diverse cancers. Interestingly, we found that the expression of NLK was correlated with the membranous expression of β-catenin in lung cancers. But, its mechanism was unclear. NLK might control Wnt signaling pathway by regulating the expression or localization of β-catenin. This hypothesis needs further study.
In conclusion, the present study demonstrated that the expression of NLK was localized in nucleus and significantly increased in lung cancers. The expression of NLK was negatively correlated with TCF4 expression and positively correlated with β-catenin membranous expression in lung cancers.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 81372497 to H.-T. Xu and Grant No. 81301930 to L.-H. Yang) and Program for Liaoning Excellent Talents in University (Grant No. LR2015067 to H.-T. Xu).
Disclosure of conflict of interest
None.
References
- 1.Brott BK, Pinsky BA, Erikson RL. Nlk is a murine protein kinase related to Erk/MAP kinases and localized in the nucleus. Proc Natl Acad Sci U S A. 1998;95:963–968. doi: 10.1073/pnas.95.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishitani T, Ishitani S. Nemo-like kinase, a multifaceted cell signaling regulator. Cell Signal. 2013;25:190–197. doi: 10.1016/j.cellsig.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between betacatenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 4.Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:1379–1389. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanei-Ishii C, Ninomiya-Tsuji J, Tanikawa J, Nomura T, Ishitani T, Kishida S, Kokura K, Kurahashi T, Ichikawa-Iwata E, Kim Y, Matsumoto K, Ishii S. Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes Dev. 2004;18:816–829. doi: 10.1101/gad.1170604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima H, Sasaki T, Ishitani T, Iemura S, Zhao H, Kaneko S, Kunimoto H, Natsume T, Matsumoto K, Nakajima K. STAT3 regulates Nemo-like kinase by mediating its interaction with IL-6-stimulated TGFbeta-activated kinase 1 for STAT3 Ser-727 phosphorylation. Proc Natl Acad Sci U S A. 2005;102:4524–4529. doi: 10.1073/pnas.0500679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ota S, Ishitani S, Shimizu N, Matsumoto K, Itoh M, Ishitani T. NLK positively regulates Wnt/beta-catenin signalling by phosphorylating LEF1 in neural progenitor cells. EMBO J. 2012;31:1904–1915. doi: 10.1038/emboj.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishitani T, Hirao T, Suzuki M, Isoda M, Ishitani S, Harigaya K, Kitagawa M, Matsumoto K, Itoh M. Nemo-like kinase suppresses Notch signalling by interfering with formation of the Notch active transcriptional complex. Nat Cell Biol. 2010;12:278–285. doi: 10.1038/ncb2028. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HH, Li SZ, Zhang ZY, Hu XM, Hou PN, Gao L, Du RL, Zhang XD. Nemo-like kinase is critical for p53 stabilization and function in response to DNA damage. Cell Death Differ. 2014;21:1656–1663. doi: 10.1038/cdd.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda J, Yokoo H, Yamada T, Kitabayashi I, Sekiya T, Ichikawa H. Nemo-like kinase suppresses a wide range of transcription factors, including nuclear factor-kappaB. Cancer Sci. 2004;95:52–57. doi: 10.1111/j.1349-7006.2004.tb03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda J, Tsuchiya A, Yamada T, Sakamoto M, Sekiya T, Hirohashi S. Nemo-like kinase induces apoptosis in DLD-1 human colon cancer cells. Biochem Biophys Res Commun. 2003;308:227–233. doi: 10.1016/s0006-291x(03)01343-3. [DOI] [PubMed] [Google Scholar]
- 12.Emami KH, Brown LG, Pitts TE, Sun X, Vessella RL, Corey E. Nemo-like kinase induces apoptosis and inhibits androgen receptor signaling in prostate cancer cells. Prostate. 2009;69:1481–1492. doi: 10.1002/pros.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui G, Li Z, Shao B, Zhao L, Zhou Y, Lu T, Wang J, Shi X, Zuo G, Zhu W, Shen A. Clinical and biological significance of nemo-like kinase expression in glioma. J Clin Neurosci. 2010;18:271–275. doi: 10.1016/j.jocn.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Wang X, Zou J, Zhang A, Wan Y, Pu P, Song Z, Qian C, Chen Y, Yang S, Wang Y. miR-92b controls glioma proliferation and invasion through regulating Wnt/beta-catenin signaling via Nemo-like kinase. Neuro Oncol. 2013;15:578–588. doi: 10.1093/neuonc/not004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Jiang Y, Lu W, Zhang Y. Nemo-like kinase associated with proliferation and apoptosis by c-Myb degradation in breast cancer. PLoS One. 2013;8:e69148. doi: 10.1371/journal.pone.0069148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Ma Z, Chen X, Zhang J. Prognostic significance of nemo-like kinase in nasopharyngeal carcinoma. Mol Med Rep. 2014;10:131–136. doi: 10.3892/mmr.2014.2190. [DOI] [PubMed] [Google Scholar]
- 17.Shen Q, Bae HJ, Eun JW, Kim HS, Park SJ, Shin WC, Lee EK, Park S, Park WS, Lee JY, Nam SW. MiR-101 functions as a tumor suppressor by directly targeting nemo-like kinase in liver cancer. Cancer Lett. 2013;344:204–211. doi: 10.1016/j.canlet.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Jung KH, Kim JK, Noh JH, Eun JW, Bae HJ, Xie HJ, Ahn YM, Park WS, Lee JY, Nam SW. Targeted disruption of Nemo-like kinase inhibits tumor cell growth by simultaneous suppression of cyclin D1 and CDK2 in human hepatocellular carcinoma. J Cell Biochem. 2010;110:687–696. doi: 10.1002/jcb.22579. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Zhang S, Wang Z, Zhang B, Wu X, Weng H, Ding Q, Tan Z, Zhang N, Mu J, Yang J, Shu Y, Bao R, Wu W, Cao Y, Liu Y. Prognostic significance of nemo-like kinase (NLK) expression in patients with gallbladder cancer. Tumour Biol. 2013;34:3995–4000. doi: 10.1007/s13277-013-0988-4. [DOI] [PubMed] [Google Scholar]
- 20.Tan Z, Li M, Wu W, Zhang L, Ding Q, Wu X, Mu J, Liu Y. NLK is a key regulator of proliferation and migration in gallbladder carcinoma cells. Mol Cell Biochem. 2012;369:27–33. doi: 10.1007/s11010-012-1365-0. [DOI] [PubMed] [Google Scholar]
- 21.Dong JR, Guo N, Zhao JP, Liu PD, Feng HH, Li Y. Inhibition of nemo-like kinase increases taxol sensitivity in laryngeal cancer. Asian Pac J Cancer Prev. 2014;14:7137–7141. doi: 10.7314/apjcp.2013.14.12.7137. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes VM, Panchapakesan SS, Braid LR, Verheyen EM. Nemo promotes Notchmediated lateral inhibition downstream of proneural factors. Dev Biol. 2014;392:334–343. doi: 10.1016/j.ydbio.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Lv L, Wan C, Chen B, Li M, Liu Y, Ni T, Yang Y, Cong X, Mao G, Xue Q. Nemo-like kinase (NLK) inhibits the progression of NSCLC via negatively modulating WNT signaling pathway. J Cell Biochem. 2014;115:81–92. doi: 10.1002/jcb.24635. [DOI] [PubMed] [Google Scholar]
- 24.Shaw-Hallgren G, Chmielarska Masoumi K, Zarrizi R, Hellman U, Karlsson P, Helou K, Massoumi R. Association of nuclear-localized Nemo-like kinase with heat-shock protein 27 inhibits apoptosis in human breast cancer cells. PLoS One. 2014;9:e96506. doi: 10.1371/journal.pone.0096506. [DOI] [PMC free article] [PubMed] [Google Scholar]


