Abstract
Backgrounds: Expression of eag1 channel (Eag1) is associated with cell malignant transformation, tumor cell metastasis and poor prognosis of the patient. This study aimed at examining whether expression of the Eag1 associated with aggressive clinicopathological feature and the molecular subtype of breast cancer. Materials and Methods: 109 patients who received breast cancer operation during January 2009 to December 2010 in Chinese-Japanese Friendship Hospital of Jilin University were recruited. We investigated the association of the Eag1 with clinicopathological features and molecular subtype of in triple negative breast cancer (TNBC) by univariate or multivariate analysis in a cross-section study. Results: The positive rate of Eag1 was 18.5% higher in TNBC compared with non-triple negative breast cancer (Non-TNBC) (P = 0.012, OR = 2.83, 95% CI = 2.16-3.47). Compared with the Eag1 negative group, the expression of Eag1 was linked to the larger tumor size (P = 0.002), advanced TNM stage (P = 0.029), high proportion of positive lymph node (87.6% vs. 65%, P = 0.014) and invasive ductal carcinoma (91% vs. 75%, P = 0.046). Conclusions: The expression of Eag1 may be partially explained the aggressive behavior of TNBC in the breast cancer tissue.
Keywords: Eag1 channel, clinicopathological features, triple negative breast cancer, association
Introduction
Breast cancer is the most frequently diagnosed cancer and becomes the leading cause of cancer death in female worldwide [1]. Furthermore, breast cancer will increase to 85 per 10,000 women by 2021 [2]. In China, the crude incidence rate and mortality rate of female breast cancer during 2003-2007 were 41.64 per 100,000, which was ranked as the top among female cancers, respectively [3], leading to a serious threat to women’s physical and mental health.
For the heterogeneity and complexity of the disease, single factor assessment is difficult to make the most suitable strategy for breast cancer treatment and predict the prognosis [4]. Based on molecular classification, breast cancer was categorized into four molecular subtypes: luminal breast cancer A, luminal breast cancer B, human epidermal growth factor receptor type 2 (HER2)-enriched breast cancer and triple negative breast cancer [5] (TNBC). However, TNBC exhibited a poor prognosis due to its aggressive biological behavior and insensitive to targeted and endocrine therapy. At present, TNBC still lack of effective treatment due to its aggressive biological behavior and insensitive to targeted and endocrine therapy. Furthermore, the patients with TNBC exhibited a poor prognosis and high mortality, compared with its non-triple-negative breast cancer (non-TNBC), particularly during the first 3-5 years of follow up [6]. In order to explore the reason of malignance for TNBC patients, it is critical to further analyze the new breast cancer target associated with clinicopathological features in breast cancer patients.
Eag1 channel (ether a-go-go potassium channel member 1, also known as Kv10.1), in physiological conditions, is restricted expression in the central nervous system, placenta, testes and prostate. However, overexpression of Eag1 has been found in many cancer, including cervical, breast, melanoma, colon, lung, and ovarian cancers [7-11]. Notably, Eag1 has been demonstrated to participate the processes of transformation, migration and metastasis of the tumor cell [12,13]. It was reported that the proportion of Eag1 positive expression in breast cancer patients was over 82% [14], so it is worthwhile to examine the association of Eag1 with the clinical progression of the tumor.
In this study, we analyze the association of Eag1 expression with clinicopathological features in breast cancer, and further evaluate the difference of eag1 channel expression between TNBC and non-TNBC, aim at investigated the role of eag1 channel in the breast cancer progression.
Materials and methods
Patients and diagnostic criteria
In this study, 109 patients who received breast cancer operation during January 2009 to December 2010 in Chinese-Japanese Friendship Hospital of Jilin University were recruited. All patients were Chinese Han population. The TNM stage was determined based on the American Joint Committee on Cancer (AJCC) criteria and the histological grade was assessed according to the modified Bloom-Richardson classification. The pathological types were assessed in accordance with the criterion of the world health organization (WHO) breast cancer pathology. Lymph node metastasis was defined as any metastasis in local region of ipsilateral axillary, supraclavicular, or internal mammary lymph nodes, or distant region of other contralateral axillary lymph nodes as well as supraclavicular lymph nodes.
Immunohistochemical staining and judgments of results
The samples of carcinoma tissue were collected from breast cancer patients undergoing surgery. Immunohistochemical staining was performed using the streptavidin biotin method (SP method) [15]. Optimal staining conditions and antibody dilutions were determined using formalin-fixed and paraffin-embedded tissue samples from human cerebral cortex for Eag1. The antibodies against Eag1 showed a good specificity. Antigen retrieval was performed in a microwave oven in 10 mM citrate buffer (pH 6.0) at 700 W for 15 min. Tissue sections were pre-incubated with normal goat serum (Zhongshanjinqiao, Beijing, China) at a 1:100 dilution in 3% bovine serum albumin/PBS. Slides were incubated overnight in a humidified chamber at 4°C with anti-Eag1 rabbit antiserum (Abcam, ab86204) at a 1:1200 dilution, followed by incubation with the EnVision Peroxidase System and DAB (Zhongshanjinqiao, Beijing, China).
Tumors with 1% or more positively nuclear-stained cells were considered positive for estrogen receptor (ER) and progesterone receptor (PR) expression; human epidermal growth factor receptor-2 (HER2) staining was scored by counting the number of cells positively stained on the membrane and expressed as a percentage of total tumor cells, according to the report of Hemmerlein et al [14]. Eag1 stains were considered positive if any (weak or strong) membranous invasive carcinoma cell staining was observed. The diagnosis was made by two pathologists who specialized in breast pathology through assessed each archival hematoxylin and eosin (H&E) stained slides.
Statistical analysis
The different distribution of the continuous variable of age between eag1 channel positive and negative groups as well as between TNBC and non-TNBC were examined by the independent t-test or one-way ANOVA. The differences of clinicopathological features, including category variables of age, histological type, and status of lymph node metastasis were assessed by chi-square test when compared the eag1 positive group with negative group. The differences clinicopathological features of histological grade, ranked tumor size and TNM stage between eag1 channel positive and negative groups were evaluated by the Wilcoxon rank sum test. Chi-square test was also used to compare the difference of eag1 channel positive rate between TNBC and non-TNBC. The associations of eag1 channel with clinicopathological features of breast cancer was assessed by the unconditional logistic regression. All statistical tests were two-sided, and P < 0.05 was considered significant. The statistical software SPSS version 19.0 (IBM Corp., Armonk, USA) was used for all statistical analyses.
Results
Clinicopathological features of the patients
A total of 109 female breast cancer cases were included in this study, with a mean age of 51.2 ± 10.4 years old, ranged from 26 to 85 years. The average tumor size was 3.4 ± 1.9 cm, ranged 2.3-5.9 cm. 85.3% (n = 93) were tumors with the size of more than 5 cm. Most of the tumors were invasive ductal carcinoma (88.1%). According to the TNM classification developed by American Joint Committee on Cancer (AJCC), nearly half of cases were diagnosed as stage II breast cancer (n = 51; 46.8%), about 41% was in stage III (n = 44; 40.4%), and the rest was in stage I (n = 14, 12.8%). For the histological classification of the breast cancer, 45 (41.3%) were in grade II, 44 (40.4%) in grade III and 20 (18.3%) in grade I. The pathology assessment indicated that 91 cases (83.5%) had involved in lymph node metastasis. As shown in Table 1, the positive rate for the expression of ER, PR, HER-2 and eag1 (Figure 1) channel were 66.9%, 59.6%, 29.4% and 81.7%, respectively.
Table 1.
Clinicopathological features and expression of biomarkers in breast cancer patients
| Clinicopathological features | N | % (range) |
|---|---|---|
| Mean age (M ± S) | 51.2 ± 10.4 | (26-85) |
| Tumor size (cm) | 3.4 ± 1.9 | (2.3-5.9) |
| < 2 | 14 | 12.8 |
| 2-5 | 80 | 73.4 |
| > 5 | 15 | 13.8 |
| Pathologic types | ||
| Invasive ductal | 96 | 88.1 |
| Others | 13 | 11.9 |
| TNM stage | ||
| I | 14 | 12.8 |
| II | 51 | 46.8 |
| III | 44 | 40.4 |
| Histologic grade | ||
| I | 20 | 18.3 |
| II | 45 | 41.3 |
| III | 44 | 40.4 |
| Lymph node metastasis | ||
| Positive | 91 | 83.5 |
| Negative | 18 | 16.5 |
| ER | ||
| Positive | 73 | 66.9 |
| Negative | 36 | 33.1 |
| PR | ||
| Positive | 65 | 59.6 |
| Negative | 44 | 40.4 |
| HER-2 | ||
| Positive | 32 | 29.4 |
| Negative | 77 | 70.6 |
| Eag1 | ||
| Positive | 89 | 81.7 |
| Negative | 20 | 18.3 |
Figure 1.
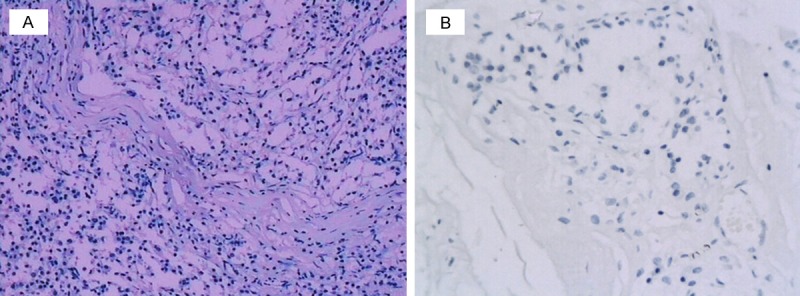
Representative Immunostaining of Eag1 in breast cancer carcinoma tissue. A. Positive staining for Eag1 in breast carcinoma tissues. B. Negative staining in breast carcinoma tissues (× 400).
Eag1 was highly expressed in TNBC
All cases of breast cancer was classified into two subgroup: TNBC and non-TNBC, based on IHC stain of ER, PR and HER-2. The results revealed that the proportion of TNBC and non-TNBC accounted 29.4% and 70.6%, respectively. The results indicated that the positive expression of Eag1 in TNBC was 18.5% higher than that in non-TNBC (P = 0.008). After adjusting for age in the unconditional logistic regression analysis, the results demonstrated that the expression of Eag1 in TNBC was still significantly higher than that in non-TNBC (P = 0.012, OR = 2.83, 95% CI = 2.14-3.47), indicated that there was a correlation between Eag1 and TNBC (Table 2).
Table 2.
Expression of Eag1 between TNBC and non-TNBC
| Eag1 | TNBC | Non-TNBC | P 1 | P 2 | OR (95% CI) |
|---|---|---|---|---|---|
| Positive (N, %) | 31 (93.8) | 58 (75.3) | 0.008 | 0.012 | 2.83 (2.16-3.47) |
| Negative (N, %) | 1 (6.2) | 19 (24.7) | |||
| Total (N, %) | 32 (100%) | 77 (100%) |
P-value from Chi-square test.
P-value from unconditional logistic regression analysis adjusting for age.
CI: confidence interval.
Expression of eag1 associated with clinicopathological features of breast cancer
The result demonstrated that the cases of eag1 channel positive expression showed a significant difference in Tumor size (P = 0.002) and TNM stage (P = 0.029) compared with the cases of eag1 channel negative expression. The proportion of 2-5 cm of tumor size in the cases of eag1 channel positive expression was the highest (79.8%), which was higher 34.8% than in the cases of eag1 channel negative expression. The proportion of TNM stage II and III in the cases of eag1 channel positive expression was higher 13.8%, 7.2% respectively than in the cases of eag1 channel negative expression. The most cases of eag1 channel positive was invasive ductal carcinoma, which showed a significant difference compared with the cases of eag1 channel negative expression (91% vs. 75%, P = 0.046). Compared with the eag1 negative expression group (65% of lymph node metastasis), the eag1 positive group (87.6% of lymph node metastasis) showed a much higher proportion of lymph node metastasis (P = 0.014). Although the histological grade II and III in eag1 channel positive group were higher than that in eag1 negative group, no difference reached statistical significance. The results of multivariate analysis demonstrated that, compared with eag1 channel negative group, the eag1 channel positive group was significantly difference in tumor size (P = 0.024, OR = 2.03, 95% CI = 1.82-2.63), pathologic types (P = 0.035, OR = 1.62, 95% CI = 1.12-2.36), TNM stage (P = 0.041, OR = 2.31, 95% CI = 1.68-3.36) and lymph node metastasis (P = 0.031, OR = 3.46, 95% CI = 2.03-4.31) (Table 3).
Table 3.
Eag1 expression associated with Clinicopathological features
| Clinicopathological features | Eag1 channel | P 1 | P 2 | OR (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| Positive | Negative | ||||
| Age (M ± S) | 52.2 ± 10.1 | 50.6 ± 9.8 | 0.362# | ||
| ≤ 40 | 22 (24.7) | 8 (40.0) | 0.167 | 0.214 | 1.23 (0.86-2.01) |
| > 40 | 67 (75.3) | 12 (60.0) | |||
| Tumor size | 0.002 | 0.024 | 2.03 (1.82-2.63) | ||
| < 2 cm | 7 (7.8) | 7 (35.0) | |||
| 2-5 cm | 71 (79.8) | 9 (45.0) | |||
| > 5 cm | 11 (12.4) | 4 (20.0) | |||
| Pathologic type | 0.046 | 0.035 | 1.62 (1.12-2.36) | ||
| Invasive ductal | 81 (91.0) | 15 (75.0) | |||
| Others | 8 (9.0) | 5 (25.0) | |||
| TNM stage | 0.029 | 0.041 | 2.31 (1.68-3.36) | ||
| I | 8 (9.0) | 6 (30.0) | |||
| II | 42 (47.2) | 9 (45.0) | |||
| III/IV | 39 (43.8) | 5 (25.0) | |||
| Histologic grade | 0.076 | 0.093 | 1.32 (0.83-1.98) | ||
| I | 13 (14.6) | 7 (35.0) | |||
| II | 37 (41.6) | 8 (40.0) | |||
| III | 39 (43.8) | 5 (25.0) | |||
| Lymph node metastasis | 0.014 | 0.031 | 3.46 (2.03-4.31) | ||
| Positive | 78 (87.6) | 13 (65.0) | |||
| Negative | 11 (22.4) | 7 (35.0) | |||
P value from Student’s t test.
P-value from Chi-square test or Wilcoxon rank sum test.
P-value from unconditional logistic regression analysis.
CI: confidence interval.
Discussion
To our knowledge, this study is the first to demonstrate that the positive rate of eag1 in TNBC was significantly higher than that in the non-TNBC, furthermore, Eag1 expression was associated with aggressive clinicopathological features, such as larger tumor size, advanced TNM stage, high proportion of positive lymph node and invasive ductal carcinoma.
Breast cancer molecular subtypes based on immunohistochemical markers have been confirmed to provide the usefulness of therapeutic and prognostic [16-19]. However, TNBC is a special subtype of breast cancer, which exhibited a poor prognosis due to its aggressive biological behavior and lack of effective treatment method, as this type of cancer is insensitive to targeted and endocrine therapy [20]. In order to explore the reason for the poor outcomes of TNBC, ongoing studies on TNBC are currently conducted clinically and experimentally. Meanwhile, many reports showed that Eag1 was overexpressed in distinct malignancies, including cervical, breast, lung, liver, prostate, colon, ovarian and gastric cancers [14,21-23]. Hemmerlein et al. have reported that Eag1 was overexpressed in breast cancer. What’s more, Eag1 has been demonstrated to participate the processes of transformation, migration and metastasis of the tumor cell [12,13]. In this study, Eag1 was expressed in 81.7% of breast cancer, which was consistent with Hemmerlein study [14].
Eag1 expression was associated with some clinical parameters in different categories of cancer, such as tumor size in head and neck cancer and lymph node metastasis in colorectal cancer [24,25]. Furthermore, Eag1 overexpression has been confirmed to correlate with the poorer prognosis, shorter overall survival in acute myelocytic leukemia (AML), ovarian, colorectal and oesophageal cancer [11,26,27]. However, no evidence was reported that eag1 expression associated with clinicopathogical features in breast cancer. Our study demonstrated that eag1 positive expression was associated with larger tumor size, advanced histological grade, more positive lymph node and invasive ductal carcinoma compared with Non-Eag1 expression, which indicated that eag1 overexpression was correlated with more aggressive clinicopathological features, which also indicated that high expression rate of Eag1 in TNBC may at least partially account for its more aggressive biological behavior.
Despite the relative small sample size and lack of the follow-up data of the patient, this study still provided the evidence that Eag1 may play an important role in the pathological process of breast cancer, indicated its potential role in the therapy for breast cancer, especially for TNBC.
Acknowledgements
This work was supported by grants of key projects in the National Science & Technology Pillar Program (No.SQ2015BA1300692) and Natural Science Foundation of Beijing Municipality to Yan He (7132027).
Disclosure of conflict of interest
None.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler RG, Anderson WF, Gail MH. Increasing breast cancer incidence in China: the numbers add up. J Natl Cancer Inst. 2008;100:1339–41. doi: 10.1093/jnci/djn330. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Sun TT, Wang N. [The incidence and mortality trends of female breast cancer in Beijing, China: between 2004 and 2008] . Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:1009–14. [PubMed] [Google Scholar]
- 4.DiGiovanna MP, Stern DF, Edgerton SM, Whalen SG, Moore D, Thor AD. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J. Clin. Oncol. 2005;23:1152–60. doi: 10.1200/JCO.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 5.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oakman C, Pestrin M, Zafarana E, Cantisani E, Di Leo A. Role of lapatinib in the first-line treatment of patients with metastatic breast cancer. Cancer Manag Res. 2010;2:13–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer R, Schönherr R, Gavrilova-Ruch O, Wohlrab W, Heinemann SH. Identification of ether à go-go and calcium-activated potassium channels in human melanoma cells. J Membr Biol. 1999;171:107–15. doi: 10.1007/s002329900563. [DOI] [PubMed] [Google Scholar]
- 8.Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–7. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding XW, Yan JJ, An P, Lü P, Luo HS. Aberrant expression of ether à go-go potassium channel in colorectal cancer patients and cell lines. World J Gastroenterol. 2007;13:1257–61. doi: 10.3748/wjg.v13.i8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz L, Ceja-Ochoa I, Restrepo-Angulo I, Larrea F, Avila-Chávez E, García-Becerra R, Borja-Cacho E, Barrera D, Ahumada E, Gariglio P, Alvarez-Rios E, Ocadiz-Delgado R, Garcia-Villa E, Hernández-Gallegos E, Camacho-Arroyo I, Morales A, Ordaz-Rosado D, García-Latorre E, Escamilla J, Sánchez-Peña LC, Saqui-Salces M, Gamboa-Dominguez A, Vera E, Uribe-Ramírez M, Murbartián J, Ortiz CS, Rivera-Guevara C, De Vizcaya-Ruiz A, Camacho J. Estrogens and human papilloma virus oncogenes regulate human ether-à-go-go-1 potassium channel expression. Cancer Res. 2009;69:3300–7. doi: 10.1158/0008-5472.CAN-08-2036. [DOI] [PubMed] [Google Scholar]
- 11.Asher V, Khan R, Warren A, Shaw R, Schalkwyk GV, Bali A, Sowter HM. The Eag potassium channel as a new prognostic marker in ovarian cancer. Diagn Pathol. 2010;5:78. doi: 10.1186/1746-1596-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegle AP, Marble DD, Wilson GF. A voltage-driven switch for ion-independent signaling by ether-à-go-go K+ channels. Proc Natl Acad Sci U S A. 2006;103:2886–91. doi: 10.1073/pnas.0505909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downie BR, Sánchez A, Knötgen H, Contreras-Jurado C, Gymnopoulos M, Weber C, Stühmer W, Pardo LA. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem. 2008;283:36234–40. doi: 10.1074/jbc.M801830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME, Martin S, Schliephacke T, Jenke M, Heinz-Joachim-Radzun , Stühmer W, Pardo LA. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41. doi: 10.1186/1476-4598-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Lee JS, Kim SI, Park BW. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–7. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, Magherini E, Mackey J, Martin M, Vogel C. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J. Clin. Oncol. 2009;27:1168–76. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, Veronesi P, Torrisi R, Montagna E, Luini A, Intra M, Gentilini O, Ghisini R, Goldhirsch A, Colleoni M. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (< 35 years) with operable breast cancer. Ann Oncol. 2010;21:1974–81. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J, Cheang MC, Mardis ER, Perou CM, Bernard PS, Ellis MJ. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–32. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J. Clin. Oncol. 2011;29:4273–8. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 20.Yuan N, Meng M, Liu C, Feng L, Hou L, Ning Q, Xin G, Pei L, Gu S, Li X, Zhao X. Clinical characteristics and prognostic analysis of triple-negative breast cancer patients. Mol Clin Oncol. 2014;2:245–251. doi: 10.3892/mco.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farias LM, Ocaña DB, Díaz L, Larrea F, Avila-Chávez E, Cadena A, Hinojosa LM, Lara G, Villanueva LA, Vargas C, Hernández-Gallegos E, Camacho-Arroyo I, Dueñas-González A, Pérez-Cárdenas E, Pardo LA, Morales A, Taja-Chayeb L, Escamilla J, Sánchez-Peña C, Camacho J. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64:6996–7001. doi: 10.1158/0008-5472.CAN-04-1204. [DOI] [PubMed] [Google Scholar]
- 22.Patt S, Preussat K, Beetz C, Kraft R, Schrey M, Kalff R, Schönherr K, Heinemann SH. Expression of ether à go-go potassium channels in human gliomas. Neurosci Lett. 2004;368:249–53. doi: 10.1016/j.neulet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Mello de Queiroz F, Suarez-Kurtz G, Stühmer W, Pardo LA. Ether à go-go potassium channel expression in soft tissue sarcoma patients. Mol Cancer. 2006;5:42. doi: 10.1186/1476-4598-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menéndez ST, Villaronga MA, Rodrigo JP, Alvarez-Teijeiro S, García-Carracedo D, Urdinguio RG, Fraga MF, Pardo LA, Viloria CG, Suárez C, García-Pedrero JM. Frequent aberrant expression of the human ether à go-go (hEAG1) potassium channel in head and neck cancer: pathobiological mechanisms and clinical implications. J Mol Med. 2012;90:1173–84. doi: 10.1007/s00109-012-0893-0. [DOI] [PubMed] [Google Scholar]
- 25.Ding XW, Luo HS, Jin X, Yan JJ, Ai YW. Aberrant expression of Eag1 potassium channels in gastric cancer patients and cell lines. Med Oncol. 2007;24:345–50. doi: 10.1007/s12032-007-0015-y. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal JR, Griesinger F, Stühmer W, Pardo LA. The potassium channel Ether à go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol Cancer. 2010;9:18. doi: 10.1186/1476-4598-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding XW, Wang XG, Luo HS, Tan SY, Gao S, Luo B, Jiang H. Expression and prognostic roles of Eag1 in resected esophageal squamous cell carcinomas. Dig Dis Sci. 2008;53:2039–44. doi: 10.1007/s10620-007-0116-7. [DOI] [PubMed] [Google Scholar]


