Abstract
Liver cancer, with a very high prevalence, is one of the most common causes of death worldwide. Galectin-9, a semi-galactoside-binding protein, was demonstrated to be involved in the formation and metastasis processes of tumors such as breast cancer, and has significant impact on the development and prognosis of tumor. In this study, 90 cases of liver cancer patients who had liver cancer resection surgery treatment, were selected. Samples of liver cancer tissues and cancer-adjacent tissues from the surgery resection of liver cancer patients, which also confirmed by pathology after operation as specimens, were obtained to detect the expression level of Galectin-9 mRNA. The comparing results showed that there were significant differences between the expression of Galectin-9 mRNA in cancer-adjacent tissues and that in cancer tissues (P < 0.05), in terms of pathology differentiation, TNM, and recurrence transfer aspects. However, there were no obvious correlations with gender, age, the size of tumor, and HBsAg. The expression of Galectin-9 mRNA has a close relationship with pathological differentiation, TNM, and recurrence metastasis. Our data presented here provide theoretical basis for new target of liver cancer diagnosis as well as potential prognosis.
Keywords: Galectin-9 mRNA, liver cancer tissues, cancer-adjacent tissues, expression
Introduction
Liver cancer, as a common neoplastic disease worldwide, currently presents the second highest fatality rate China. Furthermore, in recent years, the incidence rate of malignant liver tumors is still growing, with apparent trend of occurrence in younger people [1,2]. Because of the long time of onset for patients with liver cancer, the insufficiency of early diagnosis always results in advanced cancer once confirmed, and surgical treatment might be delayed due to deterioration. In addition, malignant liver cancer developed fast and patients generally have poor prognosis [3]. Along with the continuous development of medical diagnosis, early diagnosis rates of primary liver cancer has improved consistently, and there are also increasing researches for tumor pathogenesis [4].
Researches demonstrated that galectin-9, a semi-galactoside-binding protein, was involved in many physiological and pathological processes, and played significant role in cellular differentiation, aggregation and adhesion and other basic functions. Current studies related to galectin-9 focused on the induction of apoptosis, the probable predictions of the signaling mechanism as follows: (1) Signaling pathways of cysteine-aspartic protease-Ca and aspartic protease-Ca can be used to accelerate or facilitate the inflow of Ca2+ ion, shorten the time of cell apoptosis and speed up the process; (2) It participates in the Galectin-9-Tim-3 signaling pathways, promotes Tim-3+ and other cells related to immune apoptosis and eventually performs anti-cancer efficacy; [5] It also participates the transduction in terms of p38 MAP kinase and JNK Signaling pathways, which leads to remarkably decreased expression of IL-6 as well as other factors levels in blood, resulting in the accelerated apoptosis of myeloma cells. Some reports confirmed that Galectin-9 may also be involved in the formation and metastasis processes of tumors such as breast cancer, and has significant impact on the development and prognosis of tumor [6]. However, there are very few reports focused on the role of Galectin-9 in liver cancer. Our study presented here sought to detect the expression of galetin-9 mRNA by fluorescence quantitative PCR, discuss the correlation between the expression, the size of tumor, and pathology division, and provide theoretical basis for the future treatment of liver cancer.
Materials and methods
Resources of tissues samples
Ninety cases (65 cases of males and 25 cases of females) that patients who received liver cancer resection treatment in our hospital from October 2012 to October 2014 were retrospectively collected. The ages of patients range from 29 to 71 years old, the average age (52 ± 9); 33 cases of tumor diameter are less than 3 cm, 21 cases of tumor diameter are 3-5 cm, 36 cases of tumor diameter are more than 5 cm. In accord with the criteria of liver cancer TNM stages set up by International Union against cancer (UICC) (6th Edition), 25 cases were at stage I; 21 cases were at stage II; 42 cases were at stage III; 2 cases were at stage IV. Samples from each resection liver cancer tissues and cancer-adjacent tissues were chosen from the surgery patient, processed with RNA storage solution, and stored blow -80°C for later use.
The study protocol was approved by the Research Ethics Committee of our hospital, and all patients documented their informed consent before study commencement.
Instruments and reagents
Low temperature high speed centrifuge (TGL20M, Xiangli Scientific Instrument, Hunan), clean work station (Shenhua Biotech, Guangzhou), Homogenizer (Amplified Instrument, Shanghai), GeneAmp PCR amplification system-2720 (ABI, United States), Fluorescence quantitative PCR instrument (7700, BIO-RAD iQ5, Applied Biosystems), Trizol reagent (Invitrogen), 0.1% DEPC water (Beijing Ding Guo Chang Sheng Biology), Primer Script RT (Boli Biotechnology, Guangzhou), Reverse transcription Kit, SYBR Green Fluorescent dye Kit (Yanyu Biotechnology, Shanghai), Primers and DNA marker (Sanpo Vision Biotechnology, Beijing).
Techniques
Extraction of total RNA and cDNA synthesis
Trizol was used to extract total RNA and process reverse transcription into cDNA. Total RNA concentration was initially measured by UV spectrophotometer, and identified by agarose gel electrophoresis. 1 μg was applied for reverse transcriptase RT: 5 μl 5× PrimerScript, 1 μl PrimerScript RT Enzyme, 1 μl oligo dT primer (50 μmol/L), 1 μl Random 6 mers (100 μmol/L) were then added to a final volume of 20 μl. Reverse transcriptase reaction processes at 37°C for 15 minutes, followed by the inactivation of reverse transcriptase enzyme at 85°C for 5 seconds, and stored at -20°C for use.
Real-time PCR
A hundred μg of cDNA template was processed for PCR amplification reaction: (1) 95°C for 5 minutes; 95°C for 30 seconds, 64°C for 30 seconds, and 72°C for 30 seconds, all 35 cycles. SYBER Green chimeric fluorescent method was employed to detect Galectin-9 mRNA expression, according to the quantitative fluorescence reagent operation instructions for the specific processes: 1. 95°C 30 seconds, 2. 95°C 5 seconds, 49°C 35 seconds, all 40 cycles; All reactions were processed in 3 replicate wells.
After the reaction, Ct values of PCR amplification curve were generated, and GAPDH was selected as a reference gene The ∆Ct of the relative expression of genes was calculated based on 2-ΔΔCt method. GAPDH primer sequences were: F: 5’-CAAGAGTCGGAA CTGCCA-3’; R: 5’-AGGTGACCGCAGAA GTGGT-3’, amplified fragment length was 130 bp; Galectin-9 primer sequences were: F: 5’-CTGTCCTCCTACCTGAACCTAC-3’, R: 5’-CACACCTAGAACACACTTCGAGT-3’, amplified fragment length was 228 bp.
Observation indicators
The electrophoretic patterns of total cDNA samples after PCR amplification were recorded, and the expressions of Galectin-9 mRNA in cancer tissues and cancer-adjacent tissues were detected by using quantitative fluorescence PCR method. The specific expressions of Galectin-9 mRNA in patients cancer-adjacent tissues, and its relationship with clinical characteristics were observed and determined.
Statistical methods
SPSS 15.0 statistical software (SPSS, Chicago, IL, USA) was used for data analyses. Measurement data were presented as mean ± S.D. F test was applied for multiple sets comparison, while N-K test was applied between two groups with statistically significant differences defined as P < 0.05. Spearman correlation analysis between the expression of inflammatory factor and cognitive function was applied. Measurement data were presented as mean ± standard deviation (x̅ ± s), processed t test. χ2 test was utilized to indicate the enumeration data as rate (%) Expressed enumeration data as rate, and Kendall’s Tau-b was applied to evaluate bivariate correlation. Differences P < 0.05 indicated statistically significant.
Results
Comparison of electrophoresis results of total cDNA samples after PCR amplification
It showed that the expression levels of Galectin-9 mRNA in cancer-adjacent tissues were significantly higher than in cancer tissues, the differences were obvious, as shown in Figure 1.
Figure 1.
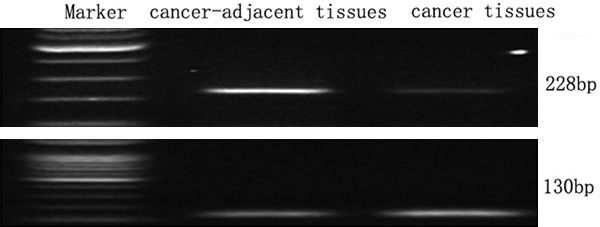
Electrophoresis of galectin-9 mRNA in cancer-adjacent tissues and cancer tissues.
Comparison of the expression of galectin-9 mRNA in cancer-adjacent tissues and cancer tissues
The detection of 90 liver cancer cases performed by fluorescence quantitative PCR determined that there were significant differences of the expression levels of Galectin-9 mRNA between cancer-adjacent tissues and cancer tissues (t=6.886, P < 0.05), the difference showed statistically significant (Table 1).
Table 1.
The expression of galectin-9 mRNA in cancer-adjacent tissues and cancer tissues
| Tissues types | Cases | Expression of galectin-9 (ΔCt values) | 2-ΔΔCt |
|---|---|---|---|
| Cancer-adjacent tissues | 90 | 6.004 ± 0.003 | 0.498 |
| Cancer tissues | 90 | 7.231 ± 0.012 | |
| T values | 6.886 | ||
| P values | < 0.05 |
Note: There was an inverse relationship between ΔCt values and the expression of mRNA.
Results of Table 2 showed significantly different expression of Galectin-9 mRNA in cancer-adjacent tissues and liver tissues based on the study of pathological differentiation, TNM, relapse and metastasis (P < 0.05); on the other hand, there was no significant correlation with age, sex, the size of tumor and HBsAg. Kendall’s Tau-b bivariate correlation analysis was shown in Table 3. Taken together, the expression of Galectin-9 mRNA in liver tissues was closely associated with patient’s pathological differentiation, TNM, recurrence and metastasis.
Table 2.
Analysis of the relationships among the expression of galectin-9 mRNA in liver cancer patients cancer-adjacent tissues and cancer tissues, and clinical characteristics
| Clinical characteristics | Cancer-adjacent tissues ΔCt values | Cancer tissues ΔCt values | T values | P values | |
|---|---|---|---|---|---|
| Sex | Male | 6.102 ± 0.021 | 7.321 ± 0.009 | 2.036 | > 0.05 |
| Female | 6.023 ± 0.011 | 7.120 ± 0.008 | |||
| Age | ≤ 65 | 5.951 ± 0.004 | 7.004 ± 0.012 | 2.114 | > 0.05 |
| > 65 | 6.123 ± 0.007 | 7.332 ± 0.008 | |||
| The size of tumor | < 3 cm | 6.104 ± 0.023 | 7.061 ± 0.031 | 2.051 | > 0.05 |
| 3-5 cm | 6.003 ± 0.011 | 6.985 ± 0.009 | |||
| > 5 cm | 5.905 ± 0.005 | 6.908 ± 0.014 | |||
| HBsAg | + | 6.231 ± 0.034 | 7.128 ± 0.044 | 2.230 | > 0.05 |
| - | 6.009 ± 0.021 | 6.995 ± 0.027 | |||
| Pathological differentiation | Well differentiated | 5.714 ± 0.035 | 7.338 ± 0.073 | 10.234 | < 0.05 |
| Moderately differentiated | 6.000 ± 0.033 | 7.206 ± 0.065 | |||
| Poorly differentiated | 6.230 ± 0.066 | 6.971 ± 0.074 | |||
| TNM | Level I | 6.039 ± 0.014 | 7.441 ± 0.010 | 10.921 | < 0.05 |
| Level II | 5.947 ± 0.054 | 7.360 ± 0.089 | |||
| Level IIII | 5.608 ± 0.037 | 7.021 ± 0.036 | |||
| Recurrence and metastasis | Yes | 6.332 ± 0.057 | 7.019 ± 0.047 | 9.107 | < 0.05 |
| No | 6.014 ± 0.007 | 6.872 ± 0.005 |
Table 3.
Correlation analysis of the bivariate of the expression of galectin-9 mRNA in liver cancer tissues
| Tissues types | Kendall’s tau-b | P values |
|---|---|---|
| Pathological differentiation | 0.228 | 0.014 |
| TNM | 0.307 | 0.011 |
| Recurrence and Metastasis | 0.239 | 0.013 |
Discussion
Galectin belongs to member protein family with amino acids sequences similar to B-galactopyranoside, which also has key Eosinophilic granulocyte characteristics. Studies have shown evidences that, in patients with melanoma, poorly differentiated pathological differentiation resulted in the decrease of positive expression of galectin-9 protein in tissues accordingly [7]. Other studies indicated that the expression of galectin-9 protein in normal cervical squamous tissues were positive, however, with the progression of cervical cancer, the positive expression of Galectin-9 protein increased in invasive cervical squamous cell carcinoma, high grade cervical intraepithelial neoplasia and low grade cervical intraepithelial neoplasia, implying that the expression of Galectin-9 may affect the onset and development of tumor [8,9].
The data of this article demonstrated that the expression galectin-9 mRNA in cancer-adjacent tissues was significantly higher than in cancer tissues, which may indicate an important role of Galectin-9 in tumor cell aggregation. Other studies in breast cancer and colorectal cancer patients also unraveled that the positive expression of galectin-9 protein in cancer tissues was lower than in adjacent tissues [10], manifesting that the expression of Galectin-9 protein may be involved in tumor progression and conduct certain functions. Therefore, it can be used as a marker of tumor malignancy. Besides, compared with adjacent tissues, the expression of galectin-9 mRNA in cancer tissues had significant differences in terms of pathologic differentiation, TNM, and recurrence and metastasis. Notwithstanding, there was not significant correlation with age, sex, the size of tumor and HBsAg. Some researches reported that the expression galectin-9 was significantly decreased or inhibited in patients with malignant melanoma [4,5,11,12], In vitro studies showed that high expression of Galectin-9 mRNA in melanoma cell displayed line colony development, while low expression led to scattered development [13-15]. We speculated that galectin-9 might perform anti-metastasis interventions through tumor cell aggregation. It has been also illuminated that the expression level of galectin-9 in patients with colorectal cancer was impacted by distant metastasis of cancer cells [16-20]. Our data indicated that the expression level of galectin-9 mRNA in liver metastases cancer patients was lower than patients without metastasis, suggesting that there are some negative correlation between the expression levels of galectin-9 and the tumor metastasis. The specific participation or influence of the development of tumor metastasis may occur, as galectin-9 interferes the combination of tumor adhesion factor with normal extracellular matrix to a certain extent.
In outline, the expression level of galectin-9 mRNA is associated with pathological differentiation, TNM levels, and relapse and metastasis. Low level of galectin-9 is closely related to the incidence and progression of liver cancer, indicating a crucial factor of pathological diagnosis of hepatocellular carcinoma and prognosis. Our findings underscore the important role of galectin-9 mRNA in hepatocellular carcinoma, and open the possibility for using galectin-9 as a novel tumor marker for early diagnosis.
Disclosure of conflict of interest
None.
References
- 1.Liu YM, Chen Y, Li JZ, Gong JP. Up-regulation of Galectin-9 in vivo results in immunosuppressive effects and prolongs survival of liver allograft in rats. Immunol Lett. 2014;162:217–22. doi: 10.1016/j.imlet.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Dolina JS, Braciale TJ, Hahn YS. Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice. Hepatology. 2014;59:1351–65. doi: 10.1002/hep.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ju Y, Shang X, Liu Z, Zhang J, Li Y, Shen Y, Liu Y, Liu C, Liu B, Xu L, Wang Y, Zhang B, Zou J. The Tim-3/galectin-9 pathway involves in the homeostasis of hepatic Tregs in a mouse model of concanavalin A-induced hepatitis. Mol Immunol. 2014;58:85–91. doi: 10.1016/j.molimm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Gu CJ, Wu H, Sheng CY, Ni QC. [Expression and prognostic value of galectin-9 in hepatocellular carcinoma patients] . Zhonghua Yi Xue Za Zhi. 2013;93:2025–8. [PubMed] [Google Scholar]
- 5.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M, Dobrinskikh E, Busson P, Polyak SJ, Hirashima M, Rosen HR. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. 2013;190:1788–96. doi: 10.4049/jimmunol.1202814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 ameliorates Con A-induced hepatitis by inducing CD4 (+) CD25 (low/int) effector TCell apoptosis and increasing regulatory T cell number. PLoS One. 2012;7:e48379. doi: 10.1371/journal.pone.0048379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, Kennedy P, Maini MK. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barjon C, Niki T, Vérillaud B, Opolon P, Bedossa P, Hirashima M, Blanchin S, Wassef M, Rosen HR, Jimenez AS, Wei M, Busson P. A novel monoclonal antibody for detection of galectin-9 in tissue sections: application to human tissues infected by oncogenic viruses. Infect Agent Cancer. 2012;7:16. doi: 10.1186/1750-9378-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberal R, Grant CR, Holder BS, Ma Y, Mieli-Vergani G, Vergani D, Longhi MS. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology. 2012;56:677–86. doi: 10.1002/hep.25682. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi Y, Fukusato T, Kobayashi Y, Akiyama S, Tamatani T, Shiga J, Mori S. High expression of eosinophil chemoattractant ecalectin/galectin-9 in drug-induced liver injury. Liver Int. 2006;26:106–15. doi: 10.1111/j.1478-3231.2005.01189.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, Wang GQ, Li HL, Wang XD. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:2503–9. doi: 10.7314/apjcp.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 13.Barjon C, Niki T, Vérillaud B, Opolon P, Bedossa P, Hirashima M, Blanchin S, Wassef M, Rosen HR, Jimenez AS, Wei M, Busson P. A novel monoclonal antibody for detection of galectin-9 in tissue sections: application to human tissues infected by oncogenic viruses. Infect Agent Cancer. 2012;7:16. doi: 10.1186/1750-9378-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–51. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 15.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351:571–6. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8831–49. doi: 10.3748/wjg.v19.i47.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Tao X, Huang CZ, Liu JF, Ye YB, Huang AM. Decreased expression of liver-type fatty acid-binding protein is associated with poor prognosis in hepatocellular carcinoma. Hepatogastroenterology. 2014;61:1321–6. [PubMed] [Google Scholar]
- 18.Sonohara F, Nomoto S, Inokawa Y, Hishida M, Takano N, Kanda M, Nishikawa Y, Fujii T, Koike M, Sugimoto H, Kodera Y. High expression of Janus kinase 2 in background normal liver tissue of resected hepatocellular carcinoma is associated with worse prognosis. Oncol Rep. 2015;33:767–73. doi: 10.3892/or.2014.3621. [DOI] [PubMed] [Google Scholar]
- 19.Dai B, Ruan B, Wu J, Wang J, Shang R, Sun W, Li X, Dou K, Wang D, Li Y. Insulin-like growth factor binding protein-1 inhibits cancer cell invasion and is associated with poor prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:5645–54. [PMC free article] [PubMed] [Google Scholar]
- 20.Gatzidou E, Mantzourani M, Giaginis C, Giagini A, Patsouris E, Kouraklis G, Theocharis S. Augmenter of liver regeneration gene expression in human colon cancer cell lines and clinical tissue samples. J BUON. 2015;20:84–91. [PubMed] [Google Scholar]


