Abstract
This study aimed to assess the relationship between serum CA724 levels and the unresectability of pancreatic adenocarcinoma. A total of 302 patients with pancreatic adenocarcinoma were analyzed for the potential association between serum CA724 levels and the unresectability of pancreatic adenocarcinoma. Serum CA724 levels in patients with unresectable pancreatic adenocarcinoma were remarkably higher than those with resectable pancreatic adenocarcinoma (P < 0.001). Patients with elevated serum CA724 levels exhibited a 12.27-fold higher risk of unresectability than those with normal serum CA724 levels after adjusting for age, sex, and tumor location (95% CI = 5.28-28.51, P < 0.001). The analysis of receiver operating characteristics demonstrated that CA724 had superior predictive value to other tumor markers (AUC was 0.77 ± 0.03, 0.65 ± 0.04, and 0.62 ± 0.04 for CA724, CA125, and CA199, respectively). CA724 appeared to be a better predictor of unresectability than CA199 and CA125.
Keywords: Pancreatic adenocarcinoma, CA724, CA125, CA199, unresectability
Introduction
Pancreatic adenocarcinoma is a highly aggressive human disease with a five-year overall survival rate of less than 4% [1]. To date, the only potential curative treatment for this disease is surgical resection. However, only 20% of pancreatic adenocarcinoma patients are eligible for radical operation because the disease has often progressed to an advanced stage at diagnosis. The preoperative assessment of resectability for pancreatic adenocarcinoma fails in accurately selecting 25% of patients who are unresectable at laparotomy [2]. Thus, accurate preoperative evaluation could provide considerable benefit to pancreatic adenocarcinoma patients. Radical and extended lymphadenectomy has been shown to benefit overall survival, but a possible trend toward increased morbidity has been implicated [3]. Identifying patients with unresectable disease would reduce morbidity and unnecessary surgical complications, such as duodenal obstruction, pain, excessive blood loss, and jaundice. Patients who are at high risk for developing unresectable tumors would be allowed to skip the delay time and be immediately subjected to systemic palliative therapy. Recent advances in chemotherapy and target therapy have improved the overall survival of pancreatic adenocarcinoma [4,5]. Furthermore, the proper identification of unresectable tumors could reduce healthcare costs.
The most frequent contraindications that preclude surgical resection include liver metastasis or peritoneal disease, as well as the superior mesenteric artery/vein, portal vein, or celiac-axis invasions by the tumor. Computed tomography (CT) is the most commonly applied imaging method for the diagnosis and selection of candidates for surgical resection. However, the accuracy of CT in predicting pancreatic adenocarcinoma resectability has been reported to range from 56%-79%, according to previous reports [6,7]. A small liver size or peritoneal metastasis and vascular involvement by the tumor cannot be detected by CT. Multidetector-row CT (MDCT) is recommended to improve the accuracy of the detection and staging of pancreatic adenocarcinoma [8]. Endoscopic ultrasound (EUS), which is affected by the presence of biliary stents, could be used as a supplementary tool to MDCT for the prediction of the resectability of pancreatic adenocarcinoma [9]. Several reports showed that gadolinium-enhanced magnetic resonance angiography holds an equal or even better accuracy than helical CT in the evaluation of pancreatic adenocarcinoma [10,11]. However, some controversy remains in the selection of CT or magnetic resonance imaging (MRI) as prior imaging tools to determine pancreatic adenocarcinoma resectability [12]. Luo et al. [13] recently reported that carbohydrate antigen (CA) 125 is useful in evaluating the resectability of pancreatic cancer. The authors considered vascular involvement and distant metastasis as indicative of unresectability. This finding still requires external validation.
Serum CA199 is the most common and effective biomarker in screening and diagnosing pancreatic cancer. Some other serum cancer-related biomarkers, such as carcinoembryonic antigen (CEA) and CA242, can be elevated in pancreatic cancer. CA724 has recently received increasing attention with regard to digestive system cancers [14,15]. Accordingly, this study aims to investigate the role of CA724 in determining the resectability of pancreatic adenocarcinoma.
Materials and methods
The sample population of this study included 302 patients with pancreatic adenocarcinoma who were newly diagnosed and treated in Zhejiang Cancer Hospital, Hangzhou, China, between October 2006 and December 2012. All patients were newly confirmed to have pancreatic adenocarcinoma and had not received treatment previously. Our study was approved by the institutional review board of the hospital. All patients provided informed consent prior to administration.
Disease extent was determined by TNM staging according to the seventh edition of the TNM classification of the American Joint Committee on Cancer [16]. CT examination was performed by MDCT. Two experienced radiologists evaluated the CT images to determine the presence or absence of vascular involvement and distant metastasis. Patients with the following characteristics [17] were considered to have unresectable tumors: (1) liver or peritoneal metastasis, (2) vascular involvement. Three arteries were included, namely, the celiac trunk, common hepatic artery, and superior mesenteric artery and three veins, namely, the portal vein, superior mesenteric vein, and splenic vein.
Pearson’s chi-squared test was used to assess the differences in demographic variables, gender, tumor location, tumor size, and serum tumor-marker level between the resectable and unresectable groups. Student’s t-test was employed to compare patient age. Multivariate forward stepwise logistic regression analysis was performed to identify serum tumor markers that correlated with the unresectability of pancreatic adenocarcinoma. The receiver operating characteristic (ROC) curves and the area under the ROC curve were used to determine the best predictive values. All statistical calculations were performed with SPSS 13.0 for Windows (Chicago, IL). P < 0.05 was considered statistically significant.
Results
A total of 302 patients with pancreatic adenocarcinoma were analyzed in this study. The characteristics of these patients are summarized in Table 1. The study population had a median age of 63 years (30-87 years). The patients comprised 188 males (62.3%) and 114 females (37.7%). Among the patients, 150 (49.7%) cases underwent curative-intent resection, including 27 (18.0%) cases of stage I and 123 (82.0%) cases of stage II. The majority of the patients (60%) underwent pylorus-preserving Whipple procedure, followed by the classic Whipple procedure (30%), whereas the remaining patients (10%) were subjected to a distal pancreatectomy with splenectomy. A total of 129 cases were found unresectable by MDCT, whereas 89 cases were concurrent with distant metastasis. The remainder (40 cases) of inoperable patients had locally advanced disease with vascular involvement at the time of evaluation. A total of 23 patients whose tumors were found resectable by CT scan underwent exploratory laparotomy. Among these patients, 20 presented with small livers or peritoneal metastasis. A total of 3 patients were found to have vascular involvement.
Table 1.
Clinicopathological features of resectable and unresectable pancreatic adenocarcinoma patients
| Characteristics | Total (n) | Resectable (n, %) | Unresectable (n, %) | P |
|---|---|---|---|---|
| Numbers of cases | 302 | 150 | 152 | |
| Age (mean ± SD, years) | 61.7 ± 9.4 | 61.6 ± 8.4 | 61.9 ± 10.2 | 0.785 |
| Gender | ||||
| Male | 188 | 84 (44.7) | 104 (55.3) | 0.026 |
| Female | 114 | 66 (57.9) | 48 (42.1) | |
| Tumor location | ||||
| Head | 188 | 86 (45.7) | 102 (54.3) | 0.186 |
| Body | 87 | 49 (56.3) | 38 (43.7) | |
| Tail | 26 | 15 (57.7) | 11 (42.3) | |
| Tumor size | ||||
| ≤ 3 cm | 104 | 54 (51.9) | 50 (48.1) | 0.648 |
| 3-6 cm | 151 | 71 (47.0) | 80 (53.0) | |
| ≥ 6 cm | 47 | 25 (53.2) | 22 (46.8) | |
| AFP | ||||
| ≤ 10 ng/ml | 286 | 144 (50.3) | 142 (49.7) | 0.978 |
| > 10 ng/ml | 16 | 8 (50.0) | 8 (50.0) | |
| CEA | ||||
| ≤ 5 ng/ml | 187 | 103 (55.1) | 84 (44.9) | 0.011 |
| > 5 ng/ml | 115 | 46 (40.0) | 69 (60.0) | |
| CA 125 | ||||
| ≤ 35 U/ml | 180 | 106 (58.9) | 74 (41.1) | 0.001 |
| > 35 U/ml | 122 | 47 (38.5) | 75 (61.5) | |
| CA 199 | ||||
| ≤ 37 U/ml | 68 | 38 (55.9) | 30 (44.1) | 0.244 |
| > 37 U/ml | 234 | 112 (47.9) | 122 (52.1) | |
| CA 242 | ||||
| ≤ 15 U/ml | 106 | 54 (50.9) | 52 (49.1) | 0.876 |
| > 15 U/ml | 196 | 98 (50.0) | 98 (50.0) | |
| CA 724 | ||||
| ≤ 6.9 U/ml | 180 | 122 (67.8) | 58 (32.2) | < 0.001 |
| > 6.9 U/ml | 122 | 28 (23.0) | 94 (77.0) |
Bold values are statistically significant (P < 0.05).
The relationship between patient characteristics and the resectability of pancreatic adenocarcinoma is presented in Table 1. The serum levels of pancreatic adenocarcinoma markers, including CA199, AFP, CEA, CA125, CA242, and CA724, were detected before the initiation of treatment. The median serum level of CA724 was 4.71 U/mL (0.10-844.0 U/mL) for the entire population. A total of 122 (40.4%) patients presented with serum CA724 levels of more than 6.9 U/mL, which is considered a high range. Serum CA724 levels in patients with unresectable pancreatic adenocarcinoma were remarkably higher than those with resectable pancreatic adenocarcinoma (P < 0.001). The median serum CA125 concentration in all patients was 23.5 U/mL; 122 patients (40.4%) exhibited elevated levels (CA125 > 35 U/mL). A significant relationship was observed between resectability and serum CA125 levels (P = 0.001). The median level of CEA was 4 ng/ml (0.5-1643.11 ng/ml). A higher serum level of CEA was detected in the resectable group than in the unresectable group (P = 0.011). However, a significant correlation was demonstrated between the resectability of pancreatic adenocarcinoma and serum AFP, CA199, and CA242 levels (P > 0.05).
A logistic regression model revealed that elevated serum CA724 and CA125 levels were 2 independent risk factors for predicting unresectability in pancreatic adenocarcinoma (Table 2). The patients with elevated serum CA724 levels were found with a 12.27-fold higher risk of unresectability than those with normal levels after adjusting for age, sex, and tumor location (95% CI = 5.28-28.51, P < 0.001). Cases with elevated levels of serum CA125 were also found with a significantly higher risk of unresectability than those with normal levels (95% CI = 1.31-6.56, P = 0.009). ROC analysis was conducted to compare the abilities of the tumor markers to predict the unresectability of pancreatic adenocarcinoma. The areas under the ROC curves were 0.77 ± 0.03, 0.65 ± 0.04, and 0.62 ± 0.04 for CA724, CA125, and CA199, respectively (Figure 1). CA724 showed the highest predictive value among the tested tumor markers.
Table 2.
Serum tumor markers and risk for unresectability in pancreatic adenocarcinoma
| Crude HR | 95% CI | P | Adjust HR | 95% CI | P | |
|---|---|---|---|---|---|---|
| AFP | ||||||
| ≤ 10 ng/ml | 1 | 1 | ||||
| > 10 ng/ml | 0.84 | 0.15-4.83 | 0.845 | 0.77 | 0.14-4.31 | 0.767 |
| CEA | ||||||
| ≤ 5 ng/ml | 1 | 1 | ||||
| > 5 ng/ml | 1.50 | 0.70-3.24 | 0.298 | 1.54 | 0.71-3.35 | 0.277 |
| CA 125 | ||||||
| ≤ 35 U/ml | 1 | 1 | ||||
| > 35 U/ml | 3.08 | 1.39-6.81 | 0.006 | 2.93 | 1.31-6.56 | 0.009 |
| CA 199 | ||||||
| ≤ 37 U/ml | 1 | 1 | ||||
| > 37 U/ml | 2.18 | 0.64-7.50 | 0.215 | 2.14 | 0.63-7.78 | 0.216 |
| CA 242 | ||||||
| ≤ 15 U/ml | 1 | 1 | ||||
| > 15 U/ml | 0.78 | 0.24-2.55 | 0.685 | 0.80 | 0.24-2.67 | 0.716 |
| CA 724 | ||||||
| ≤ 6.9 U/ml | 1 | 1 | ||||
| > 6.9 U/ml | 11.38 | 5.08-25.50 | < 0.001 | 12.27 | 5.28-28.51 | < 0.001 |
Bold values are statistically significant (P < 0.05).
Figure 1.
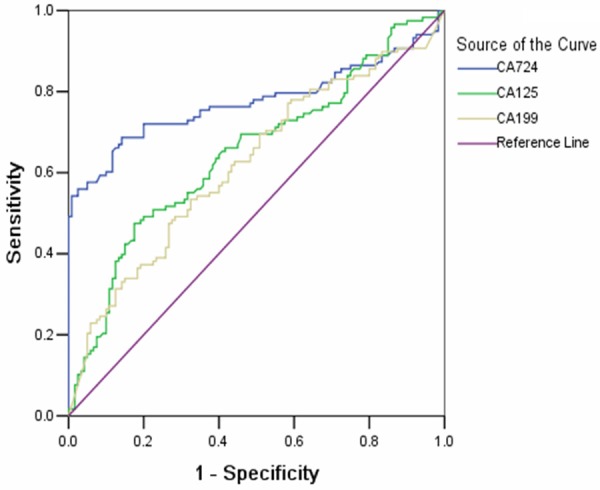
Receiver operating characteristic analysis was constructed to compare the ability of the tumor markers to predict the unresectability of pancreatic cancer. AUC was 0.77 ± 0.03, 0.65 ± 0.04 and 0.62 ± 0.04 for CA724, CA125 and CA199.
Discussion
Considerable attention has been given to the association between serum tumor marker CA724 and gastrointestinal cancer. CA724 is the most important tumor marker in gastric cancer because of its higher sensitivity and specificity than other markers [18]. However, the relationship between CA724 and pancreatic adenocarcinoma has not been well established. In this study, we found that CA724 is more sensitive in predicting the resectability of pancreatic adenocarcinoma than other tumor markers. High serum concentrations of CA724 are related to pancreatic cancer unresectability. To our knowledge, this paper reports the first large-scale study that demonstrated the relationship between CA724 and pancreatic adenocarcinoma resectability.
Numerous recent studies have explored the ability of CT, EUS, and tumor-marker detection to evaluate the resectability of pancreatic adenocarcinoma. Lee et al. [19] compared the diagnostic values of MDCT and MRI with regard to predicting resectability in a study of 116 patients. In the study, the sensitivity and specificity of MDCT were 90% and 65%, respectively. MRI exhibited a similar ability in evaluating resectability (sensitivity of 90% and specificity of 41%). EUS can also serve as an auxiliary examination to CT in assessing the concurrence of vascular involvement. In a meta-analysis by Puli et al. [20], EUS was found to detect vascular invasion with 73% sensitivity and 90% specificity. However, potential bias in these studies should be considered. The majority of patients with unresectable tumors were only evaluated by radiographic criteria, and these radiographic results were not confirmed by surgical exploration. Hence, institutional variations might exist among different studies. In our work, almost all patients underwent surgical exploration.
Some studies have used tumor markers to predict unresectability, including CA199 and CA125. CA199, a carbohydrate tumor-associated antigen, is the most important and commonly detected tumor marker in pancreatic cancer patients. Brown et al. [21] retrospectively reviewed 72 patients with pancreatic cancer. Of these patients, 65% had resectable disease. CA199 was also demonstrated to be a significant predictor for unresectability. However, in our study, CA199 was not correlated with unresectability in pancreatic adenocarcinoma, a finding consistent with another similar study [22]. This result was most likely due to the relatively small sample size in the study of Brown et al. [21]. CA125 is a member of the mucin family of glycoproteins. This marker is well known for predicting the diagnosis and survival of ovarian cancer. In a recent relatively large sample study [13], CA125 was found to be superior to CA19-9 in predicting the resectability of pancreatic cancer. In the present study, elevated serum CA724 and CA125 were 2 independent risk factors that successfully predicted unresectability. Patients with elevated serum CA724 had a 12.27-fold higher risk of unresectability than those with normal serum CA724 levels after adjusting for age, sex, and tumor location. The ROC curve analysis indicated that CA724 has a higher predictive value than CA125 and CA199. Collectively, our results suggested that CA724 may be involved in pancreatic cancer biological behavior.
Several limitations in this study must be mentioned. First, this work was designed to be a retrospective, single-center study; hence, selection bias could not be completely avoided. Furthermore, the results of this study might not be applicable to other medical settings. The evaluation of CA724, CA125, and CA199 should be standardized before clinical use. Second, the majority of patients were confirmed to have unresectable tumors by operation; thus, the number of patients with resectable tumors was relatively small. Third, our analysis focused on pancreatic adenocarcinoma, thereby excluding other histological classifications, such as endocrine pancreatic cancer.
Recent advances in molecular biology have led to a greater understanding of the genetics of pancreatic cancer. Our results showed that CA724 may be used to predict unresectable pancreatic adenocarcinoma. In the future, we hope to validate this model by analyzing a large series of pancreatic adenocarcinoma cases.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1. [DOI] [PubMed] [Google Scholar]
- 3.Michalski CW, Kleeff J, Wente MN, Diener MK, Buchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265–73. doi: 10.1002/bjs.5716. [DOI] [PubMed] [Google Scholar]
- 4.Yang ZY, Yuan JQ, Di MY, Zheng DY, Chen JZ, Ding H, Wu XY, Huang YF, Mao C, Tang JL. Gemcitabine plus erlotinib for advanced pancreatic cancer: a systematic review with metaanalysis. PLoS One. 2013;8:e57528. doi: 10.1371/journal.pone.0057528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 6.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William TL, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–33. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 7.Parsons CM, Sutcliffe JL, Bold RJ. Preoperative evaluation of pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2008;15:429–35. doi: 10.1007/s00534-007-1240-7. [DOI] [PubMed] [Google Scholar]
- 8.Manak E, Merkel S, Klein P, Papadopoulos T, Bautz WA, Baum U. Resectability of pancreatic adenocarcinoma: assessment using multidetector-row computed tomography with multiplanar reformations. Abdom Imaging. 2009;34:75–80. doi: 10.1007/s00261-007-9285-2. [DOI] [PubMed] [Google Scholar]
- 9.Bao PQ, Johnson JC, Lindsey EH, Schwartz DA, Arildsen RC, Grzeszczak E, Parikh AA, Merchant NB. Endoscopic ultrasound and computed tomography predictors of pancreatic cancer resectability. J Gastrointest Surg. 2008;12:10–6. doi: 10.1007/s11605-007-0373-y. discussion 16. [DOI] [PubMed] [Google Scholar]
- 10.Mehmet ES, Ichikawa T, Sou H, Saitou R, Tsukamoto T, Motosugi U, Araki T. Pancreatic adenocarcinoma: MDCT versus MRI in the detection and assessment of locoregional extension. J Comput Assist Tomogr. 2006;30:583–90. doi: 10.1097/00004728-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa T, Haradome H, Hachiya J, Nitatori T, Ohtomo K, Kinoshita T, Araki T. Pancreatic ductal adenocarcinoma: preoperative assessment with helical CT versus dynamic MR imaging. Radiology. 1997;202:655–62. doi: 10.1148/radiology.202.3.9051012. [DOI] [PubMed] [Google Scholar]
- 12.Tamm EP, Balachandran A, Bhosale PR, Katz MH, Fleming JB, Lee JH, Varadhachary GR. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50:407–28. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Luo G, Xiao Z, Long J, Liu Z, Liu L, Liu C, Xu J, Ni Q, Yu X. CA125 is superior to CA19-9 in predicting the resectability of pancreatic cancer. J Gastrointest Surg. 2013;17:2092–8. doi: 10.1007/s11605-013-2389-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang PY, Zhu MJ, Wang JD, Zhou XP, Quan ZW, Shen J. Xanthogranulomatous cholecystitis: a clinicopathological study of its association with gallbladder carcinoma. J Dig Dis. 2013;14:45–50. doi: 10.1111/j.1751-2980.2012.00645.x. [DOI] [PubMed] [Google Scholar]
- 15.Zou L, Qian J. Decline of serum CA724 as a probable predictive factor for tumor response during chemotherapy of advanced gastric carcinoma. Chin J Cancer Res. 2014;26:404–9. doi: 10.3978/j.issn.1000-9604.2014.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Kalra MK, Maher MM, Sahani DV, Digmurthy S, Saini S. Current status of imaging in pancreatic diseases. J Comput Assist Tomogr. 2002;26:661–75. doi: 10.1097/00004728-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Chen XZ, Zhang WK, Yang K, Wang LL, Liu J, Wang L, Hu JK, Zhang B, Chen ZX, Chen JP, Zhou ZG, Mo XM. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39:9031–9. doi: 10.1007/s11033-012-1774-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee JK, Kim AY, Kim PN, Lee MG, Ha HK. Prediction of vascular involvement and resectability by multidetector-row CT versus MR imaging with MR angiography in patients who underwent surgery for resection of pancreatic ductal adenocarcinoma. Eur J Radiol. 2010;73:310–6. doi: 10.1016/j.ejrad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Puli SR, Singh S, Hagedorn CH, Reddy J, Olyaee M. Diagnostic accuracy of EUS for vascular invasion in pancreatic and periampullary cancers: a meta-analysis and systematic review. Gastrointest Endosc. 2007;65:788–97. doi: 10.1016/j.gie.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Brown EG, Canter RJ, Bold RJ. Preoperative CA 19-9 kinetics as a prognostic variable in radiographically resectable pancreatic adenocarcinoma. J Surg Oncol. 2015;111:293–8. doi: 10.1002/jso.23812. [DOI] [PubMed] [Google Scholar]
- 22.Yovino S, Darwin P, Daly B, Garofalo M, Moesinger R. Predicting unresectability in pancreatic cancer patients: the additive effects of CT and endoscopic ultrasound. J Gastrointest Surg. 2007;11:36–42. doi: 10.1007/s11605-007-0110-6. [DOI] [PubMed] [Google Scholar]


