Abstract
The collision tumor is defined by Meyer as that arisen from the accidental meeting and eventual intermingling of two independent neoplasms, which is quite rare. Most of them occur in the junction of different epithelial types of tissue such as oral cavity, esophagogastric junction, anorectaljunction and cervix, while collision tumors occurring in the liver, gallbladder, pancreatic, urinary bladder also have been reported. Here we present a case of 55-year-old Chinese man diagnosed as a collision tumor composed of leiomyosarcoma and squamous cell carcinoma (SqCC) in the lower third part of esophagus with 6 years survival after surgery and radiotherapy.
Keywords: Collision tumor, leiomyosarcoma, squamous cell carcinoma, esophagus
Introduction
Esophageal carcinoma can be mainly divided into squamous cell carcinoma (SqCC) and adenocarcinoma histologically. Esophageal SqCC has become the predominant type of esophageal cancer in Asia [1], alcohol and tobacco use are the main risk factors, and esophageal squamous dysplasia is the precursor lesion. Leiomyosarcoma is a high grade, smooth muscle soft tissue tumor which can occur in any tissue containing smooth muscle fibers [2], but leiomyosarcomas occur in the esophagus are rare malignancies accounting for 0.1-0.5% of all malignant esophageal tumors [3], so carcinosarcoma consists of both carcinomatous (SqCC) and sarcomatous (leiomyosarcoma) elements as a collision tumor is extremely rare.
Herein, we present a 55-year-old man diagnosed of collision tumor composed of leiomyosarcoma and SqCC treated by radical esophagectomy and radiotherapy.
Case report
On Nov. 3, 2009, a 55-year-old man was admitted in our hospital with complaining of progressive dysphagia for 20 days. His tobacco smoking was 1.5 packs per day and ardent spirits drinking were about 500 ml of 40 % alcohol per day both for over 30 years. Physical examination showed no abnormality. A barium meal study showed hold-up of barium at the distal esophagus approximately 6 cm from the diaphragm consistent with an irregularly shaped filling defect with segmented narrowing in the lower third of the esophagus. Computed tomography (CT) scan revealed that a 3-cm long thickened esophageal wall with the lumen stenosis at lower third esophagus that was well enhanced, no distant metastases or enlarged lymph nodes were seen. A proximal gastrectomy with a lower esophagectomy and regional node dissections was performed subsequently, the liver, spleen and pancreas were found normal, there was no ascites.
Microscopically, the neoplasm contained spindle-shaped tumor cells (Figure 1A) invading subserosa and SqCC (Figure 1D) with frequently keratin pearls invading the muscular layer measuring 4×2.5 cm, the proximal and distal resection margins were free of tumor, no metastasis were found in 6 para esophageallymphnodes or 3 para left gastric artery lymph nodes (T2N0M0). Immunohistochemical stain shows that the spindle-shaped cells were positive for smooth muscle actin (Figure 1B) and vimentin (Figure 1C), the SqCC cells were positive for CK5/6 and p63. Then the diagnosis of collision tumor composed of leiomyosarcoma and SqCC was made.
Figure 1.
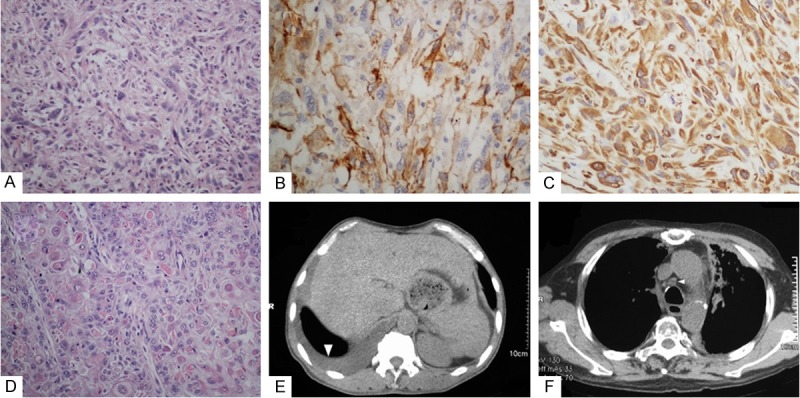
A. The portion of spindle-shaped tumor cells. B. Spindle-shaped cells were positive for smooth muscle actin. C. Spindle-shaped cells were positive for vimentin. D. The portion of SqCC with frequently keratin pearls. E. CT scan revealed right pleural effusion, laboratory examination found no tumor cells. F. CT scan shows the enlargement of mediastinal lymph node.
One month later, the patient underwent conventional technique radiotherapy with total dose of 5000cGy in 25 fractions.
On Feb. 11 in 2013, CT scan revealed right pleural effusion (Figure 1E) without any tumorcells. The patient reviewed on July 24th in 2013 found mediastinal lymph nodes recurrence (Figure 1F) then six courses of cisplatin and docetaxel were performed. At the last follow up after chemotherapy, the patient was in good condition without any recurrence or distant metastasis determined by CT scan on May 28 in 2014.
Discussion
Collision tumors are not well-recognized entities since the pathogenesis of collision tumors remains controversial, so do the diagnostic criteria. Meyer [4] emphasizes that collision tumors are two independent neoplasms meeting and eventuallyintermingling. Dodge [5] appended if both types of tumors metastasize, the two types of growth should be clearly separated in the metastasis, neither intermediate nor transitional pattern were acceptable between two neoplasms. While Spagnolo and Heenan [6] considered that two components may invade each other and produce a zone of intermediate histological appearance in the course of growth of such neoplasms. According to Milne [7], collision tumors were possibly overdiagnosed from immunohistochemistry appearance alone,genetic techniques should be used to show genetic differences in collision tumors [8,9] demonstrating that collision tumors do indeed arising from in various parts of tissue. In 1997 Iwaya T and his team [8] shared a case of carcinosarcoma composed of leiomyosarcoma and SqCC originated separately from mesenchymal precursors and epithelial, the conclusion was determined by a genetic analysis. According to our knowledge, it is the only case of collision tumor composed of leiomyosarcoma and SqCC so far, we presented another one.
We searched the literature in English published from 1980 to 2015 in the PubMed database and reference lists from candidate articles were hand-searched for additional articles to identify additional published datasets, we found 21 cases of esophageal collision tumor eventually. The patients ranged in age from 51 to 75 with an average age of 63.15, the sex ratio was 18 men to 2 women (1 literature not mentioned [7]), similar to the esophageal cancer with common pathological type (Table 1).
Table 1.
Components of collision tumor reported from 1980 to 2015
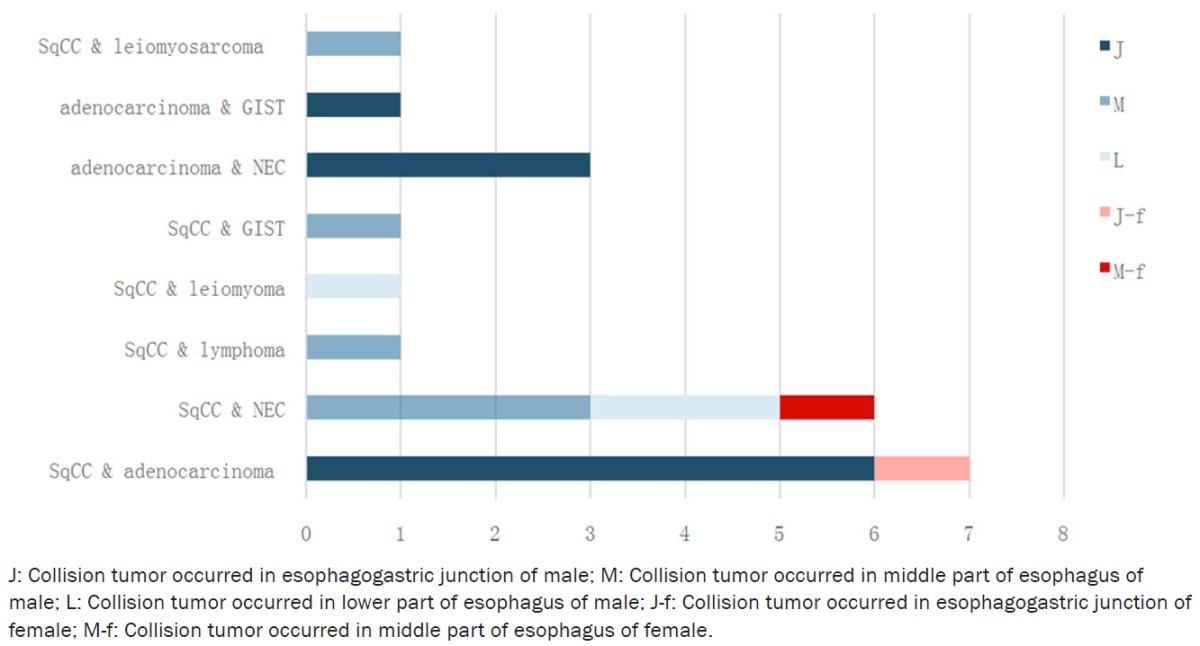
|
The correct diagnosis is significant because prompt and effective treatment usually depends on it. The diagnostic methods include light microscopy (HE stain) [10], immunohistochemistry [11], genetic/molecular analysis [8,12,13]. It is difficult but not impossible to diagnose preoperatively with endoscopicbiopsydepending on the sampled site of the collision tumor [13,14]. Collision tumors should be distinguished from composite tumors which are characterized by two divergent lineages originating from the same neoplastic clonal proliferation since it is possible that a different treatment is warranted depending on the type of collision tumor encountered.
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines recommend dose of postoperative RT is 45-50.4 Gy (1.8-2 Gy/day) for Esophageal and Esophagogastric Junction Cancers [15]. While palliative radiotherapy is considerable in the end of patient’s life which can help to alleviate a multitude of symptoms related to advanced cancer [16]. In this case, the CT scan showed the recurrence of mediastinal lymph nodes in 40 months after RT. It is considered that the high metastatic esophageal cancer with postoperative RT is related to the lower local recurrence and longer survival.
There are several potential limitations in our case report. The information of esophageal collision tumor from 1980 to 2015 may be incomplete since the restricted searching skill, the type of collision tumors may not limited to aforesaid. There is no genetic testing of specimen in this case, which can be achieved in further studies to confirm biological behavior and interaction between the two components. With the current social system and humanities, this plan of radiotherapy maybe not the best choice for this patient, since the accelerated hyperfractionation (AHF) improves local control and is responsible for a trend to an improved cause-specific survival [17]. This is a whole world problem exists not only in Asia countries.
Disclosure of conflict of interest
None.
References
- 1.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer Fabrega J. Leiomyosarcoma of the inferior vena cava: Feasibility of surgical resection. A report of two cases. Rev Esp Enferm Dig. 2015;107:458–60. [PubMed] [Google Scholar]
- 3.Almeida JM. Leiomyosarcoma of the esophagus. Chest. 1982;81:761–3. doi: 10.1378/chest.81.6.761. [DOI] [PubMed] [Google Scholar]
- 4.Meyer Beitrag zur Verstandigunguber die Namengebung in der Geschwulstlehre. ZentralblAllg Path Anat. 1919;30:291–296. [Google Scholar]
- 5.Dodge OG. Gastro-esophageal carcinoma of mixed histological type. J Pathol Bacteriol. 1961;81:459–471. doi: 10.1002/path.1700810219. [DOI] [PubMed] [Google Scholar]
- 6.Spagnolo DV, Heenan PJ. Collision carcinoma at the esophagogastric junction: report of two cases. Cancer. 1980;46:2702–2708. doi: 10.1002/1097-0142(19801215)46:12<2702::aid-cncr2820461228>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Milne AN, Carvalho R, van Rees BP, van Lanschot JJ, Offerhaus GJ, Weterman MA. Do collision tumors of the gastroesophageal junction exist? A molecular analysis. Am J Surg Pathol. 2004;28:1492–1498. doi: 10.1097/01.pas.0000138184.74496.4d. [DOI] [PubMed] [Google Scholar]
- 8.Iwaya T, Maesawa C, Tamura G, Sato N, Ikeda K, Sasaki A, Othuka K, Ishida K, Saito K, Satodate R. Esophageal carcinosarcoma: a genetic analysis. Gastroenterology. 1997;113:973–977. doi: 10.1016/s0016-5085(97)70194-x. [DOI] [PubMed] [Google Scholar]
- 9.Van Eeden S, Nederlof PM, Taal BG, Offerhaus GJ, Van Velthuysen ML. A tumour with a neuroendocrine and papillary serous component: two or a pair? J Clin Pathol. 2002;55:710–714. doi: 10.1136/jcp.55.9.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washizawa N, Kobayashi K, Kase H, Tokura N, Gotoh T, Iwasaki I, Tsujimoto S. Collision carcinoma at the esophagogastric junction. Gastric Cancer. 1999;2:240–243. doi: 10.1007/s101200050071. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Sun X, Zou Y, Meng X. Small cell type neuroendocrine carcinoma colliding with squamous cell carcinoma at esophagus. Int J Clin Exp Pathol. 2014;7:1792–1795. [PMC free article] [PubMed] [Google Scholar]
- 12.Van Eeden S, Nederlof PM, Taal BG, Offerhaus GJ, Van Velthuysen ML. A tumour with a neuroendo-crine and papillary serous component: two or a pair? J Clin Pathol. 2002;55:710–714. doi: 10.1136/jcp.55.9.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González LM, Sanz-Esponera J, Saez C, Alvarez T, Sierra E, Sanz-Ortega J. Case report: esophageal collision tumor (oat cell carcinoma and adenocarcinoma) in Barrett’s esophagus: immunohistochemical, electron microscopy and LOH analysis. Histol Histopathol. 2003;18:1–5. doi: 10.14670/HH-18.1. [DOI] [PubMed] [Google Scholar]
- 14.Ng WK, Lam KY, Chan AC, Kwong YL. Collision tumour of the oesophagus: a challenge for histological diagnosis. J Clin Pathol. 1996;49:524–526. doi: 10.1136/jcp.49.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCCN Clinical Practice Guideline. Esophageal and Esophagogastric Junction Cancers. Version 3 2015.
- 16.Jones JA, Lutz ST, Chow E, Johnstone PA. Palliative radiotherapy at the end of life: a critical review. Ca Cancer J Clin. 2014;64:296–310. doi: 10.3322/caac.21242. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura Y, Ono K, Tsutsui K, Oya N, Okajima K, Hiraoka M, Abe M. Esophageal cancer treated with radiotherapy: impact of total treatment time and fractionation. Int J Radiat Oncol Biol Phys. 1994;30:1099–1105. doi: 10.1016/0360-3016(94)90315-8. [DOI] [PubMed] [Google Scholar]


