Abstract
Epithelioid inflammatory myofibroblastic sarcoma is extremely rare and belongs to a variant of inflammatory myofibrobalstic tumor with aggressive clinical course. We describe a case of a 22 years old man presented with an abdominal huge tumor. Microscopically, the neoplasm cells were rounded and epithelioid in shape. Abundant interstitial edema and less myxoid stroma were also present together with an inflammatory infiltrate. Fluorescence in situ hybridization revealed that ALK gene presented mutation. After surgery the patient received chemotherapy with an anaplastic lymphoma kinase (ALK) inhibitor, crizotinib. The patient continues to be alive with disease for 16 months and has no recurrence. Although EIMS has a poor prognosis, this is the few successful case with sustained response of targeted therapy.
Keywords: Epithelioid inflammatory myofibroblastic sarcoma, ALK inhibitor, crizotinib, RANBP2-ALK fusion
Introduction
Inflammatory myofibroblastic tumor (IMT) is a histologically distinctive lesion that occurs primarily in the viscera and soft tissue of children and young adults. It is composed of myofibroblastic spindle cells with inflammatory infiltration, particularly of plasma cells and lymphocytes, and considered a tumor of borderline malignancy. About cytogenetics and molecular genetics findings, a high percentage of IMT is associated with ALK mutations and many are also immunoreactive for ALK. There is evidence to suggest that different fusion partners result in different patterns of ALK immunoreactivity. Recently Mariño-Enríquez A [1] et al described 11 cases of IMT, all were proposed to be a subtype of IMT with unique morphology and pattern of ALK immunoreactivity, coining the term epithelioid inflammatory myofibroblastic sarcoma (EIMS). ALK fusion proteins in EIMS are detected in the nuclear membrane with Ran-binging protein 2 (RANBP2). EIMS associated with this fusion gene often follows an aggressive clinical behavior.
We herein report a rare case of EIMS with abdominal pain and effervescence that developed a mass in the transverse colon mesentery and resulted in a sustained response by the administration of ALK inhibitor. Up to now, there are about some reports of EIMS [1-8], but cases treated with targeted therapy are rare.
Case report
A 22-year-old man gave the complaint of abdominal interrupted pain for 8 days, and the next day the abdominal mass was found when touched the stomach. He also had the constant fever for 5 days with the maximum body temperature of 39.2°C. Abdominal enhanced computed tomography (CT) revealed a huge tumor in the right abdominal cavity with heterogeneous density and scattered punctuate calcification (Figure 1), about 10.42 mm×5.95 mm in maximum section. There were the enlarged lymph nodes around the mass and few effusions in the right side of the colon, and without other abdominal nodules. The lesion was suspected to be a mesenchymal tumor, such as gastrointestinal stromal tumor. And then, excision of the abdominal tumor and part of transverse colon was performed. Intraoperative finding revealed military diffused nodes of stomach wall. The tumor located at the transverse colon mesentery, with closely adhesion of greater curvature, antrum, duodenal, transverse colon and greater omentum. It wrapped around the right gastro-omental blood vessel and was rich in blood supply. About 500 ml of hemorrhagic ascites were found in the abdominal cavity.
Figure 1.
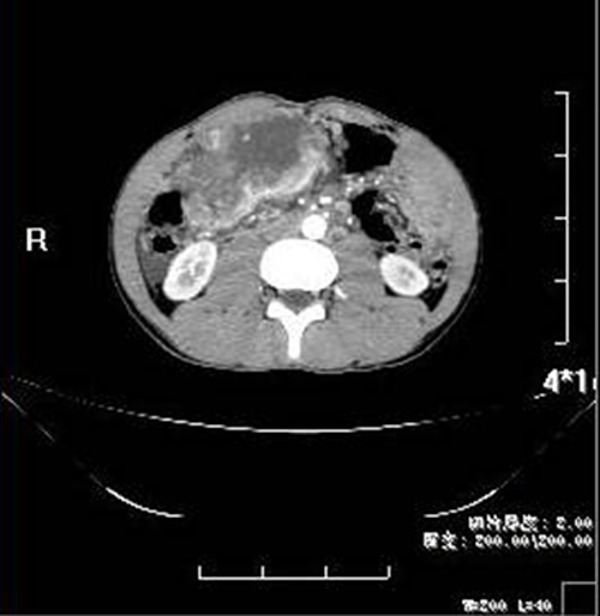
Abdominal enhanced computed tomography (CT) revealing a huge tumor in the right abdominal cavity with heterogeneous density.
Pathological and genetic studies
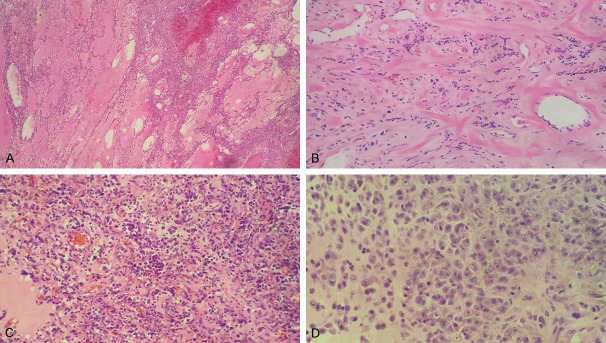
Grossly, the huge and lobulated tumor located at the transverse colon mesentery (Figure 2), measured 13 cm in maximum size. It showed a variegated appearance with mixed fleshy, hemorrhage, mucoid areas. The tumor infiltrated the adjacent colon wall. Microscopically, the tumor consisted of many different histological types, such as high cell density with prominent hemangiopericytomatous vasculature, low cell density with abundant dropsy-like and myxoid stroma, and microcapsule like structure (Figure 3A). Low cell density area contained more mixed inflammatory cells (Figure 3C), mainly neutrophils, few lymphocytes and plasma cells. The stroma was rich in capillaries and had much hemorrhage. And collagenous stroma was also observed (Figure 3B). Focal necrosis was present in high cell density. Tumor cells were rounded and epithelioid in shape with round vesicular nuclei and large nucleoli, also variable amounts of amphophilic cytoplasm (Figure 3D). There was also more spindle cell component with low density, comprising about 20% of the tumor. Mitotic activity ranged from 1 to 5 per high power field.
Figure 2.
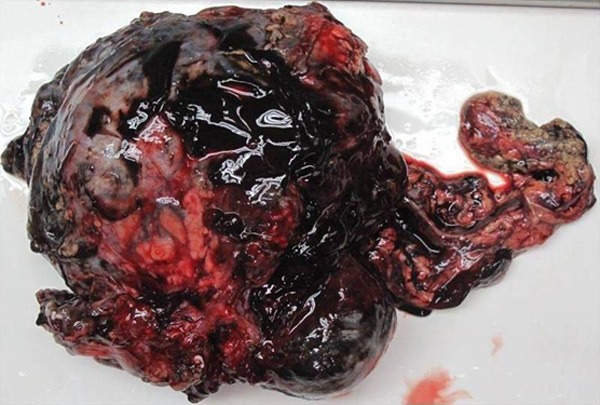
Grossly, the huge and lobulated tumor locating at the transverse colon mesentery with hemorrhage and incomplete capsule.
Figure 3.
In the region of low tumor cell density with abundant dropsy-like and microcapsule like structure (A). The region of tumor was rich in collagenous stroma and few spindle cells (B). The rounded and epithelioid tumor cells were scattered against the background of inflammatory cells (C) and unconspicuous myxoid stroma (D).
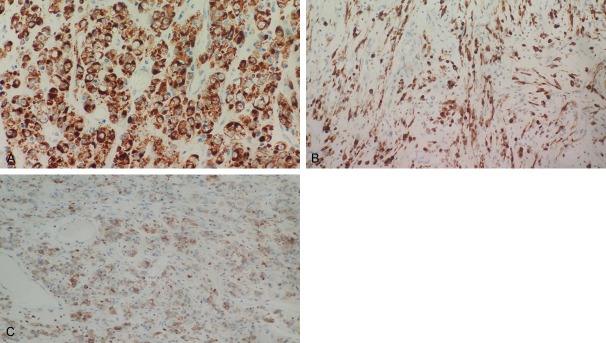
ALK was detected by immunohistochemistry and the staining was localized to the cytoplasm with perinuclear accentuation (Figure 4A). But the pattern of staining in spindle cell area wasn’t typical (Figure 4B). Focal reactivity for Desmin was detected, and CD30 showed moderate membranous staining with focally dot-like (Golgi) pattern (Figure 4C). No expression of SMA, CD34, CD117, DOG1, S-100, and cytokeratin was detected. FISH analysis showed rearrangement of ALK, identified as a set of 1 merged yellow signal, 1 separate green-labeled ALK signal and 1 red-labeled RANBP2 signal (Figure 5).
Figure 4.
The rounded and epithelioid tumor cells were positive for ALK, exhibiting a cytoplasmic pattern with perinuclear accentuation (A). But the positive staining of ALK in spindle tumor cells was only cytoplasmic pattern (B). The tumor cells were also scattered positive for CD30 (C).
Figure 5.
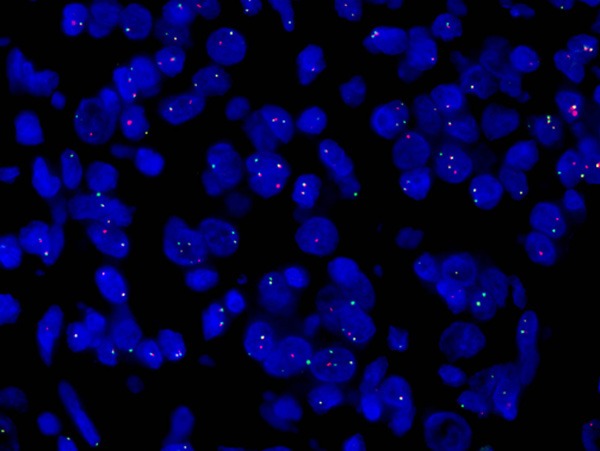
FISH analysis resulted in merged yellow signals, indicative of RANBP2-ALK fusion.
Based on these results, we diagnosed the patient as having an EIMS. One month after the operation, the patient received crizotinib treatment at a dose of 500 mg per day for 16 months. And at present, there is no recurrence and progression of disease.
Discussion
IMT is rare mesenchymal tumors composed of myofibroblastic spindle cells with kinds of inflammatory infiltration, especially of plasma cells and lymphocytes. It often arises in lung or abdominal soft tissue in children and young adults, and belongs to possess an intermediate biological potential for malignancy. Based on the report of Mariño-Enríquez A [1,6], though the tumor size, mitotic activity and presence of necrosis are not well correlated with tumor clinical outcome, the proliferation of highly atypical polygonal, round or epithelioid cells with vesicular and large nuclei are evidence of malignant transformation. That is, EIMS display the potential for a high rate of recurrence after surgery and are associated with a bad prognosis. Kimbara S et al [7] reported a case of EIMS with rapidly local recurrence after two surgical excision procedures and resistance to conventional chemotherapy. Microscopically, our case has the same characteristics, such as young patient, myxoid stroma, mixed inflammatory cells, round and epithelioid cells. But the ratio of spindle cells in the tumor is higher, about 20%. The density and type of inflammatory cells is different between high and low cells region, and neutrophils are not very abundant. At the same time, tumor stroma is rich in dropsy-like and collagenous at different region, microcapsule like structure and calcification also can be observed.
Approximately 50% of IMT harbors clonal rearrangements of the ALK gene at 2p23. This gene codes for a tyrosine kinase receptor that is a member of the insulin growth factor receptor superfamily. ALK rearrangements result in constitutive expression and activation of this gene with abnormal phosphorylation of cellular substrates. And different fusion partners result in different patterns of ALK immunoreactivity. The RANBP2-ALK fusion of EIMS causes the special staining pattern of the nuclear membrane or the cytoplasm with perinuclear accentuation, which usually exhibits an epithelioid or round cell morphology and develop a more aggressive clinical course. The patient with this fusion, whose tumor was resistant to doxorubicin, ifosfamide and imatinib, gave a durable response to crizotinib. At the same time, ALK-negative patients didn’t respond [7,9]. Our case was treated with crizotinib after one month of operation because of ALK-positive, there has no recurrence and prognosis at present. That is, the patient has a good response to crizotinib. Up to now, there have been two reported cases of patient with RANBP2-ALK fusion who achieved good response to crizotinib, suggesting that the fusion is an important target in the carcinogenesis of EIMS [7,8].
Conclusion
We report a rare case of an EIMS arising in the transverse colon mesentery treated with crizotinib. ALK inhibitor may be the new and best therapeutic strategy following surgery for EIMS, regardless of the site of origin.
Disclosure of conflict of interest
None.
References
- 1.Mariño-Enríquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, Coffin CM, Hornick JL. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant ofinflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135–44. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Jiang G, Zhang D, Zhang L, Xu J, Li S, Li W, Ma Y, Zhao A, Zhao Z. Epithelioid inflammatory myofibroblastic sarcoma with recurrence after extensive resection: significant clinicopathologic characteristics of a rare aggressive soft tissue neoplasm. Int J Clin Exp Pathol. 2015;8:5803–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol. 2015;10:106. doi: 10.1186/s13000-015-0358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, Meng YH, Lu P, Ning HY, Hong L, Kang XL, Duan MG. Epithelioid inflammatory myofibroblastic sarcoma in abdominal cavity: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:4213–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Kozu Y, Isaka M, Ohde Y, Takeuchi K, Nakajima T. Epithelioid inflammatory myofibroblastic sarcoma arising in the pleural cavity. Gen Thorac Cardiovasc Surg. 2014;62:191–4. doi: 10.1007/s11748-013-0204-x. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, Wu JM, Liau JY, Huang HY, Lo CY, Jan IS, Hornick JL, Qian X. Cytopathologic features of epithelioid inflammatory myofibroblastic sarcoma with correlation of histopathology, immunohistochemistry, and molecular cytogenetic analysis. Cancer Cytopathol. 2015;123:495–504. doi: 10.1002/cncy.21558. [DOI] [PubMed] [Google Scholar]
- 7.Kimbara S, Takeda K, Fukushima H, Inoue T, Okada H, Shibata Y, Katsushima U, Tsuya A, Tokunaga S, Daga H, Okuno T, Inoue T. A case report of epithelioid inflammatory myofibroblastic sarcoma with RANBP2-ALK fusion gene treated with the ALK inhibitor, crizotinib. Jpn J Clin Oncol. 2014;44:868–71. doi: 10.1093/jjco/hyu069. [DOI] [PubMed] [Google Scholar]
- 8.Kurihara-Hosokawa K, Kawasaki I, Tamai A, Yoshida Y, Yakushiji Y, Ueno H, Fukumoto M, Fukushima H, Inoue T, Hosoi M. Epithelioid inflammatory myofibroblastic sarcoma responsive to surgery and an ALK inhibitor in a patient with panhypopituitarism. Intern Med. 2014;53:2211–4. doi: 10.2169/internalmedicine.53.2546. [DOI] [PubMed] [Google Scholar]
- 9.Fujiya M, Kohgo Y. ALK inhibition for the treatment of refractory epithelioid inflammatory myofibroblastic sarcoma. Intern Med. 2014;53:2177–8. doi: 10.2169/internalmedicine.53.3038. [DOI] [PubMed] [Google Scholar]