Abstract
Schwannomas are usually benign tumors that arise from well-differentiated Schwann cells. They rarely occur in the retroperitoneum. Here, we present a case of a 60-year-old man with a giant retroperitoneal pelvic mass. Imageological diagnosis suggested a large heterogeneous mass of 16 cm in diameter located in the abdominopelvic retroperitoneum. Complete intralesional enucleation was achieved without any adjacent organs injury except a severe bleeding which was ceased as we applied the bilateral inferior vesical artery embolization. Final histopathological result showed the tumor was a low malignant Schwannoma. The patient’s symptoms were greatly improved after operation. Unfortunately, a local recurrence was detected at the six-month follow-up appointment with consequent losing to follow up.
Keywords: Giant malignant schwannoma, pelvic, diagnosis, complete resection
Introduction
Schwannomas, also known as neurilemmomas, are tumors composed of well-differentiated Schwann cells, which derive from glial cells of peripheral nerve sheaths [1]. Most schwannomas are benign and the malignant ones, which are usually associated with Von Recklinghausen’s disease, are much uncommon [2,3]. Schwannomas commonly occur in the head and neck, retroperitoneal, and extremities. The pelvic form is very rare, with a reported incidence of 1-3% of all schwannomas [4]. Because there are no specific clinical or radiological signs for pelvic schwannomas and they resemble a number of pelvic lesions, misdiagnosis may easily happen [5]. Surgical excision is both diagnostic and therapeutic to pelvic schwannomas. Here we report a case of giant malignant pelvic schwannoma managed surgically in our institution and review of the literature.
Case report
A 60-year-old man presented to us with the complaint of constipation and frequent micturition that had been present for 8 months. He also complained of an increasing pain and numbness of the right lower limb. Ultrasound examination performed in local hospital showed dextral severe hydronephrosis and a giant hypoecho mass located in the pelvic. The patient had already undergone a percutaneous nephrostomy to protect his renal function. His past medical history included a subtotal thyroidectomy when he aged 59 for thyroid adenoma.
On physical examination, a tough, sharp-edged, immobile mass can be palpated per-abdominally. The lower margin of the mass could not be touched while the upper extended 5 cm over the pubic symphysis. The mass was posterior to the rectum on rectal examination. The prostate could be felt separately. Laboratory studies revealed CEA, CA 19-9, blood routine examination, urea and serum biochemistry analysis were unremarkable except for a low level of hemoglobin of 101 g/L.
The ultrasound scan in our institution showed a hypoecho mass with fluid dark areas located in pelvis posterior to bladder and rectum. CT and MRI revealed a large (16 × 10 cm) inhomogeneous mass with areas of liquidation or necrosis located in the abdominopelvic retroperitoneum (Figure 1A and 1B), compressing and displacing the iliac vessels and both ureters, causing dextral moderate hydronephrosis (Figure 1C). The rectum and bladder were pushed and displaced anteriorly and superiorly (Figure 1D). A transrectal ultrasound-guided biopsy of the mass performed subsequently was inconclusive. A provisional diagnosis of low differentiative nonepithelial tumor but cannot exclude schwannoma was made. Then the patient was scheduled for open surgical exploration and resection. Due to the huge volume and abundant blood supply of the tumor, sufficient amounts of blood products including fresh frozen plasma and thrombocytes were prepared. Anorectal surgeons, vascular surgeons, and spine surgeons were invited to attend the surgery.
Figure 1.
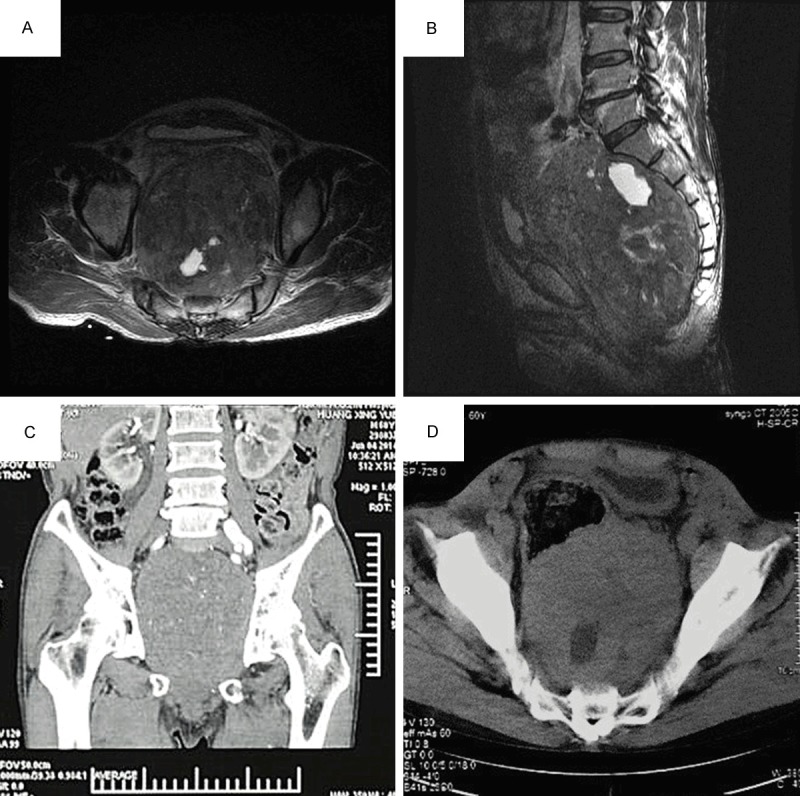
Radiologic features of the pelvic mass. A and B. A large (about 16 × 10 cm) inhomogeneous mass with areas of liquidation or necrosis located in the abdominopelvic retroperitoneum. C. The coronal plane of MRI revealed iliac vessels and both ureters were compressed, causing dextral moderate hydronephrosis. D. The rectum and bladder pushed and displaced anteriorly and superiorly.
A midline incision from umbilicus to symphysis pubis was undertaken. Intraoperative findings revealed a large encapsulated retroperitoneal mass (20 cm × 20 cm × 10 cm), occupying the entire pelvis, displacing the urinary bladder and recto-sigmoid colon to the right side. Due to its size, the peritoneum was opened over the mass. The lesion was immobile, without evidence of local invasion. And it had a cystic and a solid component. Samples of both components were sent intraoperatively for pathologic examination. The diagnosis was low malignant potential schwannoma. On account of the very large size, it was impossible to trace the originating nerve. Then the conservative intralesional enucleation was undertaken to excise the tumor into pieces. Hence, the complete specimen was achieved by a combination of sharp and blunt dissection with consequent severe haemorrhage. Haemostasis proved extremely difficult because of the limited access and poor visibility. Suturing, suture ligatures are attempted to suspend the bleeding. Finally, the bleeding was ceased as we applied the gauze compression packing hemostasis and bilateral inferior vesical artery embolization (Figure 2). 48 hours later, the wound was opened again to remove the remnant tumor and capsule. So as do the indwelled gauze and residual blood. The overall blood loss was estimated at 2200 ml. Fortunately, ureters, colon, vital nerves and bladder were preserved. 4 g cefodizime sodium was administered daily intravenously in the first postoperative week to prevent the potential infection of clostridia.
Figure 2.
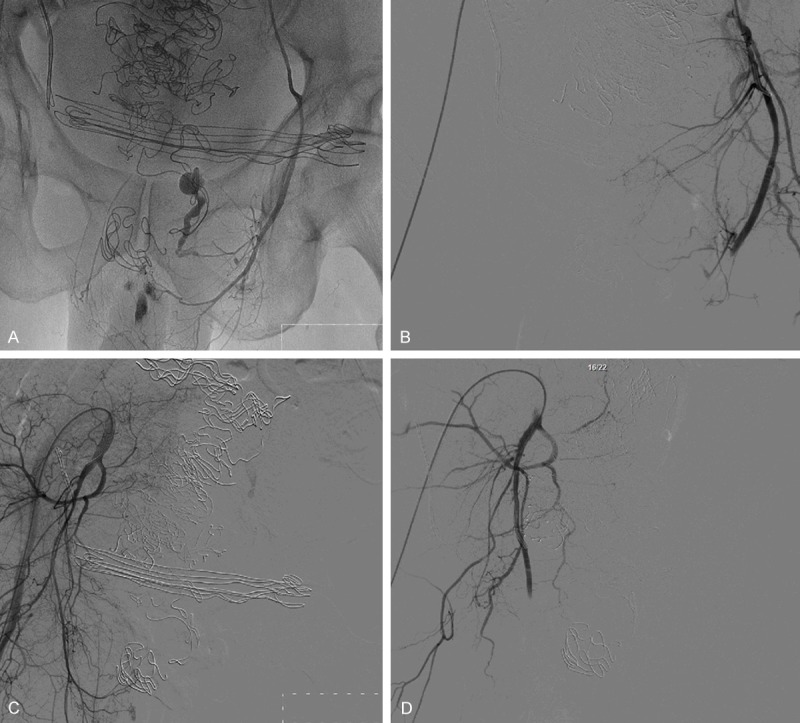
Postoperative iliac artery angiography and bilateral inferior vesical artery embolization. Many sutures can be seen on the above figures A and B are pre-embolization and post-embolization of the left inferior vesical artery, respectively. So are C and D as they show the contralateral artery.
The gross specimen was yellowish with areas of necrosis and hemorrhage. On microscopic examination, there were sheets of spindle cells that occasionally formed alternating areas of dense cellularity termed Antoni A regions (Figure 3B), and areas of myxoid matrix termed Antoni-B (AB) regions (Figure 3C). Rare atypical large nuclei and mitosis are present. Immunohistochemical studies showed cells to be sporadic positive for S100 protein, NSE (+), Ki67 (++), P53 (++), CD117 (-), CD34 (-), desmin (sporadic +), and SMA (sporadic +). The final diagnosis was a low potential malignant Schwannoma.
Figure 3.
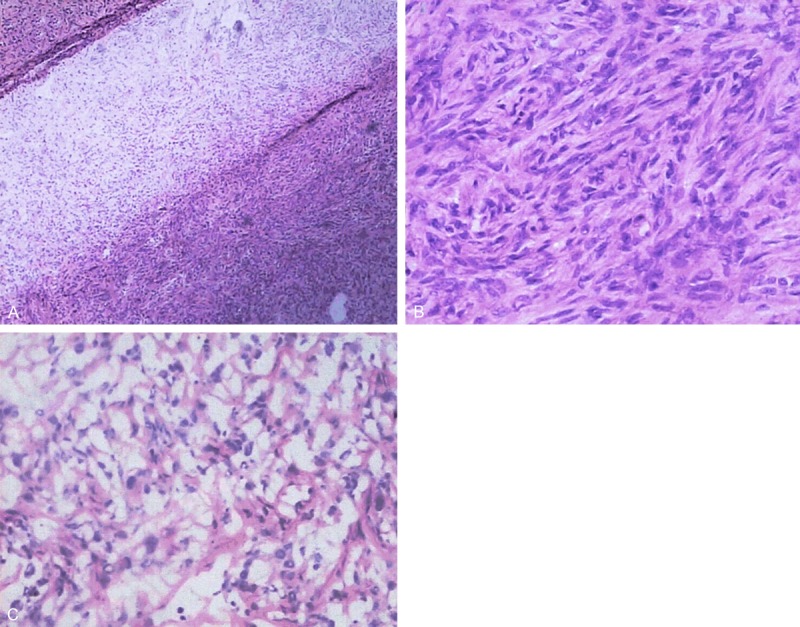
A. Antoni A regions with characteristic dense cellularity and aggregation of fibrillary, elongated cells. Antoni B regions are composed of mixoid and degenerative tissue with fewer cells and gelatinous substance (H & E, × 40). B. Antoni A area is composed of spindle cell proliferation in a palisade or turbinate pattern. Rare atypical large nuclei and mitosis are present (HE × 200). C. Antoni B area, cells loosely arranged in a myxoid background are present. (HE × 200).
The patient was uneventful during the postoperative period. Pain and numbness of the right lower limb, constipation and frequent micturition were greatly improved. But a local recurrence was detected at the six-month follow-up appointment with consequent losing to follow up.
Discussion
Pelvic schwannomas (neurilemmomas) are soft tissue neurogenic tumors that mostly arise from a sacral nerve or the hypogastric plexus and account for only a small fraction of schwannoma cases as reported [6]. Most of them are benign, but may undergo malignant changes if they are associated with von Recklinghausen’s disease [7]. The diagnosis and treatment are often delayed as those tumors show no or non-specific symptoms until they have grown to a substantial size causing compression, displacement, or invasion of adjacent structures.
Preoperative radiologic examination plays a vital role in the diagnosis of a pelvic tumor. The differential diagnoses with schwannomas include fibrosarcoma, liposarcoma, ganglioneuroma, hydatid cyst, haematoma, and connective tissue diseases [7]. As revealed by the CT scan, the tumor margins are smooth and sharp with an enhanced appearance. And liquefaction, necrosis, haemorrhage within the tumor could be present [2]. MR provides a more accurate preoperative diagnosis than CT or ultrasound when imaging the soft tissue tumors. The typical features of benign schwannoma imaged on MR include hypointensity on T1-weighted images and hyperintensity on T2-weighted ones [8-11]. However, many schwannomas showed a mixed signal on both T1- and T2-weighted images if they were associated with hemorrhagic cystic and (or) solid components [9].
As imaging findings are not specific, ultrasound-guided biopsy of large pelvic tumors is often suggested though it is mostly inaccurate and occasionally misleading due to cellular pleomorphism in areas of degeneration. But it may be helpful in suspected malignant lesions [12]. In our case, the preoperative transrectal ultrasound-guided biopsy gave us the diagnosis of low differentiative nonepithelial tumor but cannot exclude schwannoma.
Definitive diagnosis of schwannomas is based on the histological analysis with partial or mass resection [13,14]. Schwannoma is characterized by alternating areas of hyper- (Antoni A) and hypo- (Antoni B) cellularity. Clusters of parallel arranged spindle cells are contained in Antoni A areas, forming palisades, commonly known as Verocay’s bodies. Cells loosely arranged in a myxoid background are present in the Antoni B areas [2]. Malignancy is usually suggested if the mitotic figures, cellularity, nuclear atypia and tumor necrosis are shown histologically and macroscopically [2,15]. Among these factors, the presence of marked nuclear atypia and frequent mitoses are the most reliable features relating to malignancy. Typical immunohistochemical manifestations of schwannoma include immuno-positive for S-100, neuron specific enolase and vimentin, while immuno-negative for smooth muscle actins [12].
As most of the retroperitoneal tumors are in an anatomically complex and surgically inaccessible site with surrounding vital structures, it may not always be possible to obtain a complete resection with sufficient clear margins [16]. It is argued that piecemeal excision even with laparoscope is an acceptable alternative for benign schwannoma. But for a malignant schwannoma, complete excision with negative margins is still the classical treatment [2]. It has been shown that in the case of malignancy the local recurrence rate after marginal excision is 72%, versus 11.7% after wide margin resection [16,17]. Therefore, it is highly recommended to send a biopsy for frozen section to identify whether the tumor is malignant or benign before choosing the surgical approach, conservative versus aggressive ones.
Care must be taken for a meticulous dissection and separation of the iliac arteries and veins from the tumor, to control potential massive bleedings from the main retroperitoneal vessels, especially in the case of tumor adhesions or invasion [18]. A preoperative iliac artery angiography and relevant artery embolization may helpful to reduce the blood lose and prevent uncontrolled haemorrhage which may necessitate the premature termination of the operation. What is more, sufficient amounts of blood products including fresh frozen plasma and platelets have to be readily available. Multiple specialties including anorectal surgeons, vascular surgeons, spine surgeons and anesthetists should be contemplated since complications might be anticipated. It is of utmost importance to avoid injury to the adjacent visceral, vascular, and nervous structures, which may result in complications like hemorrhage and neurological deficits.
Malignant schwannoma has a high rate of recurrence and poor prognosis and is insensitive to chemotherapy and radiotherapy [2]. In our case, the patient’s symptoms were greatly improved, while with a local recurrence at the six-month follow-up appointment. The current situation is unkown since we lost follow-up with him.
In conclusion, we reported an extremely rare case of pelvic malignant schwannomas. Preoperative imaging and biopsy are helpful to diagnosis, but often inconclusive. Currently, complete surgical resection is the mainstay of treatment. Definitive diagnosis is based on histological and immunohistochemical analysis of biopsy specimens. Local recurrence and overall survival are related to negative resection margins and tumor grade.
Acknowledgements
This work is funded by grants from the Natural Science Foundation of Tianjin (No. 14JCYBJC26300) and National Key Specialty Construction of Clinical Projects, and the Natural Science Foundation of Tianjin (No. 15JCYBJC24600).
Disclosure of conflict of interest
None.
References
- 1.Dominguez J, Lobato RD, Ramos A, Rivas JJ, Gomez PA, Castro S. Giant intrasacral schwannomas: Report of six cases. Acta Neurochir (Wien) 1997;10:954–9. doi: 10.1007/BF01411305. discussion 959-60. [DOI] [PubMed] [Google Scholar]
- 2.Choudry HA, Nikfarjam M, Liang JJ, Kimchi ET, Conter R, Gusani NJ, Staveley-O’Carroll KF. Diagnosis and management of retroperitoneal ancient schwannomas. World J Surg Oncol. 2009;7:12. doi: 10.1186/1477-7819-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Gao C, Juzi JT, Hao X. Analysis of 82 cases of retroperitoneal schwannoma. ANZ J Surg. 2007;4:237–240. doi: 10.1111/j.1445-2197.2007.04025.x. [DOI] [PubMed] [Google Scholar]
- 4.Borghese M, Corigliano N, Gabriele R, Antoniozzi A, Izzo L, Barbaro M, Caporale A. [Benign schwannoma of the pelvic retroperitoneum. Report of a case and review of the literature] . G Chir. 2000;5:232–8. [PubMed] [Google Scholar]
- 5.Yi K, Wang YM, Chen J. Laparoscopic resection of an obturator schwannoma: A case report. Chin Med J (Engl) 2010;13:1804–6. [PubMed] [Google Scholar]
- 6.Chan PT, Tripathi S, Low SE, Robinson LQ. Case report--ancient schwannoma of the scrotum. BMC Urol. 2007;7:1. doi: 10.1186/1471-2490-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindal T, Mukherjee S, Kamal MR, Sharma RK, Ghosh N, Mandal SN, Das AK, Karmakar D. Cystic schwannoma of the pelvis. Ann R Coll Surg Engl. 2013;95:e1–2. doi: 10.1308/003588413X13511609956697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soderlund V, Goranson H, Bauer HC. MR imaging of benign peripheral nerve sheath tumors. Acta Radiol. 1994;35:282–6. [PubMed] [Google Scholar]
- 9.Hayasaka K, Tanaka Y, Soeda S, Huppert P, Claussen CD. MR findings in primary retroperitoneal schwannoma. Acta Radiol. 1999;40:78–82. doi: 10.1080/02841859909174408. [DOI] [PubMed] [Google Scholar]
- 10.Do-Dai DD, Ho VB, Rovira MJ, Knight RW, Twomey PA. Retroperitoneal melanotic schwannoma: Ultrasonographic features. J Clin Ultrasound. 1995;23:42–8. doi: 10.1002/jcu.1870230109. [DOI] [PubMed] [Google Scholar]
- 11.Loke TK, Yuen NW, Lo KK, Lo J, Chan JC. Retroperitoneal ancient schwannoma: Review of clinico-radiological features. Australas Radiol. 1998;42:136–8. doi: 10.1111/j.1440-1673.1998.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 12.Walczak DA, Jaguscik R, Olborski B, Falek W, Trzeciak PW. Retroperitoneal “ancient” Schwannoma-a rare case of rare location: Case report and literature review. Pol Przegl Chir. 2012;84:646–50. doi: 10.2478/v10035-012-0106-0. [DOI] [PubMed] [Google Scholar]
- 13.Schindler OS, Dixon JH, Case P. Retroperitoneal giant schwannomas: Report on two cases and review of the literature. J Orthop Surg (Hong Kong) 2002;10:77–84. doi: 10.1177/230949900201000114. [DOI] [PubMed] [Google Scholar]
- 14.Cury J, Coelho RF, Srougi M. Retroperitoneal schwannoma: Case series and literature review. Clinics (Sao Paulo) 2007;62:359–62. doi: 10.1590/s1807-59322007000300024. [DOI] [PubMed] [Google Scholar]
- 15.Wee-Stekly W, Mueller MD. Retroperitoneal tumors in the pelvis: A diagnostic challenge in gynecology. Front Surg. 2014;1:49. doi: 10.3389/fsurg.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bose B. Primary malignant retroperitoneal tumours: Analysis of 30 cases. Can J Surg. 1979;22:215–20. [PubMed] [Google Scholar]
- 17.Pinson CW, ReMine SG, Fletcher WS, Braasch JW. Long-term results with primary retroperitoneal tumors. Arch Surg. 1989;124:1168–73. doi: 10.1001/archsurg.1989.01410100070012. [DOI] [PubMed] [Google Scholar]
- 18.Herrington JL Jr, Edwards LW. Massive retroperitoneal neurilemoma, with emphasis on technical problems encountered during surgical removal. Surgery. 1965;57:366–9. [PubMed] [Google Scholar]


