Abstract
Virtually all murine plasmacytomas carry chromosomal translocations that activate c-myc. The predominant (approximately 90%) c-myc-activating chromosomal translocation in pristane (2,6,10,14-tetramethylpentadecane)-induced plasmacytomas in BALB/c mice is a reciprocal translocation t(12;15) in which an immunoglobulin heavy-chain switch sequence is joined to the 5' region of c-myc. The most common switch region involved is S alpha. We developed a direct PCR method to screen for recombinations between c-myc and S alpha. The critical step in establishing the method was the cloning and sequencing of the 5' flank of C alpha, a region with a reduced number of switch repeats that is much more favorable for designing specific PCR primers than the highly repetitive S alpha region. In applying this PCR method, we detected translocation-specific junction fragments in transplanted (10/16, 63%) and primary (5/15, 33%) plasmacytomas. Moreover, the sensitivity of a nested version of that technique allowed us to discern rare t(12;15)s in BALB/c mice in the preneoplastic stage of plasmacytoma-genesis (8/20 mice, 40%) as early as 30 days after administration of pristane. We conclude that t(12;15) is the probable primary, if not initiating, oncogenic step in plasmacytomagenesis.
Full text
PDF

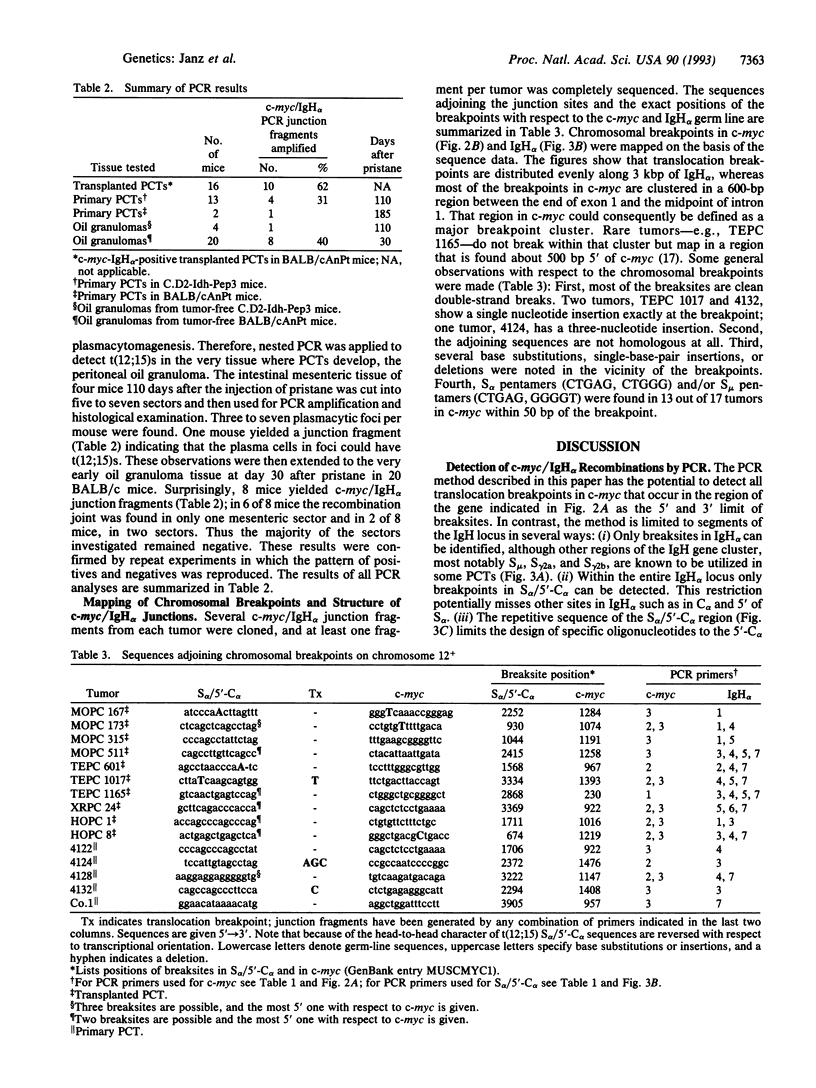
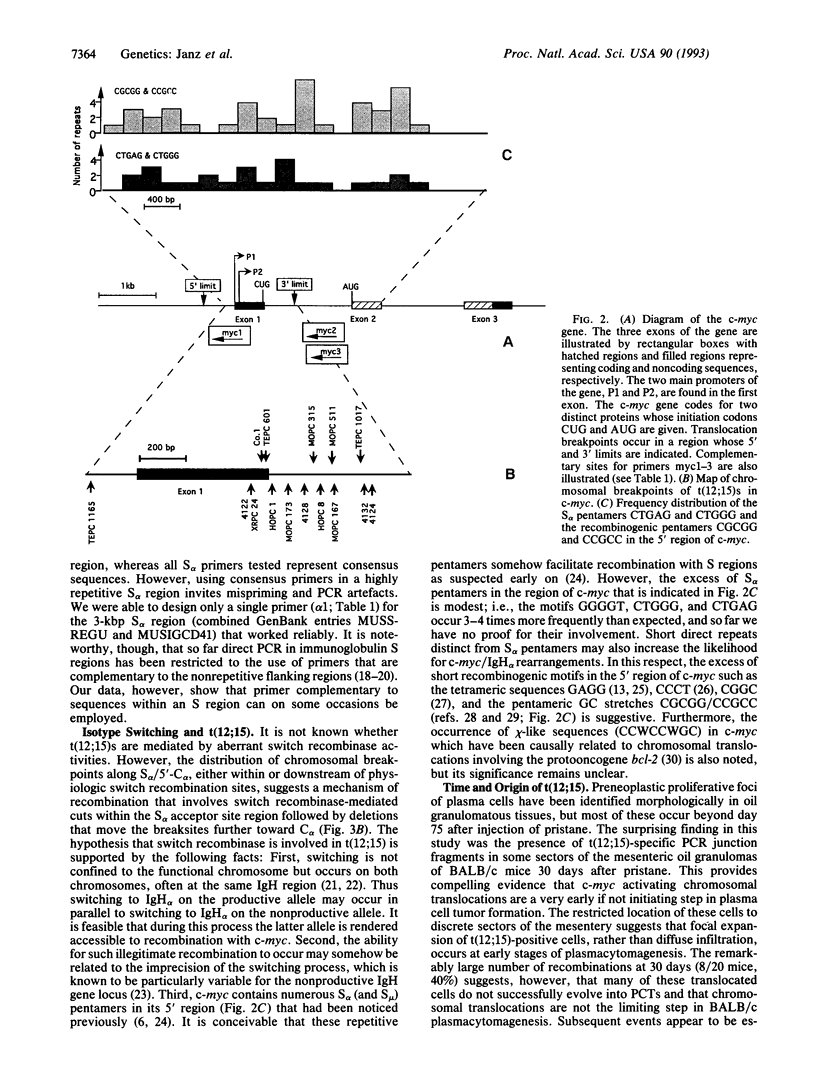
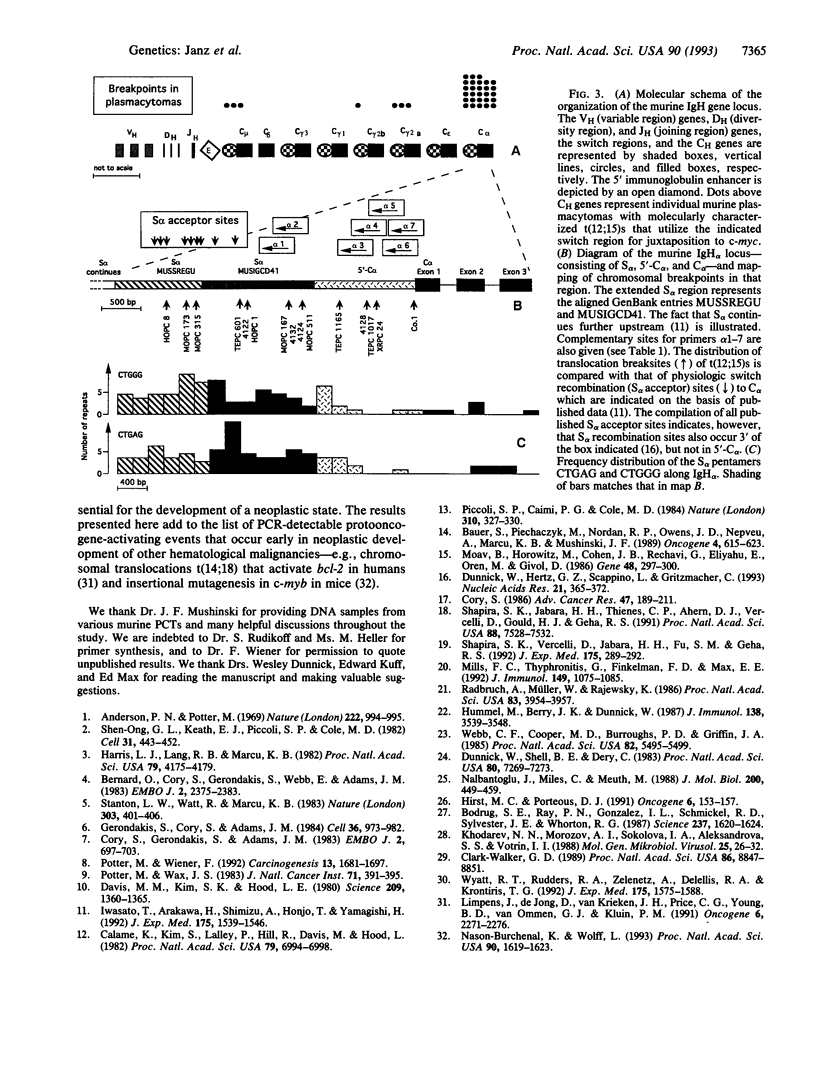
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. N., Potter M. Induction of plasma cell tumours in BALB-c mice with 2,6,10,14-tetramethylpentadecane (pristane). Nature. 1969 Jun 7;222(5197):994–995. doi: 10.1038/222994a0. [DOI] [PubMed] [Google Scholar]
- Bauer S. R., Piechaczyk M., Nordan R. P., Owens J. D., Nepveu A., Marcu K. B., Mushinski J. F. Altered myc gene transcription and intron-induced stabilization of myc RNAs in two mouse plasmacytomas. Oncogene. 1989 May;4(5):615–623. [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrug S. E., Ray P. N., Gonzalez I. L., Schmickel R. D., Sylvester J. E., Worton R. G. Molecular analysis of a constitutional X-autosome translocation in a female with muscular dystrophy. Science. 1987 Sep 25;237(4822):1620–1624. doi: 10.1126/science.3629260. [DOI] [PubMed] [Google Scholar]
- Calame K., Kim S., Lalley P., Hill R., Davis M., Hood L. Molecular cloning of translocations involving chromosome 15 and the immunoglobulin C alpha gene from chromosome 12 in two murine plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6994–6998. doi: 10.1073/pnas.79.22.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D. In vivo rearrangement of mitochondrial DNA in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8847–8851. doi: 10.1073/pnas.86.22.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S. Activation of cellular oncogenes in hemopoietic cells by chromosome translocation. Adv Cancer Res. 1986;47:189–234. doi: 10.1016/s0065-230x(08)60200-6. [DOI] [PubMed] [Google Scholar]
- Cory S., Gerondakis S., Adams J. M. Interchromosomal recombination of the cellular oncogene c-myc with the immunoglobulin heavy chain locus in murine plasmacytomas is a reciprocal exchange. EMBO J. 1983;2(5):697–703. doi: 10.1002/j.1460-2075.1983.tb01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Dunnick W., Hertz G. Z., Scappino L., Gritzmacher C. DNA sequences at immunoglobulin switch region recombination sites. Nucleic Acids Res. 1993 Feb 11;21(3):365–372. doi: 10.1093/nar/21.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick W., Shell B. E., Dery C. DNA sequences near the site of reciprocal recombination between a c-myc oncogene and an immunoglobulin switch region. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7269–7273. doi: 10.1073/pnas.80.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S., Cory S., Adams J. M. Translocation of the myc cellular oncogene to the immunoglobulin heavy chain locus in murine plasmacytomas is an imprecise reciprocal exchange. Cell. 1984 Apr;36(4):973–982. doi: 10.1016/0092-8674(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Harris L. J., Lang R. B., Marcu K. B. Non-immunoglobulin-associated DNA rearrangements in mouse plasmacytomas. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4175–4179. doi: 10.1073/pnas.79.13.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M. C., Porteous D. J. Molecular cloning of a rearranged HRAS1 oncogene in chromosome mediated gene transfer associated with elevated tumorigenicity. Oncogene. 1991 Jan;6(1):153–157. [PubMed] [Google Scholar]
- Hummel M., Berry J. K., Dunnick W. Switch region content of hybridomas: the two spleen cell Igh loci tend to rearrange to the same isotype. J Immunol. 1987 May 15;138(10):3539–3548. [PubMed] [Google Scholar]
- Iwasato T., Arakawa H., Shimizu A., Honjo T., Yamagishi H. Biased distribution of recombination sites within S regions upon immunoglobulin class switch recombination induced by transforming growth factor beta and lipopolysaccharide. J Exp Med. 1992 Jun 1;175(6):1539–1546. doi: 10.1084/jem.175.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodarev N. N., Morozov A. Iu, Sokolova I. A., Aleksandrova S. S., Votrin I. I. Spetsifichnost' fragmentatsii DNK plazmidy pBR322 Ca/Mg-zavisimoi éndonukleazoi kletochnykh iader limfotsitov cheloveka. Mol Gen Mikrobiol Virusol. 1988 Sep;(9):26–32. [PubMed] [Google Scholar]
- Limpens J., de Jong D., van Krieken J. H., Price C. G., Young B. D., van Ommen G. J., Kluin P. M. Bcl-2/JH rearrangements in benign lymphoid tissues with follicular hyperplasia. Oncogene. 1991 Dec;6(12):2271–2276. [PubMed] [Google Scholar]
- Mills F. C., Thyphronitis G., Finkelman F. D., Max E. E. Ig mu-epsilon isotype switch in IL-4-treated human B lymphoblastoid cells. Evidence for a sequential switch. J Immunol. 1992 Aug 1;149(3):1075–1085. [PubMed] [Google Scholar]
- Moav B., Horowitz M., Cohen J. B., Rechavi G., Eliyahu E., Oren M., Givol D. Structure and activity of the translocated c-myc in mouse plasmacytoma XRPC-24. Gene. 1986;48(2-3):297–300. doi: 10.1016/0378-1119(86)90089-2. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Miles C., Meuth M. Insertion of unique and repetitive DNA fragments into the aprt locus of hamster cells. J Mol Biol. 1988 Apr 5;200(3):449–459. doi: 10.1016/0022-2836(88)90535-9. [DOI] [PubMed] [Google Scholar]
- Nason-Burchenal K., Wolff L. Activation of c-myb is an early bone-marrow event in a murine model for acute promonocytic leukemia. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1619–1623. doi: 10.1073/pnas.90.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli S. P., Caimi P. G., Cole M. D. A conserved sequence at c-myc oncogene chromosomal translocation breakpoints in plasmacytomas. 1984 Jul 26-Aug 1Nature. 310(5975):327–330. doi: 10.1038/310327a0. [DOI] [PubMed] [Google Scholar]
- Potter M., Wax J. S. Peritoneal plasmacytomagenesis in mice: comparison of different pristane dose regimens. J Natl Cancer Inst. 1983 Aug;71(2):391–395. [PubMed] [Google Scholar]
- Potter M., Wiener F. Plasmacytomagenesis in mice: model of neoplastic development dependent upon chromosomal translocations. Carcinogenesis. 1992 Oct;13(10):1681–1697. doi: 10.1093/carcin/13.10.1681. [DOI] [PubMed] [Google Scholar]
- Radbruch A., Müller W., Rajewsky K. Class switch recombination is IgG1 specific on active and inactive IgH loci of IgG1-secreting B-cell blasts. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3954–3957. doi: 10.1073/pnas.83.11.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Jabara H. H., Thienes C. P., Ahern D. J., Vercelli D., Gould H. J., Geha R. S. Deletional switch recombination occurs in interleukin-4-induced isotype switching to IgE expression by human B cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7528–7532. doi: 10.1073/pnas.88.17.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Vercelli D., Jabara H. H., Fu S. M., Geha R. S. Molecular analysis of the induction of immunoglobulin E synthesis in human B cells by interleukin 4 and engagement of CD40 antigen. J Exp Med. 1992 Jan 1;175(1):289–292. doi: 10.1084/jem.175.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Webb C. F., Cooper M. D., Burrows P. D., Griffin J. A. Immunoglobulin gene rearrangements and deletions in human Epstein-Barr virus-transformed cell lines producing different IgG and IgA subclasses. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5495–5499. doi: 10.1073/pnas.82.16.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. T., Rudders R. A., Zelenetz A., Delellis R. A., Krontiris T. G. BCL2 oncogene translocation is mediated by a chi-like consensus. J Exp Med. 1992 Jun 1;175(6):1575–1588. doi: 10.1084/jem.175.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]