Abstract
Objective: To determine the expression of NIMA-related kinase NEK2 and evaluate its clinical value in colon cancer. Method: Sixty specimens of colon cancer, 30 specimens of paracancerous colon tissues and 10 specimens of normal colon tissues conventionally resected in surgery at the Second Affiliated Hospital of Nantong University from February 2006 to February 2014 were collected. These tissues were detected for the expression of Nek2 using Western Blot and immunohistochemical staining. The relationship between Nek2 protein expression and the clinicopathology and prognosis of colon tissues was discussed. Results: The expression level and positive expression rate of Nek2 protein in the colon cancer were obviously higher than that in the paracancerous tissues and normal colon tissues. They were also significantly higher in the paracancerous tissues than in the normal tissues (P<0.05). Statistical analysis revealed that Nek2 protein expression was not obviously correlated with gender, age and tumor size, but obviously correlated with degree of differentiation (P=0.008), TNM staging (P=0.000), lymph node metastasis (P=0.022) and tumor invasion (P=0.011). With the plotting of Kaplan-Meier survival curve, it could be seen that Nek2 protein expression was not significantly correlated with survival (P=0.0048). High Nek2 protein expression may be an independent risk factor for colon cancer (HR=0.227, 95% CI 0.101-0.510). Conclusion: High Nek2 protein expression reflects the malignant behavior of colon cancer. Playing important roles in the occurrence of colon cancer, Nek2 protein expression has diagnostic and prognostic value in colon cancer.
Keywords: Colon cancer, Nek2, expression, prognosis
Introdution
Colon cancer is one of the common malignancies of the digestive system [1]. The incidence of colon cancer worldwide is climbing every year, and the pathogenesis of colon cancer remains unclear [2,3]. Never in mitosis gene a (NIMA)-related protein kinase (NEK) family consists of regulators of mitosis and known as the third family of mitotic kinases [4]. As the representative of the NEK family, Nek2 is mainly involved in the regulation of G2/M check points, promoting the maturity of centrosomes and affecting chromosomal enrichment and the formation of spindle bodies [5]. Abnormality of Nek2 protein expression may indicate malignant transformation. Nek2 is over-expressed in various tumors, which causes multipolar division of centrosomes [6]. The existing studies on Nek2 mainly focus on the human tumor cell lines, prostate cancer, testicular seminoma, primary breast cancer and cholangiocarcinoma, but few of them detect the colon cancer specimens [6-9]. We applied Western Blot and immunohistochemical staining to the detect Nek2 protein expression in colon cancer, paracancerous tissues and normal colon tissues, analyzed the change of its expression in various colon tissues, and explored the relationship between Nek2 protein expression and clinicopathologic parameters and prognosis of colon cancer, providing experimental basis for the subsequent study on the mechanism of Nek2 acting in tumors.
Materials and methods
General data
Sixty specimens of colon cancer were conventionally resected from patients with colon cancer at Department of General Surgery, the Second Affiliated Hospital of Nantong University from February 2006 to February 2014. The clinicopathological data of these cases were reviewed, including gender, age, tumor size, degree of differentiation, TNM staging, lymph node metastasis and invasion (Table 1). No cases received preoperative chemotherapy and radiotherapy, and those who died from other diseases or accidents were excluded. All specimens were subjected to HE staining and diagnosed as colon cancer by two pathologists. For paracancerous specimens, 10 cm-margin of healthy looking tissues were collected from 30 cases, and for normal colon specimens, diseased colonic mucosal specimens were collected from 10 cases. All specimens were divided into two parts. One was fixed in 10% neutral formaldehyde, embedded in paraffin and sliced to 5 cm thickness. The other was cryopreserved in the fridge at -80°C. Informed consent was obtained from all cases or their relatives. The experimental protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Nantong University.
Table 1.
Expression of Nek2 in colon cancer tissues, para-carcinoma tissue and normal colon tissues
| Group | Case | Score of Nek2 expression | |||
|---|---|---|---|---|---|
|
| |||||
| - | + | ++ | +++ | ||
| NT | 30 | 22 | 6 | 2 | 0 |
| PT | 30 | 12 | 13 | 3 | 2 |
| CT | 60 | 4 | 17 | 18 | 21 |
P<0.05, NT, Normal colon tissues, PT Para-carcinoma tissue and CT colon cancer tissues.
Reagents
Nek2 mouse anti-human monoclonal antibody (Abcam, USA), HRP-conjugated goat anti-mouse IgG (H+L) (Beyotime Institute of Biotechnology, China), biotinylated goat anti-mouse IgG ELISA kit (Wuhan Boster Biological Technology Co., Ltd, China), anti-β-actin monoclonal antibody (Beyotime Institute of Biotechnology, China), horseradish peroxidase-conjugated goat anti-mouse IgG Western Blot kit (Beyotime Institute of Biotechnology, China).
Western Blot
The specimens were thawed and added with tissue lysis buffer to prepare the homogenate. After high-speed centrifugation, the supernatant was collected, mixed with loading buffer, heated, and then placed in the fridge at -20°C. The separating gel and stacking gel were prepared for electrophoresis, and the proteins separated by electrophoresis were transferred to the PVDF membrane. The membrane was sealed with defatted milk powder and incubated with Nek2 mouse anti-human monoclonal antibody at 4°C overnight. The membrane was washed and incubated with HRP-conjugated goat anti-mouse IgG. The membrane was washed again and ECL reagent was added for color development. Western Blot was repeated, and the images were scanned and analyzed by gel image analysis system. The gray scale ratio of specific proteins to the internal reference (β-actin) was calculated as a measure of the expression level.
Immunohistochemical staining
The paraffin-embedded sections were baked in the oven at 65°C for 2 h and then subjected to conventional dewaxing. The specimens were washed with distilled water three times, incubated in 3% H2O2 at room temperature for 10 min, and washed with distilled water again. Antigen recovery was performed using Tris-EDTA buffer at medium to high pressure for 3 min. After washing with PBS buffer, the specimens were incubated with Nek2 monoclonal antibody (1:100) at 4°C overnight. The specmens were washed with PBS buffer again, and the biotin-labeled working solution of secondary antibody was added dropwise to incubate the cells at room temperature for 60 min. Then the specimens were washed with PBS buffer and reacted with DAB color development solution. Hematoxylin counterstain was peformed, and the specimens were washed with distilled water, dehydrated, transparentized and sealed.
Judgment of results
For each section, 10 high-power fields (×400) were selected randomly, and 500-1000 cells were counted in each field. The scores of 0-3 were assigned according to staining intensity and the number of positive cells. The cells not stained were scored as 0, light yellow 1, pale brown 2, and dark brown 3; number of positive cells <10% was scored as 0, 10%-45% 1, 45%-70% 2, and >70% 3. The two scores were added up and the sum was divided into 4 levels: the total score of 1 was considered negative (-), the score of 2 weakly positive (+), the score of 3-4 positive (++), and the score of 5-6 strongly positive (+++).
Statistical process
Statistical analyses were performed using SPSS 19.0 software and GraphPad Prism 5, and P<0.05 indicated significant difference. The results of immunohistochemistry were analyzed with χ2 test and Spearman rank correlation test. The means between the groups were compared by variance analysis and Spearman rank correlation test. Kaplan-Meier method was used to plot the survival curves, which were compared by the log rank test.
Results
Detection of Nek2 protein expression in colon cancer, paracancerous tissues and normal colon tissues by Western Blot
Western Blot showed that the Nek2 protein expressions in colon cancer tissues (CT) (0.950±0.068) and paracancerous tissues (PT) (0.460±0.028) were obviously higher than that in normal colon tissues (NT) (0.310±0.036) (P=0.0011, P=0.00314). The Nek2 protein expression in colon cancer tisues was also significantly higher than that in the paracancerous tissues (P=0.0027) (Figure 1).
Figure 1.
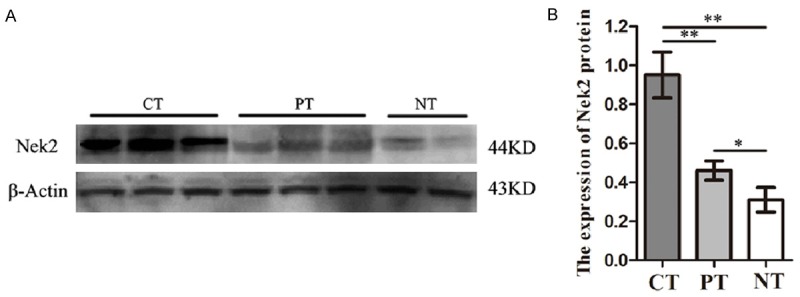
Expression of Nek2 in colon cancer tissues, para-carcinoma tissue and normal colon tissues detected by Western blot. A. Expression of Nek2 protein level in different tissues (CT Colon cancer tissues, PT Para-carcinoma tissues, NT Normal colon tissues). B. A graphical representation of the Nek2 protein level expression profiles in (b) *P<0.05 **P<0.005.
Detection of Nek2 protein expression in colon cancer, paracancerous tissues and normal colon tissues by immunohistochemical method
Immunohistochemical results (Figure 2) indicated that the positive Nek2 protein signals were mainly localized to the cytoplasm and also in the nuclei. Nek2 protein expression was mostly negative in normal colon tissues, with negative expression rate of 66.67% (22/30); weakly positive (20%, 6/30) and positive expressions (6.67%, 2/30) were also detected occasionally. The paracancerous tissues were mainly negative or weakly positive for Nek2 protein, with negative expression rate and weakly positive expression rate of 40.00% (12/30) and 43.33% (13/30), respectively; the positive expression (10%, 3/30) and strongly positive expression (6.67%, 2/30) were less frequent. In the colon cancer tissues, the overall positive expression rate was 93.33% (56/60), including strongly positive expression rate of 35% (21/60) and positive expression rate of 30% (18/60); however, the negative expression rate was only 6.67% (4/60). Statistically, Nek2 protein expression in the colon cancer tissues was much higher than that in paracancerous tissues and normal colon tissues (P<0.05, Table 1).
Figure 2.
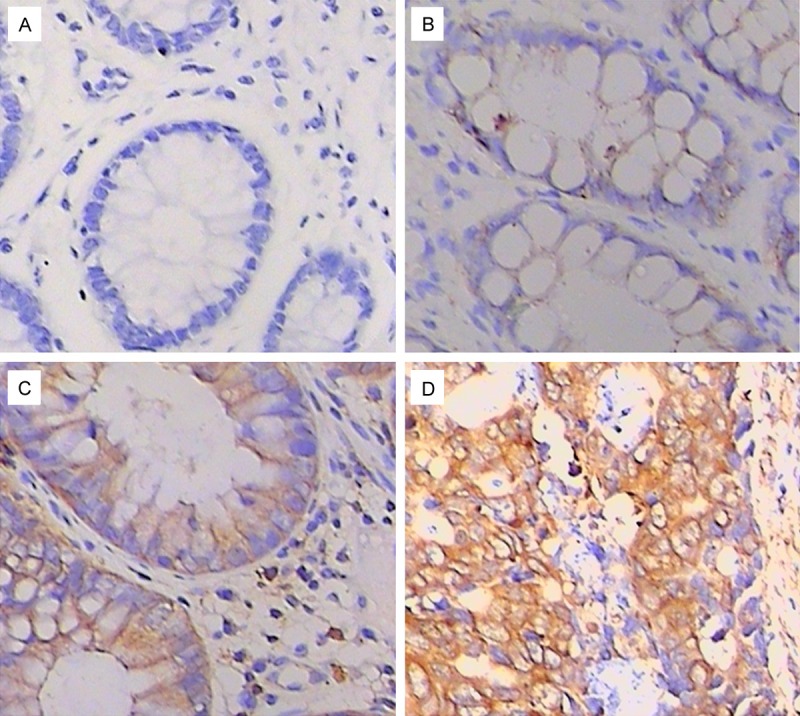
Expression of Nek2 in colon cancer tissues, para-carcinoma tissue and normal colon tissues detected by IHC (DAB × 400). A. Normal colon tissues. B. Para-carcinoma tissues. C and D. Colon cancer tissues.
Correlation between Nek2 protein expression in colon cancer tissues and clinicopathological features
The clinical data of 60 cases were reviewed. Based on Nek2 protein expression in immunohistochemical staining, the colon cancer specimens were divided into high expression group (+++, ++) and low expression group (+, -). Results showed that Nek2 protein expression was not obviously correlated with age, gender and tumor size, but with degree of differentiation (P=0.008), TNM staging (P=0.000), lymph node metastasis (P=0.022) and invasion (P=0.011) (Table 2).
Table 2.
Ralationship between Nek2 expression and clinicopathological characteristics of colon cancer
| Nek2 expression | |||||
|---|---|---|---|---|---|
|
|
|||||
| Clinical Parameters | N | Low expression (-, +) | high expression (++, +++) | Χ2 | P |
| Gender | 21 | 39 | |||
| Male | 32 | 12 | 20 | 1.841 | 0.175 |
| Female | 28 | 9 | 19 | ||
| Age | |||||
| <55 | 25 | 10 | 15 | 0.471 | 0.493 |
| ≥55 | 35 | 11 | 24 | ||
| Tumor diameter | |||||
| <5 cm | 39 | 14 | 25 | 0.039 | 0.843 |
| ≥5 cm | 21 | 7 | 14 | ||
| Differentiation | |||||
| High | 15 | 10 | 5 | 9.551 | 0.008** |
| Moderately | 19 | 6 | 13 | ||
| Low | 26 | 5 | 21 | ||
| TNM stage | |||||
| I~II | 24 | 15 | 9 | 13.297 | 0.000** |
| III~IV | 36 | 6 | 30 | ||
| Lymph node metastasis | |||||
| Yes | 20 | 11 | 9 | 5.275 | 0.022* |
| No | 40 | 10 | 30 | ||
| Invasion | |||||
| Mucosa and muscle | 22 | 15 | 7 | 6.406 | 0.011* |
| Serous | 38 | 6 | 32 | ||
P<0.05;
P<0.01.
Correlation between Nek2 protein expression and prognosis in colon cancer
Sixty cases were followed up for 2-81 months, during which 33 cases died. The average survival for cases showing high Nek2 protein expression (+++, ++) and low expression (+, -) was 66 months and 42 months, respectively. According to Kaplan-Merier survival curves, the prognosis of cases with high Nek2 protein expression was worse than cases with low expression, and the difference was of staistical significance (P=0.0048) (Figure 3). Thus high Nek2 protein expression may be an independent prognostic factor in colon cancer (HR=0.227, 95% CI 0.101 to 0.510).
Figure 3.
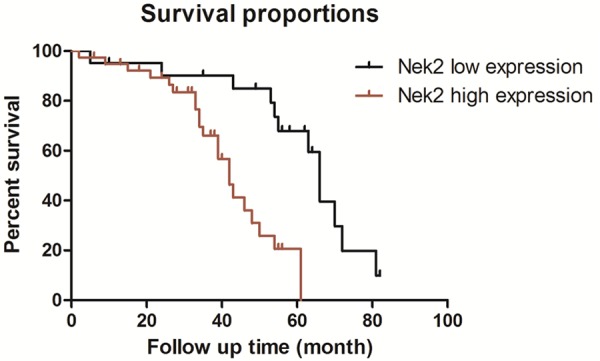
Kaplan-Meier survival curves for colon cancer patients with high or low expression of Nek2.
Discussion
Centrosomes are microtubule-organizing centers in human cells and also the centers of cell division [10]. It is found that centrosomes are closely related to tumor occurrence [11]. Damaged centrosomes affect the localization of spindle bodies and hence the cell movement. As a result, the cell cycle cannot be completed [12]. As a representative of NEK family, Nek2 is generally known as a regulator of the centrosome structure. Human Nek2 contains amino terminal of the kinase domain and carboxyl terminal of the uncatalyzed regulatory domain [13]. Nek2 over-expression may play important roles in the occurrence and development of tumors by several mechanisms: the over-expression of Nek2 and its homologues causes the aggregation of centrosomes and excessively facilitates mitotic progress, finally leading to the splitting of the chromatin. Aberrant Nek2 expression can affect the centromere protein; over-production of centrosomes resulting from Nek2 over-expression may lead to the proliferation of multinucleate cells [12,14].
Upregulation of Nek2 mRNA was first discovered in Ewing sarcoma, and later in non-Hodgkin’s lymphoma and diffuse large B-cell lymphoma [15-17]. More and more studies have confirmed that Nek2 is upregulated in breast cancer, cholangiocarcinoma and testicular seminoma [6,8,9]. The upregulation of exogenous Nek2 can facilitate the cell cycle and proliferation of tumor cells [18]. We detected Nek2 protein expression in 60 specimens of colon cancer surgically resected, 30 specimens of paracancerous tissues and 10 specimens of normal colon tissues. It was found that Nek2 protein expression in colon cancer tissues was obviously upregulated compared with the paracancerous tissues and normal colon tissues. Moreover, Nek2 protein expression differed significantly with TNM staging in colon cancer, and the expression was much higher in TNM stage III-IV than in stage I-II. The Nek2 expression also varied with the degree of differentiation. The expression in lowly differentiated colon cancer tissues was significantly higher than that in moderately and highly differentiated tissues, and the lowest expression was detected in the highly differentiated tissues. Nek2 protein showed a much higher expression in colon cancer tissues with lymph node metastasis compared with tissues without lymph node metastasis. In addition, the expression was higher for cases where the tumors had infiltrated the serosa compared with those where the tumors infiltrated the mucosa and muscles. No significant differences were found among cases differing in gender, age or tumor size. Nek2 expression was closely correlated with the depth of infiltration, TNM stage, lymph node metastasis and degree of differentiation. Therefore, Nek2 over-expression may predict facilitated progression of colon cancer, and Nek2 is considered the candidate prognostic marker and therapeutic target.
The formation of colon cancer involves multiple factors interacting over a long period of time. The progression from colonic epithelial hyperplasia to atypical hyperplasia and finally to colon cancer is mediated by the mutation of oncogenes and the inactivation of tumor suppressor genes [19]. We found that Nek2 over-expression was associated with the occurrence and metastasis of colon cancer. It was speculated that Nek2 may be the oncogene regulating the centrosomes, thereby being carcinogenic in colon cancer. However, the specific mechanism of carcinogenesis of Nek2 in colon cancer needs further study.
Acknowledgements
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
References
- 1.Van Cutsem E, Costa F. Progress in the adjuvant treatment of colon cancer: has it influenced clinical practice? JAMA. 2005;294:2758–2760. doi: 10.1001/jama.294.21.2758. [DOI] [PubMed] [Google Scholar]
- 2.Sabbagh C, Cosse C, Chauffert B, Nguyen-Khac E, Joly JP, Yzet T, Regimbeau JM. Management of colon cancer in patients with cirrhosis: A review. Surg Oncol. 2015;24:187–93. doi: 10.1016/j.suronc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Z, Gao S, Yang Z, Xie H, Zhang C, Lin B, Wu L, Zheng S, Zhou L. Single nucleotide polymorphisms in the metastasis-associated in colon cancer-1 gene predict the recurrence of hepatocellular carcinoma after transplantation. Int J Med Sci. 2014;11:142–150. doi: 10.7150/ijms.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letwin K, Mizzen L, Motro B, Ben-David Y, Bernstein A, Pawson T. A mammalian dual specificity protein kinase, Nek1, is related to the NIMA cell cycle regulator and highly expressed in meiotic germ cells. EMBO J. 1992;11:3521–3531. doi: 10.1002/j.1460-2075.1992.tb05435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 1998;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbagallo F, Paronetto MP, Franco R, Chieffi P, Dolci S, Fry AM, Geremia R, Sette C. Increased expression and nuclear localization of the centrosomal kinase Nek2 in human testicular seminomas. J Pathol. 2009;217:431–441. doi: 10.1002/path.2471. [DOI] [PubMed] [Google Scholar]
- 7.Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res. 2004;64:7370–7376. doi: 10.1158/0008-5472.CAN-04-0960. [DOI] [PubMed] [Google Scholar]
- 8.Cappello P, Blaser H, Gorrini C, Lin DC, Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, Youngson B, Done SJ, Mak TW. Role of Nek2 on centrosome duplication and aneuploidy in breast cancer cells. Oncogene. 2014;33:2375–2384. doi: 10.1038/onc.2013.183. [DOI] [PubMed] [Google Scholar]
- 9.Kokuryo T, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M. Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res. 2007;67:9637–9642. doi: 10.1158/0008-5472.CAN-07-1489. [DOI] [PubMed] [Google Scholar]
- 10.Attar N. Parasite biology: Dual-core centrosomes power cell division. Nat Rev Microbiol. 2015;13:252. doi: 10.1038/nrmicro3470. [DOI] [PubMed] [Google Scholar]
- 11.D’Assoro AB, Barrett SL, Folk C, Negron VC, Boeneman K, Busby R, Whitehead C, Stivala F, Lingle WL, Salisbury JL. Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res Treat. 2002;75:25–34. doi: 10.1023/a:1016550619925. [DOI] [PubMed] [Google Scholar]
- 12.Hayward DG, Fry AM. Nek2 kinase in chromosome instability and cancer. Cancer Lett. 2006;237:155–166. doi: 10.1016/j.canlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene. 2002;21:6184–6194. doi: 10.1038/sj.onc.1205711. [DOI] [PubMed] [Google Scholar]
- 14.Weiss MM, Kuipers EJ, Postma C, Snijders AM, Pinkel D, Meuwissen SG, Albertson D, Meijer GA. Genomic alterations in primary gastric adenocarcinomas correlate with clinicopathological characteristics and survival. Cell Oncol. 2004;26:307–317. doi: 10.1155/2004/454238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wai DH, Schaefer KL, Schramm A, Korsching E, Van Valen F, Ozaki T, Boecker W, Schweigerer L, Dockhorn-Dworniczak B, Poremba C. Expression analysis of pediatric solid tumor cell lines using oligonucleotide microarrays. Int J Oncol. 2002;20:441–451. [PubMed] [Google Scholar]
- 16.de Vos S, Hofmann WK, Grogan TM, Krug U, Schrage M, Miller TP, Braun JG, Wachsman W, Koeffler HP, Said JW. Gene expression profile of serial samples of transformed B-cell lymphomas. Lab Invest. 2003;83:271–285. doi: 10.1097/01.lab.0000053913.85892.e9. [DOI] [PubMed] [Google Scholar]
- 17.Gu Z, Zhou W, Huang J, Yang Y, Wendlandt E, Xu H, He X, Tricot G, Zhan F. Nek2 is a novel regulator of B cell development and immunological response. Biomed Res Int. 2014;2014:621082. doi: 10.1155/2014/621082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee K, Wolgemuth DJ. The NIMA-related kinase 2, Nek2, is expressed in specific stages of the meiotic cell cycle and associates with meiotic chromosomes. Development. 1997;124:2167–2177. doi: 10.1242/dev.124.11.2167. [DOI] [PubMed] [Google Scholar]
- 19.Ding YL, Zhou Y, Xiang L, Ji ZP, Luo ZH. Expression of glioma-associated oncogene homolog 1 is associated with invasion and postoperative liver metastasis in colon cancer. Int J Med Sci. 2012;9:334–338. doi: 10.7150/ijms.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]


