Abstract
A novel system for simultaneous detection of pathogenic bacteria and fungi in pathological samples was developed using a real-time polymerase chain reaction (PCR) system. This system, designated the “multi-microbial real-time PCR”, has the potential to simultaneously detect 68 bacterial and 9 fungal species in a 96-well plate format. All probe-primer sets were designed to produce amplicons smaller than 210 bp using formalin-fixed paraffin-embedded samples as input. The specificity and sensitivity of each probe-primer set were tested against DNA extracted from pure cultures of specific pathogens. The multi-microbial real-time PCR system revealed profiles of microorganism infection in lung samples collected at autopsy from 10 patients with acquired immunodeficiency syndrome. Staphylococcus aureus was the most common microbe detected (n=8), but with low copy numbers. High copy numbers of Pseudomonas aeruginosa were detected in the lung samples with abscess (n=6). Enterococcus faecium (n=6), Elizabethkingia meningoseptica (n=4), and Candida albicans (n=4) were also frequently detected. In addition, a latent infection of Mycobacterium tuberculosis was detected in one case of pneumonia. In conclusion, this multi-microbial real-time PCR system can be useful for detecting bacteria and fungi in pathological specimens from patients with uncertain diagnoses.
Keywords: Real-time PCR, bacteria, fungi, detection, pathological sample, FFPE sample
Introduction
In pathological samples, bacteria and fungi are detectable using a conventional microscope. Hematoxylin and eosin staining and special staining techniques, such as periodic acid-Schiff staining, Gram staining, silver staining, and immunohistochemistry, are useful tools for characterizing microbes; however, these stains are not sufficiently specific to identify individual species. In clinical samples, conventional culture is required to identify microbial species, a technique that is not available for formalin-fixed paraffin-embedded (FFPE) samples.
Polymerase chain reaction (PCR) is widely used to identify pathogens from clinical samples. Real-time PCR can be used to both to accurately quantify microbial DNA and to identify species of pathogen using specific primers. Several papers describe real-time PCR systems capable of identifying bacterial or fungal DNA that are the most frequent causative agents of sepsis [1] or acute gastroenteritis [2,3]. However, the number of bacterial species that can be detected in a sample is limited by the fluorescence wavelength, and some rare, but important, infectious species were not included in these systems. Microarray can be used to simultaneously identify microbial species from samples with large numbers of bacteria or fungi, but its sensitivity is much lower than real-time PCR, even if amplified DNA is used for the assay [4]. Current sequencing technologies are remarkably advanced, and next generation sequencing (NGS) can analyze highly complex populations of sequences quickly, enabling massively parallel analyses [5]. Despite its promise, however, the high cost of equipment and the necessary time investment has prevented NGS from being useful in clinical laboratories. We previously established the multi-virus real-time PCR system to detect viruses in pathological specimens from patients with uncertain diagnoses [6]. This system is able to simultaneously detect more than one hundred human pathogenic viruses using a multiplex Taqman real-time PCR system. In addition, all probe-primer sets were designed to produce amplicons of less than 210 bp from viral genomes in FFPE samples.
In present study, we established a novel system for simultaneous detection of pathogenic bacteria and fungi in pathological samples using real-time PCR. We designate this system the “multi-microbial real-time PCR” system as it is analogous in design to the multivirus real-time PCR system we previously developed. The multi-microbial real-time PCR system could simultaneously assay for 68 bacterial species and 9 fungal species, all common human pathogens, in a 96-well plate. The specificity and sensitivity of each probe-primer set was estimated and confirmed by testing against standard lab strains. Using this system, we quantified the bacteria and fungi present in FFPE samples of infectious diseases. Finally, we investigated lung specimens from 10 cadavers with acquired immunodeficiency syndrome (AIDS) to reveal the profile of microbial infection in each case.
Materials and methods
Probe and primer sets
A total of 68 bacteria and 9 fungi were chosen as targets (Table 1). The choice of bacterial strains was made based on associations with human diseases and prevalence among humans. Probe-primer sets for each target were designed using Primer Express 2.0 (Applied Biosystems, Foster City, CA; Table S1). For some species, the designs of probe-primer sets published elsewhere were employed in our system. To detect short DNA fragments extracted from FFPE samples, primers were designed so that amplicons would be less than 210 bp. Probes and primers were synthesized by Sigma Genosys (Sigma-Aldrich, St. Louis, MO). All probes were labeled with 6-carboxy fluoresce in (FAM) and 6-carboxytetramethylrhodamine (TAMRA). The sensitivity of each probe-primer set was confirmed by detection of at least 10 copies of a positive control PCR amplicon using conventional TaqMan real-time PCR (Applied Biosystems).
Table 1.
List of Target Bacteria and Fungi
| Bacteria |
|
|
| Firmicutes |
| Bacillales: Staphylococcus aureus, Bacillus anthracis, Listeria monocytogenes. |
| Lactobacilales: Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus mutans, Streptococcus sobrinus, Streptococcus sanguinis, Streptococcus oralis, Streptococcus salivaris, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium. |
| Clostridiales: Clostridium tetani, Clostridium difficile, Peptostreptococcus anaerobius. |
| Actinobacteria |
| Actinomyces, Propionibacterium acnes. |
| Corynebacteriales: Corynebacterium diphteriae, Mycobacterium tuberculosis, Mycobacterium laprae, Mycobacterium chelonae, Mycobacterium kansasii, Mycobacterium avium complex, Nocardia asteroides. |
| Bacteroides |
| Bacteroides fragilis, Elizabethkingia meningoseptica. |
| Proteobacteria |
| Francisella tularensis, Stenotrophomonas maltophilia, Legionella pneumophila, Aeromonas hydrophila, Haemophilus influenzae. |
| Campylobacteriales: Campylobacter jejuni, Helicobacter cinaedi, Helicobacter pylori. |
| Rickettsiales: Rickettsia prowazekii, Rickettsia japonica, Orientia tsutsugamushi. |
| Rhizobiales: Bartonella henselae, Brucella. |
| Bukholderiales: Bordetella pertussis, Burkhoderia mallei, Burkhoderia cepacia. |
| Neisseriales: Neisseria gonorrhoeae, Neisseria meningitidis. |
| Pseudomonadales: Moraxella catarrhalis, Pseudomonas aeruginosa, Acinetobacter baumannii. |
| Vibrionales: Vibrio cholerae, Vibrio parahaemolyticus, Vibrio vulnificus. |
| Enterobacteriales: Escherichia coli, Salmonella enterica, Shigella, Klebsiella pneumonia, Yersinia pestis, Yersinia enterocolitica, Citrobacterfreundii, Proteus mirabilis, Morganella morganii, Providencia. |
| Tenericutes |
| Mycoplasma pneumoniae. |
| Fusobacteria |
| Fusobacterium nucleatum. |
| Spirochaetes |
| Leptospira interrogans, Treponema pallidum. |
| Chlamydiae |
| Chlamydia psittaci, Chlamydia trachomatis, Chlamydia pneumoniae. |
|
|
| Fungi |
|
|
| Aspergillus fumigatus, Aspergillus nigar, Aspergillus flavus, Cryptococcus, Candida albicans, Histoplasma, Trichosporon, Mucor, Coccidioides. |
Establishment of the multi-microbialreal-time PCR system
A TaqMan real-time PCR protocol was designed to detect specific bacterial and fungal species in a 96-well plate format. A Quantitect Probe PCR kit (Qiagen, Hilden, Germany), MicroAmp Optical 96-Well Reaction Plates (Applied Biosystems), and MicroAmp Optical Adhesive Film (Applied Biosystems) were used as 2× master mix, 96-well plates, and adhesive film, respectively. Each well contained a probe-primer set (Table S1), and each 96-well plate contained each probe-primer set for simultaneous detection of the 68 bacteria and 9 fungi listed in Table 1. To estimate microbial quantities, nine wells (A1-A9) from each plate contained a mixture of the glutathione S-transferase gene probe-primer set with dilutions of control plasmids (101-108 copies) to generate a standard curve [6]. To enable routine use of the system, 10 µl/well of 2× probe-primer mix was stored in 96-well plates at -20°C. DNA samples (50 ng per well) were added to the 2× master mix, and 10 µl were added to each well of a reaction plate and mixed with the 2× probe-primer mix. Real-time PCR was performed in an ABI PRISM 7900HT (Applied Biosystems), an Mx3005P (Stratagene, La Jolla, CA), or a 7500 real-time PCR system (Applied Biosystems). The PCR conditions were 95°C for 15 min, followed by 40 cycles of 94°C for 15 sec and 60°C for 1 min. Microbial quantities were calculated based on the standard curves generated from the plasmids in wells A1-A9.
Gene expression image
A gene expression image was produced with TreeView and Cluster software by Michael Eisen, University of California at Berkeley [7].
Positive control DNA samples
The control DNA samples were provided by the DNA Bank of the Riken Bio Resource Center (Tsukuba, Ibaraki, Japan) or purchased from the American Type Culture Collection (ATCC, Manassas, VA). Some control DNAs were also kindly provided by researchers in the National Institute of Infectious Diseases (Table S2).
Clinical samples
The study protocol was approved by the Institutional Review Board, National Institute of Infectious Diseases, Japan (Approval No. 569). Pathological samples were collected from anonymized samples stored in our department. Lung tissues were taken at autopsy from 10 patients with AIDS. All lung tissue samples were immediately frozen and stored at -80°C. Nine patients were male and one was female. The mean age of the patients was 41.8 years (range: 26-62 years). No patients had received highly active anti-retroviral therapy. The CD4 count of each patient was below 4 counts/µl.
DNA extraction
Frozen samples were homogenized with a Multi-Beads Shocker (Yasui Kikai, Tokyo, Japan) in TEN buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, pH 8.0, 100 mM NaCl) with 100 ng/ml proteinase K and 0.1% sodium dodecyl sulfate. DNA was extracted from the homogenized tissues using the phenol-chloroform method. A QIAamp DNA FFPE tissue kit (Qiagen) was used for FFPE samples.
Histology and immunohistochemistry
In FFPE sections, bacteria/fungi were characterized by their morphology in hematoxylin-eosin stain, periodic acid-Schiff stain, acid-fast stain, Grocott’s methenamine silver stain, and/or by immunohistochemistry. Immunohistochemistry was performed using primary antibodies specific to the bacteria/fungi listed in Table 2.
Table 2.
Detection of bacteria and fungi in FFPE samples
| Sample No. | Human/mouse | Organ | Autopsy/biopsy | Confirmed microbe | Copy number in MMRP | Copy number by single real-time PCR | Other microbes detected in MMRP (copy No.) | Methods of confirmation (antibody) |
|---|---|---|---|---|---|---|---|---|
| 1 | Clinical sample | Lymph node | Autopsy | Cryptococcus | 2,918 | 5,404 | E.meningoseptica (52), P.acnes (52), S.maltophilia (15), Providencia (15), S.aureus (12) | IHC (novocastra: NCL-CN) |
| 2 | Clinical sample | Paranasal sinus | Biopsy | A.fumigatus | 1,732 | 2,329 | P.acnes (58), E.meningoseptica (26), S.maltophilia (25), S.aureus (15) | IHC (LSBio: LS-C121391) |
| 3 | Clinical sample | Skin | Autopsy | M.tuberculosis | 37 | 17 | E.meningoseptica (42), S.maltophilia (20), H.cinaedi (18), P.acnes (14) | IHC (LSBio: LS-C72963) |
| 4 | Clinical sample | Heart | Autopsy | L.monocytogenes | 1,997 | 699 | S.aureus (30), P.acnes (30), E.meningoseptica (20) | IHC (In-house) |
| 5 | Clinical sample | Pharynx | Biopsy | T.pallidum | 3,888 | 481,841 | See Table S2 | IHC (Abcam: ab20923) |
| 6 | Clinical sample | Tongue | Autopsy | P.acnes | 65 | 193 | S.maltophilia (16) | Gram |
| 7 | Clinical sample | Nasal mucosa | Autopsy | C.albicans | 22 | 75 | E.meningoseptica (60), S.aureus (34), P.acnes (14), S.maltophilia (12) | PAS, Grocott |
| 8 | Mouse model | Liver | - | S.pneumoniae | 214 | 1,474 | See Table S2 | Injected with isolated microbe |
| 9 | Mouse model | Liver | - | C.tetani | 92 | 1,329 | See Table S2 | Injected with isolated microbe |
| 10 | Mouse model | Liver | - | C.difficile | 286 | 118 | C.tetani (1446), Trichosporon (88), E.meningoseptica (84), P.acnes (47), R.japonica (35), B.pertussis (24), P.anaerobius (18), B.cepacia (18), S.maltophilia (16), C.freudii (14), C.albicans (12) | Injected with isolated microbe |
| 11 | Mouse model | Liver | - | H.cinaedi | 827 | 216 | See Table S2 | Injected with isolated microbe |
| 12 | Mouse model | Liver | - | M.pneumoniae | 144,418 | 592,381 | See Table S2 | Injected with isolated microbe |
| 13 | Mouse model | Liver | - | Y.pestis | 315,300 | 238,439 | E.meningoseptica (34), M.leprae (29), P.acnes (20) | Injected with isolated microbe |
IHC: immunohistochemistry, PAS: Periodic acid-Schiff stain, MMRP: multi-microbial real-time PCR; A.fumigatus: Aspergillus fumigatus, B.cepacia: Burkhoderia cepacia, B.pertussis: Bordetella pertussis, C.albicans: Candida albicans, C.freundii: Citrobacter freundii, C.tetani: Clostridium tetani, E.meningoseptica: Elizabethkingia meningoseptica, H.cinaedi: Helicobacter cinaedi, L.monocytogenes: Listeria monocytogenes, M.leprae: Mycobacterium leprae, M.tuberculosis: Mycobacterium tuberculosis, P.anaerobius: Peptostreptococcus anaerobius, P.acnes: Propionibacterium acnes, R.japonica: Rickettsia japonica, S.aureus: Staphylococcus aureus, S.maltophilia: Stenotrophomonas maltophilia, Y.pestis: Yersinia pestis.
Results
Establishment and validation of multi-microbial real-time PCR
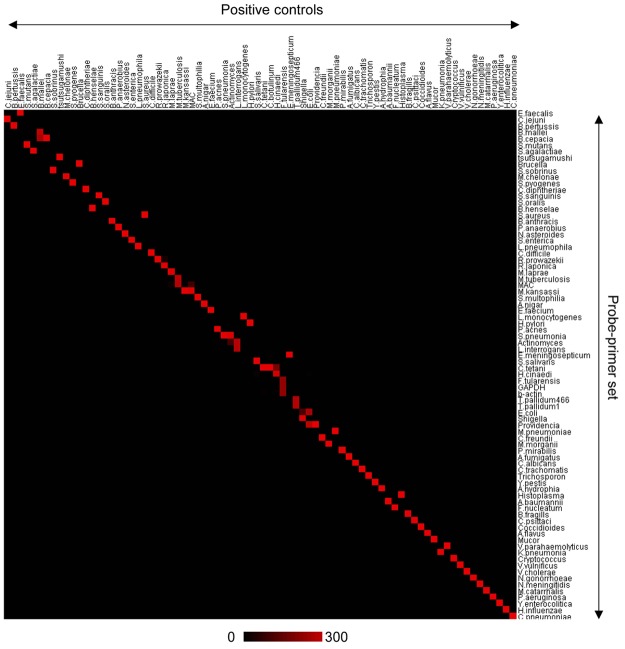
To validate the sensitivity and specificity of each probe and primer set used in the multi-microbial real-time PCR system, we examined DNA samples extracted from control bacteria and fungi (Figure 1 and Table S2). Each probe and primer set amplified a gene fragment from the specific target microbe. The specificity of each probe-primer set was calculated by dividing the copy number of the target microbe in the positive control by the sum of all control samples. With only 9 exceptions, the probe-primer sets demonstrated more than 95% specificity. Of the probe-primer sets with low specificity, some had amplified DNA from closely related bacterial families. For example, the probe-primer set for Mycobacterium avium complex (MAC) amplified gene fragments from nocardia asteroids and some members of the Mycobacteriaceae family, including Mycobacterium tuberculosis, Mycobacterium leprae, Mycobacterium kansasii, and Mycobacterium avium, but not Mycobacterium chelonae. A probe-primer set for Mycobacterium kansasii also reacted with MAC. The probe-primer set for Neisseria gonorrhoeae also amplified a gene fragment of Neisseria meningitidis. The probe-primer set for Providencia had low specificity (21%) because of cross-reactions with the enterobacteriae family, including Escherichia coli, Salmonella enterica, Proteus mirabilis, and Morganella morganii; however, these probe-primer sets failed to detect Providencia spp. Yersinia pestis, Yersinia enterocolitica, and Citrobacter freundii were not amplified with the probe-primer set for Providencia, although they are members of the Enterobacteriae family. The probe-primer set for Clostridium tetani cross-reacted with Helicobacter cinaedi. The set for Rickettsia prowazekii cross-reacted with Rickettsia japonica. The set for Burkholderia cepacia cross-reacted with Burkholderia mallei, although the set for B.mallei did not react with B.cepacia. Similarly, the set for E.coli cross-reacted with Shigellaspp, although a set for Shigella spp. did not react with E.coli. All other probe-primer sets demonstrated high specificity (>95%), and no, or very low cross-reaction with off-target bacteria/fungi controls. Some FFPE samples from a mouse model of bacterial infection were positive for bacteria other than the target bacteria (Table S2). The multi-microbial real-time PCR system also detected human internal control genes, such as glyceraldehyde-3-phosphate dehydrogenase and beta-actin (Tables S1 and S2), which were tested to determine if there was an inhibitory effect in FFPE samples on the PCR.
Figure 1.
Validation of the multi-microbial real-time PCR assay. A gene expression image produced using TreeView software based on the results of the multi-microbial real-time PCR for control samples is shown. A horizontal line shows each probe-primer set and a vertical line is one sample of the positive control. A scale bar indicates copy number of color. Details of positive controls are provided in Table S2.
Detection of bacteria and fungi in FFPE samples
To evaluate if the multi-microbial real-time PCR method is able to detect microbes in pathological samples, FFPE samples from patients with infectious diseases were tested (Table 2; Figure 2). The multi-microbial real-time PCR detected at least 7 bacteria/fungi from the FFPE samples, including Cryptococcussp, Aspergillussp, M.tuberculosis, Listeria monocytogenes, and Treponema pallidum. The presence of these microbes in the FFPE samples was confirmed by immunohistochemistry or additional methods (Figure 2). The copy number of each microbe was also determined by a single, real-time PCR specific to each microbe. Copy numbers calculated using our multi-microbial real-time PCR were comparable to those determined by the individual real-time PCR (Table 2). In addition, six microbes were detected in FFPE samples from mouse models (Table 2, Table S2). Together, the multi-microbial real-time PCR successfully detected DNA from at least 13 microbial species in FFPE samples.
Figure 2.
Immunohistochemical and pathological detection of bacteria and fungi in formalin-fixed paraffin-embedded (FFPE) samples. The presence of microbes in FFPE samples identified as containing Cryptococcus sp (A and B), Mycobacterium tuberculosis (C and D), Aspergillus (E and F), Listeria (G and H), and Treponema pallidum (I and J) were confirmed by immunohistochemistry using specific antibodies to each microbe. Propionibacterium acnes (K and L) was confirmed by Gram stain.
Detection of bacteria and fungi in the lungs of AIDS patient autopsy samples
Using the multi-microbial real-time PCR, we investigated the presence of bacteria and fungi in 10 lung samples from autopsies of AIDS patients. The multi-microbial real-time PCR detected 17 bacterial DNA and one fungal DNA in 9 cases (Table 3). The multi-microbial real-time PCR also revealed copy numbers of each bacterium. The most commonly detected species was Staphylococcus aureus (n=8), but copy numbers were low in all cases. All samples positive for Pseudomonas aeruginosa (n=6) showed high bacterial copy numbers. Enterococcus faecalis (n=6), Elizabethkingia meningoseptica (n=4) and Candida albicans (n=4) were occasionally detected. In some cases, the microbes detected in the samples correlated with the patients’ clinical diagnoses. For example, a high copy number of MAC was detected in a clinical case of atypical mycobacterial disease (Case No. 5). In Case No. 9, P.aeruginosa and E.faecalis were detected in lung abscesses by our system, while P.aeruginosa had been cultivated from patient sputum culture. Case No. 6 was from a patient with sepsis, the cause of which had been unclear in his lifetime; however, our system detected a high copy number of S.aureus from lung samples. E.coli, K.pneumoniae, and P.aeruginosa were detected in case No. 7, a patient whose lung showed organized pneumonia.
Table 3.
Detection of bacteria/fungi in the lung of AIDS autopsies
| Case No. | Clinical diagnosis | Pathological diagnosis | Detected bacteria by multibacteria real-time PCR (Copy number) |
|---|---|---|---|
| 1 | Toxoplasma encephalitis, PCP | Blonchial pneumonia, Adrenal CMV infection, Epididymis small abscess | P.aeruginosa (4476), S.aureus (171), C.albicans (66), F.nucleatum (56), S.maltophilia (26) |
| 2 | PCP, SLE | PCP, CMV pneumonia, SLE | S.aureus (44), S.maltophilia (41), P.acnes (20), E.faecium (14) |
| 3 | No Data | No Data | P.aeruginosa (20406), E.faecalis (174), E.meningoseptica (55), C.albicans (42), S.maltophilia (23) |
| 4 | CMV infection, Pulmonary edema, DIC | Systemic CMV infection | Not detected |
| 5 | CMV retinaitis, CMV colitis, Atypical mycobacterial disease, Chronic blonchial pneumonia, Kidney disfarction, Liver mass | Atypical mycobacterial disease, ML, CMV infection | MAC (18721), P.aeruginosa (6746), M.kansasii (4468), S.aureus (167), S.maltophilia (97), Providencia (42), E.meningoseptica (33), M.morganii (32), C.albicans (30), P.acnes (22), E.faecalis (10) |
| 6 | ML, Cryptococcus meningitis, CMV retinaitis, Aspiration pneumonitis | ML, Cryptococcus meningitis, CMV infection, sepsis | S.aureus (38515), E.faecalis (70) |
| 7 | PML, Aspiration pneumonitis, Hemophilia A, Oral candidiasis, Chronic hepatitis C | PML, Organized pneumonia | E.coli (7217224), Providencia (95254), K.pneumoniae (87912), P.aeruginosa (5784), E.faecalis (352), S.aureus (36), E.meningoseptica (31) |
| 8 | PCP, CMV pneumonia | Severe pneumonia (PCP and CMV pneumonia) | M.tuberculosis (125), E.meningosepticum (36), S.aureus (21) |
| 9 | Kidney disfunction induced drug, ML, Lung abscess (P.aeruginosa), KS, CMV infection, Amoeba liver abscess, adrenal disfanction | ML, CMV infection, Lung abscess, KS | P.aeruginosa (5891), E.faecalis (3423), S.aureus (45), B.fragills (26) |
| 10 | No Data | No Data | P.aeruginosa (727389), Providencia (65430), E.faecalis (4093), S.oralis (749), E.faecium (734), C.albicans (202), P.acnes (70), S.maltophilia (52), S.aureus (20) |
CMV: cytomegalovirus, KS: Kaposi’s sarcoma, ML: malignant lymphoma, PCP: Pneumocystis pneumonia, PML: progressive multifocal leukoencephalopathy, SLE: systemic lupus erythematosus; B.fragills: Bacteroides fragills, C.albicans: Candida albicans, E.coli: Escherichia coli, E.faecalis: Enterococcus faecalis E.faecium: Enterococcus faecium, E.meningoseptica: Elizabethkingia meningoseptica, F.nucleatum: Fusobacterium nucleatum, K.pneumoniae: Klebsiella pneumoniae, M.kansasii: Mycobacterium kansasii, M.morganii: Morganella morganii, M.tuberculosis: Mycobacterium tuberculosis, MAC: Mycobacterium avium complex, P.acnes: Propionibacterium acnes, P.aeruginosa: Pseudomonas aeruginosa, S.aureus: Staphylococcus aureus, S.maltophilia: Stenotrophomonas maltophilia, S.oralis: Streptococcus oralis.
Discussion
In the present study, we developed a new real-time PCR system, designated the “multi-microbial real-time PCR”, with the potential to detect 68 bacterial and 9 fungal species simultaneously, even in FFPE samples. This system detected human pathogenic bacteria and fungi in the lungs of diseased AIDS patients who had not received any anti-retroviral therapy. The sensitivity and specificity of the multi-microbial real-time PCR system are equivalent to those of standard real-time PCR systems. Moreover, once the system is established, it follows simple protocols and is completed rapidly, requiring only 2 hours obtaining results. The system is flexible, in that new probe-primer sets can be incorporated; thus, methods for detecting new species of microbe can be established quickly.
To detect DNA sequences in FFPE samples, PCR amplicons should be less than 300 bp, because DNA is usually fragmented by formalin fixation [8,9]. Many probe-primer sets were designed specifically for this study, because there are few reports describing real-time PCR for detection of bacterial/fungal genomes in FFPE samples. The 16S rRNA gene is encoded by genomes of almost all bacteria, and variable regions in this gene have been identified. Thus, while there are some known shortcomings, using the 16S rRNA sequence is a promising strategy to identify bacterial pathogens [10]. The 23S rRNA gene has more sequence variations between bacterial species than the 16S [11]. More recently, the locus between the 16S and 23S regions, the so-called internal transcribed region, has been targeted, because it contains greater variability than either 16S or 23S rRNA, allowing even better discrimination of bacterial species [12]. In the present study, we designed probe-primer sets targeting mainly 16S rRNA, 23S rRNA, and the internal transcribed regions. High specificities were achieved in the cross-reaction analysis, with few exceptions. Generally, to categorize a bacterial strain, it is necessary to amplify and sequence more than 1 kbp of the 16S rRNA region [10]. The use of amplicons shorter than 210 bp may be the main reason for the cross-reactions observed in our system. High copy numbers in target bacteria will assist identification of microbes in cases where cross-reaction is suspected. Although there is some room to improve the specificity of the probe-primer sets for reducing the cross-reactivities, the system as currently designed will function as a system for preliminary screening.
Systems for simultaneous molecular detection of bacteria and fungi DNA in blood samples have been previously reported [13-15]. Among them, Septifast® is a commercialized detection system that can detect 25 pathogens in clinical samples using real-time PCR [16]. Septifast® is useful for screening blood samples for bacterial and fungal infections [17-20]. However, there is no report describing the ability of Septifast® to detect bacterial DNA in FFPE samples. Our multi-microbial real-time PCR can detect a greater number of bacterial/fungal species than Septifast®, and can detect DNA from pathogens even in FFPE samples in which DNA had degraded into fragments of less than 300 bp. This provides a large advantage in screening for pathogens in pathological samples. Generally, FFPE samples at autopsy were fixed in formalin for a longer time than FFPE biopsy samples, suggesting a greater degradation of DNA. Our system identified microbial DNA not only in biopsy FFPE samples, but also in autopsy FFPE samples. Our system provides a useful tool for safe detection of pathogens in FFPE samples, even after long periods of storage. In addition to FFPE samples, blood and other fluid samples, such as cerebral spinal fluid, pharyngeal swab, throat washing, and pleural and peritoneal effusions may be also suitable for our system. Various types of samples will be tested by our system to confirm its suitability in the future.
Using the multi-microbial real-time PCR, we detected 17 bacterial and one fungal species from 10 lung samples from autopsies of AIDS patients. Basically, a pathogen detected with a high copy number in Septifast® system is correlated with the severity of sepsis [21]. On the other hand, when small quantities of bacteria are detected, it is difficult to determine whether they are associated with disease as a pathogen or a colonization event. A series of studies using bacterial DNA sequencing approaches have shown that healthy lungs harbor a unique steady-state microbiota, which is very different from other organs, such as the skin and gut [22]. Slight alterations to the microbiota in the lung could underlie susceptibility to lung disease. Additionally, the detection of bacteria may be complicated by previous treatments with antibiotics. Thus, when bacteria are detected in low copy numbers, we need to consider the whole picture, including the copy numbers of other bacteria, the patient’s history of antibiotics usage, and the condition of host’s immunity.
We have established a multi-microbial real-time PCR system with the potential to detect 68 bacteria and 9 fungi simultaneously from FFPE samples and preserved frozen samples from cadavers. Further research is required to confirm the utility of the system to probe additional patient samples, such as blood and other fluids. This system may provide a powerful tool for screening clinical samples for the presence of bacteria and fungi, particularly pathological samples, including FFPE samples from patients with uncertain diagnoses. In addition, this system is inherently flexible, allowing for addition of additional species of microbe on an as-needed basis. Use of this method in combination with a previously developed multivirus real-time PCR system will be considered for generation of a real-time PCR system detecting almost all human pathogens, which will compensate for some of the disadvantages of next generation sequencing-based assays.
Acknowledgements
The authors thank Drs. Michio Suzuki, Koichi Imaoka, Shuji Ando, Hideaki Ohno, Masanori Kai, Tetsu Mukai, Masatomo Morita, Jiro Mitobe, Hidenobu Senpuku, Hideyuki Takahashi, Noriyo Nagata, Kazunari Kamachi, Keigo Shibayama, Atsuko Horino, Makoto Ohnishi, and Haruo Watanabe of the National Institute of Infectious Diseases for providing DNA extracted from bacteria/fungi for use as controls. This work was partly supported by Health and Labor Sciences Research Grants (no. H24-AIDS-I-003, H25-Shinko-Ippan 015 and H25-Shinko-Shitei 006 to HK) from the Ministry of Health, Labor and Welfare, Research Program on HIV/AIDS Grants, Japan Agency for Medical Research and Development, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 24659212 to HK).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Dark P, Blackwood B, Gates S, McAuley D, Perkins GD, McMullan R, Wilson C, Graham D, Timms K, Warhurst G. Accuracy of LightCycler(®) SeptiFast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review and meta-analysis. Intensive Care Med. 2015;41:21–33. doi: 10.1007/s00134-014-3553-8. [DOI] [PubMed] [Google Scholar]
- 2.Onori M, Coltella L, Mancinelli L, Argentieri M, Menichella D, Villani A, Grandin A, Valentini D, Raponi M, Russo C. Evaluation of a multiplex PCR assay for simultaneous detection of bacterial and viral enteropathogens in stool samples of paediatric patients. Diagn Microbiol Infect Dis. 2014;79:149–154. doi: 10.1016/j.diagmicrobio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Van Lint P, De Witte E, De Henau H, De Muynck A, Verstraeten L, Van Herendael B, Weekx S. Evaluation of a real-time multiplex PCR for the simultaneous detection of Campylobacter jejuni, Salmonella spp. , Shigella spp./EIEC, and Yersinia enterocolitica in fecal samples. Eur J Clin Microbiol Infect Dis. 2015;34:535–542. doi: 10.1007/s10096-014-2257-x. [DOI] [PubMed] [Google Scholar]
- 4.Lee DY, Shannon K, Beaudette LA. Detection of bacterial pathogens in municipal wastewater using an oligonucleotide microarray and real-time quantitative PCR. J Microbiol Methods. 2006;65:453–467. doi: 10.1016/j.mimet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Bertelli C, Greub G. Rapid bacterial genome sequencing: methods and applications in clinical microbiology. Clin Microbiol Infect. 2013;19:803–813. doi: 10.1111/1469-0691.12217. [DOI] [PubMed] [Google Scholar]
- 6.Katano H, Kano M, Nakamura T, Kanno T, Asanuma H, Sata T. A novel real-time PCR system for simultaneous detection of human viruses in clinical samples from patients with uncertain diagnoses. J Med Virol. 2011;83:322–330. doi: 10.1002/jmv.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich D, Uhl B, Sailer V, Holmes EE, Jung M, Meller S, Kristiansen G. Improved PCR performance using template DNA from formalinfixed and paraffin-embedded tissues by overcoming PCR inhibition. PLoS One. 2013;8:e77771. doi: 10.1371/journal.pone.0077771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 10.Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol. 2007;45:2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rantakokko-Jalava K, Nikkari S, Jalava J, Eerola E, Skurnik M, Meurman O, Ruuskanen O, Alanen A, Kotilainen E, Toivanen P, Kotilainen P. Direct amplification of rRNA genes in diagnosis of bacterial infections. J Clin Microbiol. 2000;38:32–39. doi: 10.1128/jcm.38.1.32-39.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann LE, Hunfeld KP, Emrich T, Haberhausen G, Wissing H, Hoeft A, Stüber F. A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol. 2008;197:313–324. doi: 10.1007/s00430-007-0063-0. [DOI] [PubMed] [Google Scholar]
- 13.Altun O, Almuhayawi M, Ullberg M, Ozenci V. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol. 2013;51:4130–4136. doi: 10.1128/JCM.01835-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellinghausen N, Kochem AJ, Disque C, Muhl H, Gebert S, Winter J, Matten J, Sakka SG. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J Clin Microbiol. 2009;47:2759–2765. doi: 10.1128/JCM.00567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liesenfeld O, Lehman L, Hunfeld KP, Kost G. Molecular diagnosis of sepsis: New aspects and recent developments. Eur J Microbiol Immunol (Bp) 2014;4:1–25. doi: 10.1556/EuJMI.4.2014.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang SS, Hsieh WH, Liu TS, Lee SH, Wang CH, Chou HC, Yeo YH, Tseng CP, Lee CC. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis - a systemic review and meta-analysis. PLoS One. 2013;8:e62323. doi: 10.1371/journal.pone.0062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosiewski T, Flis A, Sroka A, Kedzierska A, Pietrzyk A, Kedzierska J, Drwila R, Bulanda M. Comparison of nested, multiplex, qPCR; FISH; SeptiFast and blood culture methods in detection and identification of bacteria and fungi in blood of patients with sepsis. BMC Microbiol. 2014;14:313. doi: 10.1186/s12866-014-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdino E, Ruggiero T, Allice T, Milia MG, Gregori G, Milano R, Cerutti F, De Rosa FG, Manno E, Caramello P, Di Perri G, Ghisetti V. Combination of conventional blood cultures and the SeptiFast molecular test in patients with suspected sepsis for the identification of bloodstream pathogens. Diagn Microbiol Infect Dis. 2014;79:287–292. doi: 10.1016/j.diagmicrobio.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Herne V, Nelovkov A, Kutt M, Ivanova M. Diagnostic performance and therapeutic impact of LightCycler SeptiFast assay in patients with suspected sepsis. Eur J Microbiol Immunol (Bp) 2013;3:68–76. doi: 10.1556/EuJMI.3.2013.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dierkes C, Ehrenstein B, Siebig S, Linde HJ, Reischl U, Salzberger B. Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis. BMC Infect Dis. 2009;9:126. doi: 10.1186/1471-2334-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler I, Josefson P, Olcen P, Molling P, Stralin K. Quantitative data from the SeptiFast real-time PCR is associated with disease severity in patients with sepsis. BMC Infect Dis. 2014;14:155. doi: 10.1186/1471-2334-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsland BJ, Gollwitzer ES. Hostmicroorganism interactions in lung diseases. Nat Rev Immunol. 2014;14:827–835. doi: 10.1038/nri3769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.