Abstract
WISP1, a Wnt-induced secreted protein, has been found to have anticancer activity. ALL is a leading cause of death. Here we investigate the WISP1 effects on ALL Jurkat cells. Cell viability was assessed by CCK-8. Cell cycle and apoptosis were detected by flow cytometry. Mitochondrial membrane potential (MMP) was monitored using TMRM. Generation of reactive oxygen species (ROS) was quantified using DCFH-DA. Western blot was used to detect the expression of cell proliferation and apoptosis related genes. The results showed that knockdown of WISP1 significantly inhibited proliferation of Jurkat cells. Parallelly, cell cycle distribution was increased at G1 phase and apoptotic rate was induced after WISP1 knockdown. Furthermore, knockdown of WISP1 induced apoptosis of Jurkat cells was also associated with loss of MMP and generation of ROS. Western blot results showed that the protein expression p-AKT, PCNA, CDK1, P-ERK, CDK2, VEGF, VEGFR2 and Bcl2 were decreased, while the expression of Bax was up-regulated. In conclusion, WISP1 plays an important role in proliferation and apoptosis of Jurkat cells in mitochondria dependent pathway, the specific mechanisms need further study.
Keywords: ALL, WISP1, proliferation, apoptosis, MMP, ROS
Introduction
Leukemia is a hematopoietic malignancy that results from the clonal proliferation of bone marrow cells with impaired differentiation, regulation, and cell death. There are four main types of leukemia: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL) and chronic myeloid leukemia (CML) [1]. The acute leukemia arise from neoplastic transformation of hematopoietic stem cells or progenitors with aberrant differentiation and proliferation [2]. These cells accumulate in the bone marrow and cause suppression of the growth and differentiation of normal blood cells. ALL is a malignant disorder of lymphoid progenitor cells that originated from B- and T-lymphoid cells, affects both children and adults [3-5]. At present, the therapy methods may involve chemotherapy, radiation therapy, targeted therapy and bone marrow transplant. The ALL survival rates vary by the age that 85% in children and 50% in adults [6]. Thus, it is important to search for additional approaches to treat ALL.
CCN family is defined by the first three members of the family including cysteine-rich protein 61/CCN1, Connective tissue growth factor/CCN2 and nephroblastoma over-expressed gene/CCN3 [7,8]. The CCN family currently are six members also include WISP1/CCN4, WISP2/CCN5 and WISP3/CCN6 [9]. The CCN proteins have been implicated in the pathogenesis of diverse diseases involving skeletal development, wound healing, fibrosis, and cancer [10]. WISP1 (Wnt induced secreted protein 1) is a matricellular protein that allocated to the CCN family, and mainly expressed during organ development and under diseased conditions [11]. WISP1 could regulate survival, proliferation, and migration of diverse cell types and thus play a role in wound repair, angiogenesis, and tumorigenesis [12-14]. WISP1 has been found in a variety of cancers. In prostate cancer, the WISP1 investigated as a potential target for inhibiting growth and spread to bone [15]. Induction of WISP1expression also correlates invasive with breast cancer oncogenesis [16]. Therefore WISP1 could serve as potential therapeutic targets for drug development against tumors. However, the role of WISP1 in ALL has not been explored. Thus, in this research, we determined the anti-cancer effects of knockdown WISP1in ALL Jurkat cell line.
Materials and methods
Cell culture, transfection and treatment
Human leukemia cells HL-90, U937, K562, Jurkat, Raj and Molt4 were purchased from American Type Culture Collection (ATCC) and cultured in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin solution in a 37°C atmosphere of 5% CO2. Cells were transfected with negative control siRNA or siRNA-WISP1 that knockdown of WISP1 were performed with Lipofectamine (Invitrogen, CA, USA). The transfected cells were then used in the follow assays.
Quantitative reverse transcription (qRT)-PCR
Total RNA extracted with Trizol reagent (Invitrogen, CA, USA) and was reverse transcribed to cDNA using reverse transcriptase (Thermo, USA). A SYBR Green PCR kit (Thermo, USA) was used for the amplification. The primers of WISP1 and GAPDH were as follow: WISP1: F 5’ GAAGCAGTCAGCCCTTATG 3’, R 5’ CTTGGGTGTAGTCCAGAAC 3’; GAPDH: F 5’ CACCCACTCCTCCACCTTTG 3’, R 5’ CCACCACCCTGTTGCTGTAG 3’. The analysis of all samples were carried out followed the instructions.
Cell viability assay (CCK-8)
Proliferation assays were performed using the CCK-8 kit (Dojindo, Gaithersburg, MD, USA). Cells were seeded in 96-well plates at 1×103 cells/well, and incubated for 24, 48 and 72 hours, respectively. 10 μl CCK-8 reagent was added in each well, and then plates were incubated at 37°C for 1 h before measuring the absorbance at 450 nm using a microplate reader. Experiments were repeated at least three times.
Cell cycle assay
For the cell cycle analysis, 5×105 of cells were harvested after transfection at the indicated time. Cells were fixed with 75% (v/v) cold ethanol, washed twice with PBS, and then staining with 50 μg/ml propidium iodide (PI) with 0.1% RNase A for 30 min. The cells’ DNA content were performed with a a FACScan flow cytometer (FACSCalibur, BD Biosciences).
Flow cytometric analysis of apoptosis and mitochondria membrane potential (MMP)
After transfection for 48 hours, cells were stained with Annexin V-fluorescein isothiocyanate/PI and apoptosis rates were analyzed using a flow cytometer (FACSCalibur, BD Biosciences). Mitochondria Membrane Potential (MMP) was determined by measuring TMRM retention. Cells were adjusted to a density of 2×105/ml and stained with TMRM for 20 min at 37°C. Cells were then washed twice with cold PBS and analyzed by flow cytometer.
Reactive oxygen species (ROS)
The generation of ROS was assessed by flow cytometry. In briefly, (2×105 cells/ml) were cultured and washed with PBS and re-suspended in complete medium followed by incubation with 50 μM dihydrorhodamine DHE (Vigorous Biotechnology, Beijing). ROS fluorescence intensity was determined by flow cytometer.
Western blotting
Collecting cells and lysing cells completely in lysis buffer at 4°C. After centrifugation, the protein content determined using a BCA Protein Assay Kit (Thermo, USA). Total proteins were separated by SDS-PAGE and then transferred onto polyvinylidene membranes (Millipore, Bedford, Mass). The blotted membranes were incubated with primary antibodies and then with secondary antibody. All the primary antibodies were purchased from Abcam (USA), Santa (USA) or CST (USA). The dilutions of the primary antibodies were as follows: P-AKT (1:800, CST, USA); AKT (1:1000, CST, USA); PCNA (1:5000, Abcam, USA); CDK1 (1:200, Abcam, USA); p-ERK (1:1000, CST, USA); ERK (1:1000, CST, USA); CDK2 (1:1000, Abcam, USA); VEGF (1:1000, Abcam, USA); VEGFR2 (1:1000, Abacm, USA); Bcl2 (1:100, Santa, USA); Bax (1:150, Santa, USA); WISP1 (1:500, Abcam, USA). The secondary antibody: Goat anti-rabbit IgG (1:1000, Beyotime, China), Goat anti-mouse IgG (1:1000, Beyotime, China). GAPDH (1:1500, CST, USA) was used as the loading control.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA). All the data were presented as means ± standard deviation of the number of experiments indicated in the legends. P<0.05 was considered statistically significant.
Results
Silence of WISP1 inhibits Jurkat cell proliferation
To study the biological role of WISP1 in cell leukemias. Six different leukemic cell lines HL-90, U937, K562, Jurkat, Raj and Molt4 were used to detect the mRNA and protein expression level (Figure 1A, 1B). The results showed that Jurkat cells had the highest WISP1 expression level than that of other cell lines. Then we selected Jurkat cells for further study. We used siRNA to knockdown the expression of WISP1 in Jurkat cells (Figure 1C). CCK-8 assay was performed to detect the cell proliferation. The results indicated that silence of WISP1 significantly inhibited the cell proliferation (Figure 1D).
Figure 1.
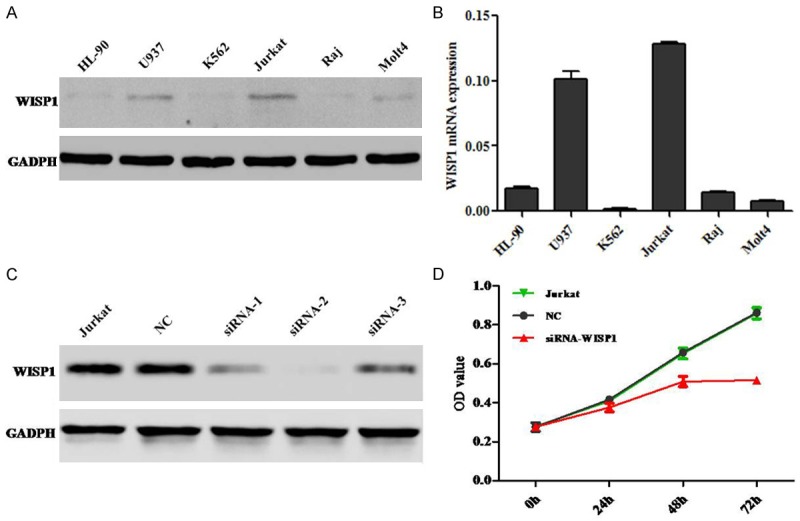
Silence of WISP1 inhibits cell proliferation of leukemia Jurkat cell line. A. The protein expression of WISP1 in leukemia cell lines. B. The mRNA expression of WISP1 in leukemia cell lines. C. Western blot analysis of WISP1 protein expression after Jurkat cells was transfected with siRNA. D. Cell proliferation data were analyzed. The data presented mean ± SD from three separate experiments.
Silence of WISP1 promoted the G1 phase in cell cycle distribution
The cell cycle distribution of Jurkat cells was detected by flow cytometry. The results indicated that WISP1 knockdown could dramatically increase G1 phase cell numbers when compared to negative control (NC) (Figure 2).
Figure 2.
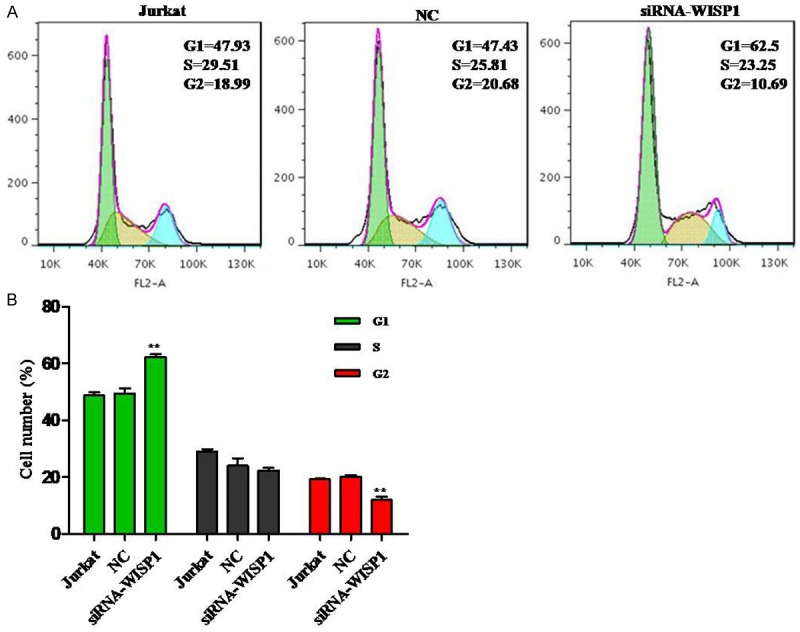
Silence of WISP1 arrests the cell cycle ratios at G1 phase of Jurkat cells. A. A data representative, cell cycle was analyzed by PI staining. B. Data pooled from three independent experiments show the percentage of cell cycle number cells. **P<0.01.
Silence of WISP1 induces apoptosis in the Jurkat cell line
We evaluated the apoptotic function of WISP1 in Jurkat cells by Annexin V-FITC/PI staining assay. As shown in Figure 3, flow cytometry analysis revealed that silence of WISP1 in Jurkat cells significantly induced cell apoptosis by 39.7% compared with NC.
Figure 3.
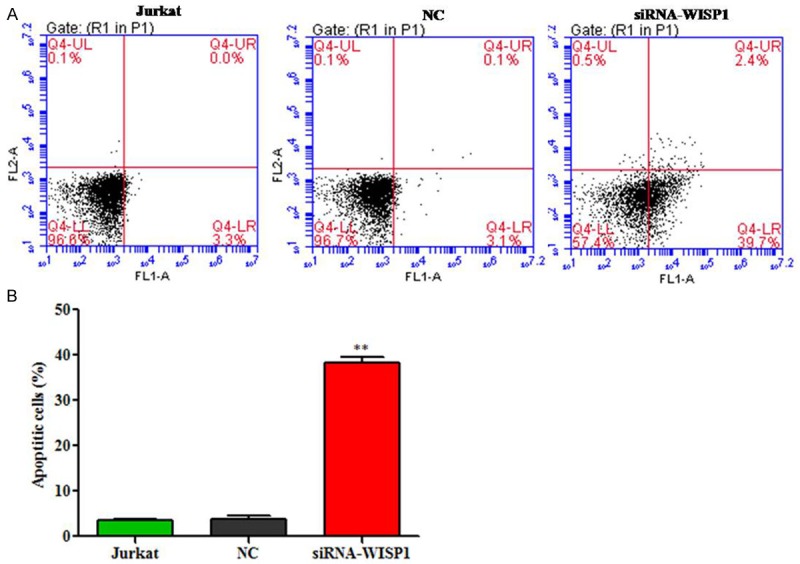
Silence of WISP1 induces apoptosis in acute leukemia cells. A. Jurkat cells transfected and stained with Annexin V and PI. B. The data are representative of three separate experiments. **P<0.01.
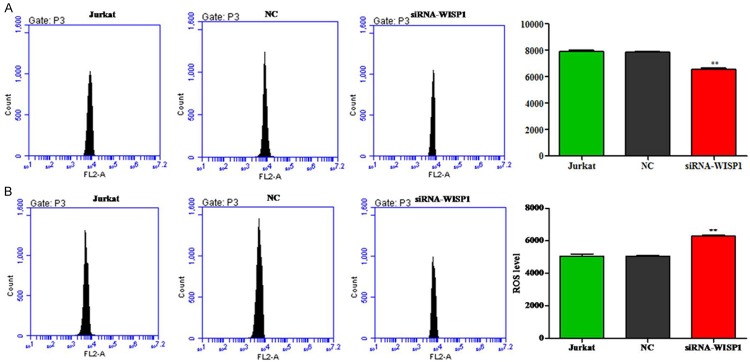
Silence of WISP1 decreases the MMP in Jurkat cells
The fluorescence intensity in the silence of WISP1 Jurkat cells was significantly reduced compared to the control group (Figure 4A). This data revealed that WISP1 could influence the mitochondrial membrane potential in Jurkat cells.
Figure 4.
Silence of WISP1 decreases mitochondria membrane potential and increases the intracellular ROS in Jurkat cells. A. Cells were transfected with WISP1, then incubated with TMRM and analyzed by flow cytometry, data pooled from three independent experiments show the fluorescence intensity. B. Data pooled from three independent experiments show the ROS level. **P<0.01.
Silence of WISP1 increases the ROS level in Jurkat cells
Fluorescence probe DCFH-DA was used to determine the levels of ROS production in Jurkat cells. As shown in Figure 4B, when knockdown of WISP1 in Jurkat, the ROS level was significant increase in the intracellular than the control cells.
Western blot
To clarify the mechanism of Jurkat cells after WISP1 knockdown, the proliferation and apoptosis related proteins were determined by Western blot. As shown in Figure 5, knockdown WISP1 could down-regulate the expression of p-AKT, PCNA, CDK1, P-ERK, CDK2, VEGF, VEGFR2 and Bcl2 compared to the NC group, whereas Bax protein expression was up-regulated.
Figure 5.
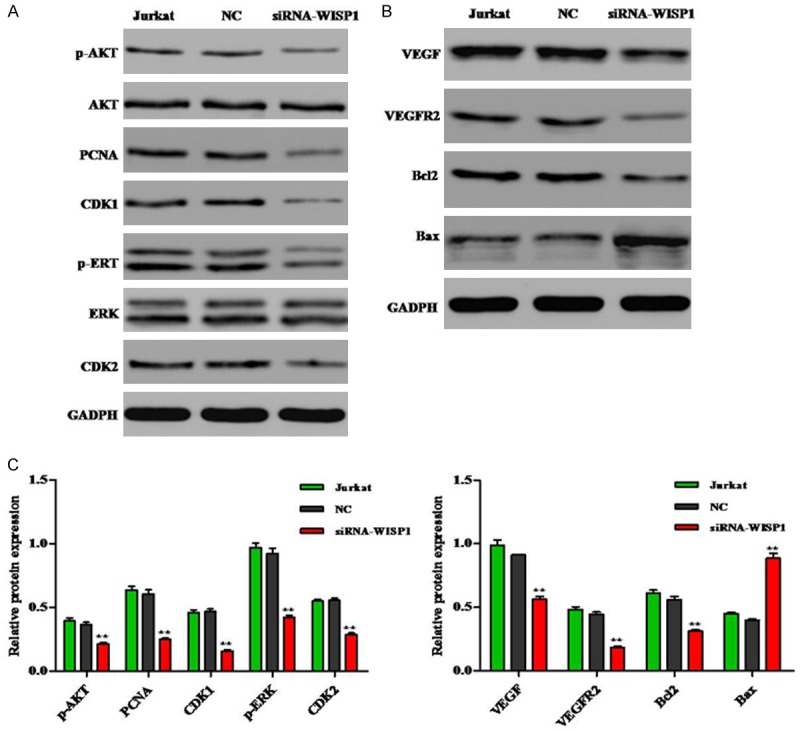
Effects of knockdown WISP1 on the activation of related proteins in Jurkat cells. A, B. Representative western blot results. C. The related proteins p-AKT, PCNA, CDK1, P-ERK, CDK2, VEGF, VEGFR2 and Bcl2 expression in Jurkat cells after PYGB knockdown was down-regulated compared to the NC group. Protein expression of Bax was up-regulated. **P<0.01.
Discussion
Recently, many studies have confirmed that WISP1 is an important therapy target for cancer treatment [11,17,18]. However, the correlation between its expression and function of WISP1 in ALL currently remain unknown. Given the accumulating evidence and information, we hypothesized that WISP1 also plays an important role in ALL carcinogenesis. To clarify this hypothesis, the current study was performed to investigate the expression and biological role of WISP1 in ALL.
In this study, first, we detected WISP1 expression in leukemia cells. Our data revealed that both mRNA and protein levels of WISP1 were highly elevated in Jurkat cells compared with levels in the corresponding cells. As so we used Jurkat cells in this research. According with previous reports, our study confirmed that knockdown of WISP1 significantly inhibited proliferation of Jurkat cells. While, cell cycle distribution at G1 phase was promoted and apoptotic rates was induced, which suggested that induction of apoptosis might be the main mechanism of anti-proliferative effect of WISP1 in Jurkat cells.
Apoptosis, a process of programmed cell death (PCD), is the common mechanism for target chemotherapies that induce cancer cells to die or sensitize them to established therapy [19]. There are two established pathways that result in apoptosis: cell death receptor pathway and the mitochondria initiated pathway. By our data, the loss of MMP causes an increase in the permeability of the mitochondrial membrane. ROS production and consequent oxidative stress have long been implicated in cell apoptosis, previous study has found that mitochondria are the major organelle where ROS was generated [20]. As expected, the intracellular ROS was significantly increased when Jurkat cell knockdown WISP1. Therefore, mitochondria dependent pathway plays important roles in knockdown WISP1 promoted apoptosis in Jurkat cells. Moreover, western blot assay was performed to determine the proteins expression of P-AKT, PCNA, CDK1, P-ERK, CDK2, VEGF, VEGFR2, Bcl2 and Bax. Cell proliferation related proteins PCNA, CDK1 and CDK2 are increased. Bcl2 family play important role in mitochondria-mediated apoptosis. Bax is a pro-apoptotic protein and Bcl2 is anti-apoptotic protein [21,22]. In our study, Knockdown of WISP1 can down-regulate Bcl2, whereas up-regulate the Bax. Vascular endothelial growth factor (VEGF) is a key regulator of physiological angiogenesis, while VEGFR2 is one of the receptor to mediate the biological effects of VEGF [23]. In our results, the VEGF and VEGFR2 expression were down-regulated when the WISP1 knockdown in Jurkat cells. Moreover, our result showed that knockdown of WISP1 can down-regulate both expression of P-AKT and P-ERK, which indicated that WISP1 can inhibit the proliferation of Jurkat cells via P13K/AKT and ERK signaling pathways.
In conclusion, knockdown of WISP1 inhibit proliferation and induce apoptosis of Jurkat cells in mitochondria dependent pathway, which may provide new safe and effective options for the treatment of ALL in the future.
Disclosure of conflict of interest
None.
References
- 1.Cancer IAfRo. World cancer report 2014. Geneva: WHO; 2014. [Google Scholar]
- 2.Gowda C, Dovat S, editors. Impact of Genetic Targets on Cancer Therapy. Springer; 2013. Genetic targets in pediatric acute lymphoblastic leukemia; pp. 327–340. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 4.Kamazani FM, Mehdiabadi GB, Aghaeipour M, Vaeli S, Amirghofran Z. The Expression and Prognostic Impact of CD95 Death Receptor and CD20, CD34 and CD44 Differentiation Markers in Pediatric Acute Lymphoblastic Leukemia. Iran J Pediatr. 2014;24:371–380. [PMC free article] [PubMed] [Google Scholar]
- 5.Scott LM. Lymphoid malignancies: Another face to the Janus kinases. Blood Rev. 2013;27:63–70. doi: 10.1016/j.blre.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–9. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perbal BV, Takigawa M. CCN proteins: a new family of cell growth and differentiation regulators. Imperial College Press; 2005. [Google Scholar]
- 8.Brigstock D. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 9.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen PC, Cheng HC, Yang SF, Lin CW, Tang CH. The CCN family proteins: modulators of bone development and novel targets in bone-associated tumors. Biomed Res Int. 2014;2014:437096. doi: 10.1155/2014/437096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berschneider B, Königshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease. Int J Biochem Cell Biol. 2011;43:306–309. doi: 10.1016/j.biocel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1-and β-catenin-responsive oncogene. Genes Dev. 2000;14:585–595. [PMC free article] [PubMed] [Google Scholar]
- 13.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, Delafontaine P, Chandrasekar B. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010;22:809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono M, Inkson CA, Sonn R, Kilts TM, de Castro LF, Maeda A, Fisher LW, Robey PG, Berendsen AD, Li L. WISP1/CCN4: a potential target for inhibiting prostate cancer growth and spread to bone. PLoS One. 2013;8:e71709. doi: 10.1371/journal.pone.0071709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klinke DJ II. Induction of Wnt-inducible signaling protein-1 correlates with invasive breast cancer oncogenesis and reduced type 1 cell-mediated cytotoxic immunity: a retrospective study. PLoS Comput Biol. 2014;10:e1003409. doi: 10.1371/journal.pcbi.1003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278:11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- 18.Tian C, Zhou ZG, Meng WJ, Sun XF, Yu YY, Li L, Luo HZ, Yang L, Zhou B, Gu J. Overexpression of connective tissue growth factor WISP-1 in Chinese primary rectal cancer patients. World J Gastroenterol. 2007;13:3878. doi: 10.3748/wjg.v13.i28.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burz C, Berindan-Neagoe I, Balacescu O, Irimie A. Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol. 2009;48:811–821. doi: 10.1080/02841860902974175. [DOI] [PubMed] [Google Scholar]
- 20.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 21.Strasser A, Huang DC, Vaux DL. The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochim Biophys Acta (BBA)-Rev Cancer. 1997;1333:F151–F178. doi: 10.1016/s0304-419x(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 22.Antonsson B. Bax and other pro-apoptotic Bcl-2 family” killer-proteins” and their victim the mitochondrion. Cell Tissue Res. 2001;306:347–361. doi: 10.1007/s00441-001-0472-0. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]