Key points
Intrinsic cardiac (IC) neurons undergo differential morphological and phenotypic remodelling that reflects the site of myocardial infarction (MI).
Afferent neural signals from the infarcted region to IC neurons are attenuated, while those from border and remote regions are preserved post‐MI, giving rise to a ‘neural sensory border zone’.
Convergent IC local circuit (processing) neurons have enhanced transduction capacity following MI.
Functional network connectivity within the intrinsic cardiac nervous system is reduced post‐MI.
MI reduces the response and alters the characteristics of IC neurons to ventricular pacing.
Abstract
Autonomic dysregulation following myocardial infarction (MI) is an important pathogenic event. The intrinsic cardiac nervous system (ICNS) is a neural network located on the heart that is critically involved in autonomic regulation. The aims of this study were to characterize structural and functional remodelling of the ICNS post‐MI in a porcine model (control (n = 16) vs. healed anteroapical MI (n = 16)). In vivo microelectrode recordings of basal activity, as well as responses to afferent and efferent stimuli, were recorded from intrinsic cardiac neurons. From control 118 neurons and from MI animals 102 neurons were functionally classified as afferent, efferent, or convergent (receiving both afferent and efferent inputs). In control and MI, convergent neurons represented the largest subpopulation (47% and 48%, respectively) and had enhanced transduction capacity following MI. Efferent inputs to neurons were maintained post‐MI. Afferent inputs were attenuated from the infarcted region (19% in control vs. 7% in MI; P = 0.03), creating a ‘neural sensory border zone’, or heterogeneity in afferent information. MI reduced transduction of changes in preload (54% in control vs. 41% in MI; P = 0.05). The overall functional network connectivity, or the ability of neurons to respond to independent pairs of stimuli, within the ICNS was reduced following MI. The neuronal response was differentially decreased to ventricular vs. atrial pacing post‐MI (63% in control vs. 44% in MI to ventricular pacing; P < 0.01). MI induced morphological and phenotypic changes within the ICNS. The alteration of afferent neural signals, and remodelling of convergent neurons, represents a ‘neural signature’ of ischaemic heart disease.
Key points
Intrinsic cardiac (IC) neurons undergo differential morphological and phenotypic remodelling that reflects the site of myocardial infarction (MI).
Afferent neural signals from the infarcted region to IC neurons are attenuated, while those from border and remote regions are preserved post‐MI, giving rise to a ‘neural sensory border zone’.
Convergent IC local circuit (processing) neurons have enhanced transduction capacity following MI.
Functional network connectivity within the intrinsic cardiac nervous system is reduced post‐MI.
MI reduces the response and alters the characteristics of IC neurons to ventricular pacing.
Abbreviations
- ANS
autonomic nervous system
- IC
intrinsic cardiac
- ICNS
intrinsic cardiac nervous system
- IVC
inferior vena cava
- LCN
local circuit neuron
- LV
left ventricle/ventricular
- MI
myocardial infarction
- RV
right ventricle/ventricular
- VIP
vasoactive intestinal peptide
- VIV GP
ventral interventricular ganglionated plexus
- VT
ventricular tachyarrhythmia
Introduction
Sudden cardiac death due to ventricular arrhythmias is one of the leading causes of mortality in the world, resulting in an estimated 4–5 million deaths each year (Zipes & Wellens, 1998; Chugh et al. 2008). Dysregulation of the autonomic nervous system (ANS) following myocardial infarction (MI) plays a crucial role in the genesis of arrhythmias and the progression of heart failure (Vaseghi & Shivkumar, 2008; Shen & Zipes, 2014; Fukuda et al. 2015). The cardiac neuraxis is responsible for the dynamic regulation of cardiac electrical and mechanical function (Ardell, 2004; Armour, 2004), and involves neural networks located from the level of the heart (Armour, 2008) to that of the insular cortex (Oppenheimer & Hopkins, 1994; Gray et al. 2007).
At the organ level, the intrinsic cardiac nervous system (ICNS) comprises a distributed network of ganglia and interconnecting nerves (Armour, 2008). The ICNS, in concert with higher neuraxial centres (intrathoracic extracardiac ganglia, spinal cord, brain stem and cortex), regulates cardiac excitability and contractile function on a beat‐to‐beat basis (Ardell, 2004; Armour, 2004; Beaumont et al. 2013). The ICNS contains all the neuronal elements necessary for intracardiac reflex control independent of higher centres (Murphy et al. 2000), namely sensory neurons, adrenergic and cholinergic efferent postganglionic neurons, as well as interposed local circuit neurons (LCNs) (Armour, 2008; Beaumont et al. 2013). The largest subpopulation, LCNs, account for the intra‐ and interganglionic communication that occurs among neurons within the ICNS and is responsible for local information processing (Armour, 2008; Beaumont et al. 2013).
Cardiac diseases, such as MI, adversely impact the myocardium and its associated neural components (Vracko et al. 1991; Cao et al. 2000; Kember et al. 2013; Ajijola et al. 2015). Neural signals conveying cardiac injury are transduced by cardiac afferents to multiple levels of the cardiac neuraxis (Armour, 1999). Remodelling within the cardiac neuraxis and its processing of that sensory signal post‐MI (Wang et al. 2014) contribute to neurohumoral activation (Zucker et al. 2012) and the potential for sudden cardiac death (Fukuda et al. 2015). Intrinsic cardiac (IC) neurons from humans with ischaemic heart disease contain inclusions and vacuoles, and display degenerative changes in their dendrites and axons (Hopkins et al. 2000). In vitro intracellular studies of IC neurons derived from chronic MI animals show enhanced excitability, altered synaptic efficacy, and adaptive changes in neurochemical phenotypes and neuromodulation (Hardwick et al. 2014). However, there is limited knowledge on the functional consequences of such changes on neural signalling in vivo, in the context of a healed infarct.
The objectives of this study were to (1) examine morphological and phenotypic remodelling within the ICNS, and (2) directly evaluate remodelling of ICNS processing of afferent and efferent (sympathetic and parasympathetic) inputs, and their integration by LCNs following MI.
In the present study, we show that MI leads to morphological and neurochemical changes within certain IC ganglia. This structural remodelling is paralleled by functional alterations in the processing of afferent and efferent neural signals by the ICNS and a decrease in overall functional network connectivity, or the ability of neurons to respond to independent pairs of stimuli. The heterogeneity in afferent neural signals, along with the remodelling of convergent neurons, could play an important role in the genesis of arrhythmias and progression to heart failure. Characterization of this adverse neural signature in ischaemic heart disease has the potential to serve as a marker of disease progression, and can potentially be used for monitoring and evaluating therapies targeting the ANS.
Methods
Ethical approval
Yorkshire pigs with normal hearts (n = 16; 8 male and 8 female; 49 ± 3 kg) and Yorkshire pigs with healed anteroapical MI (n = 16; 6 male and 10 female; 46 ± 2 kg) were used in this study. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the UCLA Chancellor's Animal Research Committee.
Creation of myocardial infarction
MI was induced as previously described (Nakahara et al. 2011). Briefly, animals were sedated with telazol (8 mg kg−1, i.m.), intubated and ventilated. General anaesthesia consisted of isoflurane (1–2%, inhalation). A 12‐lead electrocardiogram and arterial pressure were monitored. Left femoral arterial access was obtained, and a guidewire (0.035‐inch Amplatz Super Stiff Guidewire with J‐Tip; Boston Scientific, Marlborough, MA, USA) was placed into the left main coronary artery under fluoroscopy. A 3 mm angioplasty balloon catheter (FoxCross PTA Catheter; Abbot Vascular, Temecula, CA, USA) was then advanced over the guidewire and inflated at approximately the third diagonal coronary artery that arose from the left anterior descending coronary artery. Thirty seconds after balloon inflation, a 5 ml suspension of saline containing 1 ml polystyrene microspheres (Polybead, 90 μm diameter; Polysciences Inc., Warrington, PA, USA) was injected distally into the artery through the central lumen of the catheter. Occlusion of the artery was visualized by contrast angiography, and acute MI was confirmed by the presence of ST‐segment elevations in the limb and precordial leads.
Experimental protocol post‐myocardial infarction
Healed MI animals were studied 42 ± 2 days post‐MI. MI and age‐matched control animals were sedated with telazol (8 mg kg−1, i.m.), intubated and ventilated. General anaesthesia was maintained with isoflurane (1–2%, inhalation). Depth of anaesthesia was monitored by haemodynamic indices, jaw tone and pedal withdrawal reflex; anaesthesia was adjusted as necessary. Right femoral venous access was obtained for maintenance fluid administration, and right femoral arterial access for monitoring arterial pressure. A median sternotomy was performed to expose the heart, as well as the stellate ganglia, inferior vena cava (IVC), and descending thoracic aorta. A lateral incision of the neck was performed to expose the cervical vagi and carotid arteries. Snare occluders were placed around the vessels (IVC, aorta and carotid arteries), and stimulating electrodes placed around (vagi) or into (stellate ganglia) autonomic efferent neural structures. Following the completion of surgery, general anaesthesia was changed to α‐chloralose (50 mg kg−1 i.v. bolus with 10 mg kg−1 h−1 continuous i.v. infusion). Body temperature was monitored and maintained via heating pads. Acid–base status was evaluated hourly; respiratory rate and tidal volume were adjusted and bicarbonate was infused as necessary to maintain blood gas homeostasis. At the completion of the experiments, animals were killed by an overdose of sodium pentobarbital (100 mg kg−1, i.v.) followed by potassium chloride (150 mg kg−1, i.v.) to arrest the heart.
Recording intrinsic cardiac neuronal activity
A linear microelectrode array (MicroProbes, Gaithersburg, MD, USA) was used to record the in vivo activity generated by neurons in the ventral interventricular ganglionated plexus (VIV GP) (Fig. 1 A). The linear microelectrode array consisted of 16 platinum–iridium electrodes (25 μm diameter electrodes with an exposed tip of 2 mm; impedance 0.3–0.5 MΩ at 1 kHz) (Fig. 1 B). The electrode was embedded in the VIV GP, which lies near the origin of the left anterior descending coronary artery from the left main coronary artery (Fig. 1 A) (Arora et al. 2003). The linear microelectrode array was attached to a flexible cable, thereby allowing it to be semi‐floating. The electrode wires, as well as earth and reference electrodes, were connected to a 16‐channel microelectrode amplifier with a headstage pre‐amplifier (Model 3600; A‐M Systems Inc., Carlsborg, WA, USA). For each channel, filters were set to 300 Hz to 3 kHz with a gain of 5000. An electrode was sewn to the right atrial myocardium to provide a reference right atrial electrogram. Neuronal waveform, electrocardiogram, right atrial electrogram, and haemodynamic data were input to a data acquisition system (Power1401; Cambridge Electronic Design, Cambridge, UK). Data were analysed offline using the software Spike2 (Cambridge Electronic Design), as previously described (Beaumont et al. 2013).
Figure 1. Methods: intrinsic cardiac neuronal recording .
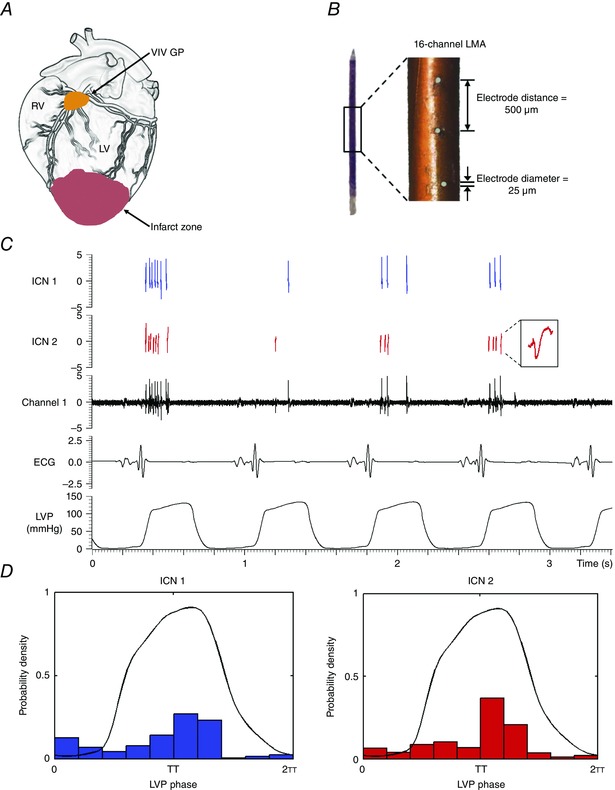
A, schematic diagram showing location of ventral interventricular ganglionated plexus (VIV GP), from which neuronal activity was recorded. B, 16‐channel linear microelectrode array (LMA) used to record in vivo activity of multiple individual neurons contained within the VIV GP. C, representative trace showing the activity of two intrinsic cardiac (IC) neurons (ICN 1 and ICN 2) identified from a single electrode (channel 1) of the LMA. ICN 2 inset is an expanded version of identified neuron. D, basal activity of the neurons from panel C in relation to the cardiac cycle. Note that the activity of both neurons is clustered predominantly during systole. RV, right ventricle; LV, left ventricle; ECG, electrocardiogram; LVP, left ventricular pressure.
Left ventricular haemodynamic assessment
A pressure catheter (Mikro‐Tip; Millar Instruments, Houston, TX, USA) was placed into the left ventricular (LV) chamber via the left femoral artery and connected to a control unit (PCU‐2000; Millar Instruments). LV systolic function was evaluated by end‐systolic pressure and maximum rate of pressure change (dP/dt maximum). LV diastolic function was evaluated by end‐diastolic pressure and minimum rate of chamber pressure change (dP/dt minimum).
Afferent neural input assessment
In order to determine the capacity of IC neurons to transduce mechanosensory afferent inputs, epicardial mechanical stimuli (gentle touch) were applied for 10 s at the following four sites: right ventricular (RV) outflow tract, RV apex, LV mid‐anterior wall, and LV apex. Transient (30 s) occlusions of the IVC and aorta were then performed using a snare occluder in order to determine the capacity of neurons to transduce acute changes in preload and afterload, respectively.
Efferent neural input assessment
In order to determine which IC neurons receive parasympathetic and sympathetic efferent inputs, bipolar spiral cuff electrodes (PerenniaFlex Model 304; Cyberonics Inc., Houston, TX, USA) were placed around the cervical vagi and bipolar needle electrodes inserted into the stellate ganglia bilaterally. A stimulator with a photoelectric isolation unit (S88 and PSIU6; Grass Technologies, Warwick, RI, USA) was used to modulate efferent inputs to IC neurons. For each vagus nerve, threshold was defined as the current necessary to evoke a 10% decrease in heart rate or blood pressure (20 Hz frequency, 1 ms pulse width). For each stellate ganglion, threshold was defined as the current necessary to evoke a 10% increase in heart rate or blood pressure (4 Hz frequency, 4 ms pulse width). Each vagus nerve and stellate ganglion was then stimulated individually for 1 min at threshold current and a frequency of 1 Hz. This was done in order to assess direct inputs to the ICNS independent of any changes in cardiac function. Transient (1 min) occlusion of the bilateral carotid arteries (caudal to carotid sinus) was then performed using a snare occluder to determine the capacity of the carotid baroreflex to modulate efferent inputs to IC neurons.
Epicardial pacing
In order to determine the capacity of IC neurons to respond to cardiac electrical stimulation, a bipolar pacing electrode (St Jude, St Paul, MN, USA) was placed at various epicardial sites and pacing (6 mA current; 2 ms pulse width) was performed at 10% above baseline heart rate for 10 captured beats. The following four sites were paced: (1) right atrial appendage, (2) RV outflow tract, (3) RV apex, and (4) LV apex.
Ventricular tachyarrhythmia inducibility
In a separate set of control (n = 8) and healed anteroapical MI animals (n = 8), ventricular tachyarrhythmia (VT) inducibility was evaluated by programmed ventricular stimulation (EPS320; Micropace, Canterbury, New South Wales, Australia) at two different cycle lengths (600 and 400 ms) with up to three extra stimuli (200 ms minimum) from two different sites (RV apex and LV anterior wall epicardium).
Tissue processing
Following completion of IC neuronal recording, animals were killed and hearts immediately excised. The fat pads containing the VIV GP, dorsal interventricular ganglionated plexus, right marginal artery ganglionated plexus and right atrial ganglionated plexus were removed, rinsed in cold (4°C) saline, and transferred to cold 10% phosphate‐buffered formalin (Fisher Scientific, Pittsburgh, PA, USA) for 4 days. Afterwards, the tissue was transferred to 70% ethanol (Sigma‐Aldrich, St Louis, MO, USA) and paraffin embedded within 3 days. Sections of 4 μm thickness were cut from the paraffin blocks.
Histological staining
IC neuronal size was determined from haematoxylin and eosin stained sections (cat. no. H345‐25; Fisher Scientific, Pittsburgh, PA, USA) using computerized morphometric analysis (Aperio ImageScope; Leica Biosystems, Buffalo Grove, IL, USA).
Immunohistochemical stains
IC neuronal adrenergic phenotype was quantified by tyrosine hydroxylase immunoreactivity (1:2000 dilution; cat. no. ab112; Abcam, Cambridge, MA, USA); neuronal cholinergic phenotype by choline acetyltransferase immunoreactivity (1:200 dilution; cat. no. AB144‐P; Millipore, Billerica, MA, USA); and vasoactive intestinal peptide (VIP) immunoreactivity by anti‐VIP antibody (cat. no. 20077; ImmunoStar, Hudson, WI, USA). Secondary detection was performed with Dako EnVision+ System–HRP labelled polymer anti‐rabbit (cat. no. K4003; Dako North America Inc., Carpinetria, CA, USA) for tyrosine hydroxylase and VIP at 1:500, and polyclonal rabbit anti‐goat immunoglobulins/biotinylated (E0466, Dako) for choline acetyltransferase. Secondary immunoreactivity was detected by diaminobenzidine (Life Technologies, Grand Island, NY, USA) as per the manufacturer's recommended protocol for all stains. The slides were then scanned digitally and analyses performed on the electronic images. All neurons present in the slides were quantified using computerized image analysis (Aperio ImageScope) at ×20–40 magnifications. Staining and quantification of the groups were performed in a blinded fashion.
Data analysis: signal processing of multi‐unit intrinsic cardiac neuronal activity
Artifact removal and IC neuronal identification were performed using off‐line analysis, as previously described (Fig. 1 C) (Beaumont et al. 2013). Briefly, recorded neuronal activity was contaminated by endogenous electrical artifact arising from the activity of the adjacent atrial and ventricular myocardium, as well as by exogenous electrical artifact arising from stimulation of autonomic efferent nerves. Simultaneously occurring activity displaying similar waveforms in more than three adjacent channels of the linear microelectrode array was also considered to be artifact. After identification, artifacts were removed from all channels by blanking, or setting the amplitudes of the time interval containing them to zero. This process resulted in a maximum loss of 3% of the total signal. Following artifact removal, individual units were sorted using principal component analysis of waveform shapes (Beaumont et al. 2013).
Data analysis: monitoring the activity of individual intrinsic cardiac neurons
For epicardial mechanical stimuli and autonomic efferent nerve stimulations, IC neuronal activity was compared 1 min before the stimuli (baseline) vs. during the stimuli. For vascular occlusions and pacing, neuronal activity was compared at baseline vs. during the stimuli, as well as at baseline vs. 1 min after the stimuli (recovery). After each stimulus, we waited at least 5 min for neuronal activity and haemodynamics to return to baseline levels before proceeding. IC neurons were functionally classified as afferent, efferent or convergent based on their response characteristics to the cardiovascular stimuli (Fig. 2 B and C). Afferent neurons were defined as those that responded solely to epicardial mechanical stimuli and/or occlusion of the IVC or aorta. Efferent neurons were defined as those that responded solely to stimulation of autonomic efferent nerves (vagus nerve or stellate ganglia) and/or occlusion of the bilateral carotid arteries. Neurons that responded to activation of both afferent and efferent inputs were defined as convergent (Beaumont et al. 2013).
Figure 2. Analytics and functional classification of intrinsic cardiac neurons .
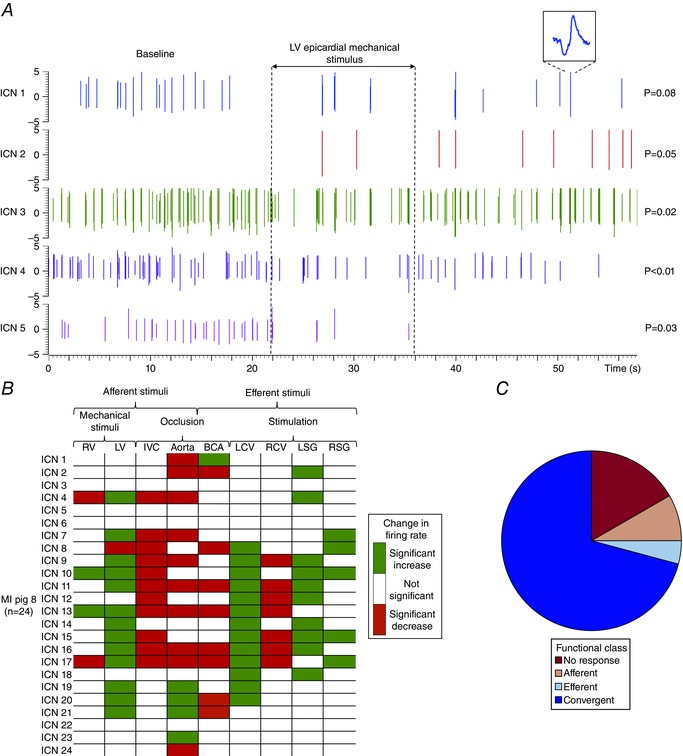
A, spiking activity recorded from 5 IC neurons in a control heart. Vertical dotted lines indicate the onset and offset of LV epicardial mechanical stimulus. ICN 1 inset is an expanded version of identified neuron. Note that subpopulations of neurons showed an increase (ICN 2), a decrease (ICN 3, 4 and 5), or no change in activity (ICN 1) from baseline. The significance levels of induced changes in activity are shown to the right of each trace. P values were derived based on the analysis described in the Methods. B, summary of evoked changes in neuronal activity in response to cardiovascular stimuli in a myocardial infarction (MI) animal. Horizontal rows represent the response of an individual neuron to a given stimulus (vertical columns). Green indicates significant increases in activity (P < 0.05); red indicates significant decreases (P < 0.05). C, functional classification of neurons depicted in panel B. Neurons were classified as afferent, efferent, or convergent based on their responses to the cardiovascular stimuli. Afferent neurons were defined as those that responded solely to epicardial mechanical stimuli of the RV or LV; transient occlusion of the inferior vena cava (IVC); and/or transient occlusion of the descending thoracic aorta. Efferent neurons were defined as those that responded solely to electrical stimulation of the left (LCV) or right cervical vagus nerve (RCV); electrical stimulation of the left (LSG) or right stellate ganglia (RSG); and/or transient occlusion of the bilateral carotid arteries (BCA). Neurons that responded to activation of both afferent and efferent inputs were defined as convergent.
Data analysis: conditional probability
Conditional probability analysis was used to determine whether an IC neuron that responded to one stimulus also responded to another stimulus, as previously described (Beaumont et al. 2013). The potential for a functional relationship between stimulus X and stimulus Y was quantified within neurons identified in each animal as a conditional probability that a neuron that responded to stimulus Y also responded to stimulus X. The conditional probability (probability: response to Y | response to X) was estimated as the number of neurons that responded to both stimulus X and stimulus Y, divided by the number of neurons that responded to stimulus X.
Statistics
The significance level of changes in the firing rate of each IC neuron between baseline vs. stimulus and recovery interval was assessed using a statistical test developed for cortical neurons based on the Skellam distribution (Shin et al. 2010). This test has been previously validated for the study of IC neurons (Beaumont et al. 2013). A χ2 test was used to compare the neuronal response and VT inducibility in MI vs. control animals. Wilcoxon's signed‐rank test or the Mann–Whitney U test was used to compare neuronal firing frequencies, resting haemodynamic indices, as well as morphological and phenotypic changes in neurons in MI vs. control animals. Data are represented as means ± standard error of the mean. P ≤ 0.05 was considered to be statistically significant. Statistical analyses were performed using SigmaPlot 12.0 (Systat Software Inc., San Jose, CA, USA).
Results
Healed anteroapical MI animals were in a chronic compensated state and not in overt heart failure, as evidenced by similar resting haemodynamic indices such as LV end‐diastolic pressure (3 ± 1 vs. 4 ± 1 mmHg (P = 0.59)) and LV dP/dt maximum (1436 ± 112 vs. 1426 ± 151 mmHg s−1 (P = 1.00)) in MI vs. control animals, respectively. Figure 3 J and K illustrates a typical pattern of scar formation induced by the microembolization technique. Since ventricular fibrillation is a terminal event, VT inducibility in this model was evaluated in a separate set of animals. While none of the control animals (n = 8) were inducible, 75% of the MI animals (n = 8) developed VTs (P < 0.01).
Figure 3. Myocardial infarction induces morphological and phenotypic remodelling of intrinsic cardiac neurons .
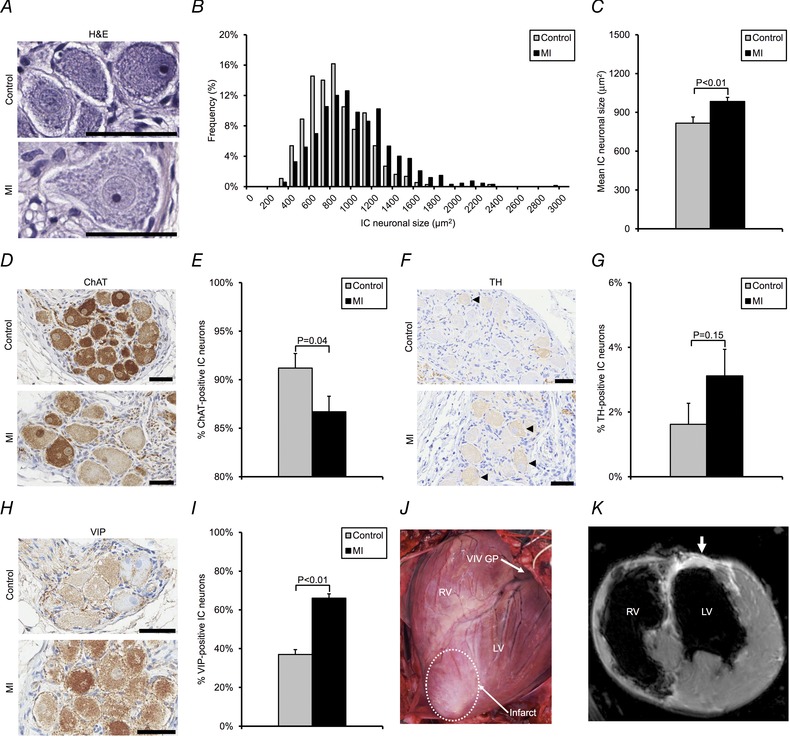
A, photomicrographs showing haematoxylin and eosin (H&E)‐stained neurons from the VIV GP in control vs. MI animals. B, histogram of intrinsic cardiac (IC) neuronal size distribution in control vs. MI animals. C, mean neuronal size in control vs. MI animals. D, photomicrographs showing VIV GP stained with choline acetyltransferase (ChAT) in control vs. MI animals. ChAT catalyses the synthesis of acetylcholine and was used to identify putative cholinergic neurons. E, percentage of ChAT‐positive neurons in control vs. MI animals. F, photomicrographs showing VIV GP stained with tyrosine hydroxylase (TH) in control vs. MI animals. TH catalyses the rate‐limiting step in the synthesis of noradrenaline and was used to identify putative adrenergic neurons (black arrowheads). G, percentage of TH‐positive neurons in control vs. MI animals. H, photomicrographs showing VIV GP stained with vasoactive intestinal peptide (VIP) in control vs. MI animals. VIP is a modulator of cardiac function and a marker of putative afferent neurons. I, percentage of VIP‐positive neurons in control and MI animals. J, image of a porcine heart with a healed anteroapical MI. The location of the VIV GP in relation to the infarct scar (white dotted line) is shown. K, corresponding short‐axis cardiac magnetic resonance image of the heart. White arrow indicates areas of delayed hyperenhancement resulting from scar tissue. Data in C, E, G and I are presented as means ± standard error of the mean (SEM). The Mann–Whitney U test was used in C, E, G and I to determine significance between groups. Scale bars in A, D, F and H represent 50 μm.
Myocardial infarction induces differential intrinsic cardiac neuronal enlargement and phenotypic changes
Histological and immunohistochemical analyses were performed on VIV GP neurons for size, adrenergic–cholinergic phenotype, and VIP phenotype in order to evaluate potential morphological and neurochemical changes induced by MI. VIP is a modulator of cardiac function and a putative afferent marker (Weihe & Reinecke, 1981; Brum et al. 1986; Frase et al. 1987; Rytel et al. 2015).
VIV GP neurons from MI animals were significantly larger than those from controls (946 ± 23 vs. 755 ± 22 μm2, respectively; P < 0.01) (Fig. 3 A–C). A histogram of neuronal size distribution from MI and control animals is shown in Fig. 3 B. Neuronal enlargement was observed in the VIV GP, dorsal interventricular ganglionated plexus, and right marginal artery ganglionated plexus, which exert preferential influence over the ventricles, but was not observed in the right atrial ganglionated plexus, which exerts preferential influence over the atria (Fig. 4 A–C).
Figure 4. Myocardial infarction induces differential morphological and neurochemical remodelling of intrinsic cardiac neurons .
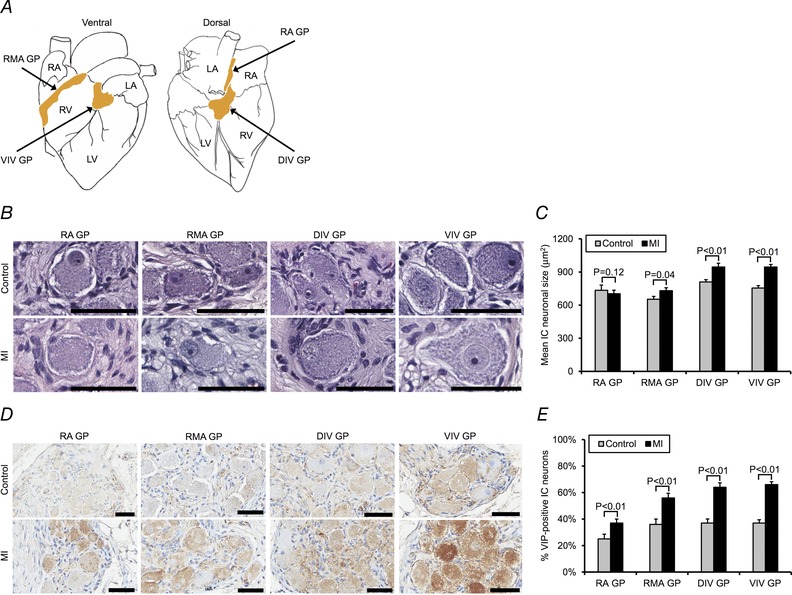
A, schematic diagram showing location of right atrial ganglionated plexus (RA GP), right marginal artery ganglionated plexus (RMA GP), dorsal interventricular ganglionated plexus (DIV GP), and VIV GP. The RA GP and RMA GP exert preferential influence over the right atrium and RV, respectively, whereas the DIV GP and VIV GP exert preferential influence over the LV. B, photomicrographs showing H&E‐stained neurons from the ganglionated plexi (GPs) studied in control vs. MI animals. C, mean IC neuronal size in the GPs in control vs. MI animals. D, photomicrographs showing the GPs stained with VIP in control vs. MI animals. E, percentage of VIP‐positive neurons in the GPs in control vs. MI animals. Data in C and E are presented as means ± SEM. The Mann–Whitney U test was used in C and E to determine significance between groups. Scale bars in B and D represent 50 μm. RA, right atrium; LA, left atrium.
There was a significant decrease in the percentage of IC neurons expressing choline acetyltransferase in MI animals compared with controls (87 ± 2% vs. 91 ± 2%, respectively; P = 0.04) (Fig. 3 D and E). In contrast, there was no significant difference in tyrosine hydroxylase expression in MI relative to control animals (3 ± 1% vs. 2 ± 1%, respectively; P = 0.15) (Fig. 3 F and G). VIP expression was significantly increased in MI vs. control animals (66 ± 2% vs. 37 ± 3%, respectively; P < 0.01) (Fig. 3 H and I). VIP expression was also significantly increased in all the other ganglionated plexi (GPs) studied (Fig. 4 D and E).
Functional characterization of intrinsic cardiac neurons post‐myocardial infarction
The in vivo activity of neurons from the VIV GP was recorded in control and MI animals using a microelectrode array in order to evaluate functional changes in neuronal response characteristics induced by MI (Figs 1 and 2). In eight control animals, the activity generated by 118 IC neurons from the VIV GP was studied (average: 15 ± 3 neurons per animal) (Fig. 5 A, left panel). In eight MI animals, the activity generated by 102 neurons was studied (average: 10 ± 2 neurons per animal) (Fig. 5 A, right panel). The spontaneous firing rates of neurons were derived from pooling data from baseline intervals. The average spontaneous firing rate of the neurons from control animals was 0.31 Hz (range: 0–4.42 Hz), while the average of those from MI animals was 0.21 Hz (range: 0–1.59 Hz). The distribution was overall similar in both states, with more than 90% of neurons firing below 1 Hz.
Figure 5. Myocardial infarction induces no overall change in functional or temporal characteristics of intrinsic cardiac neurons .
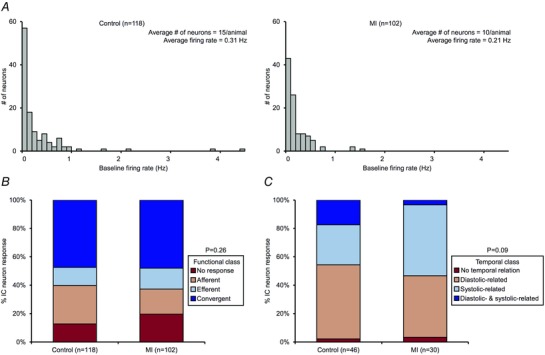
A, histogram of baseline firing rates of IC neurons identified in control vs. MI hearts. B, functional classification of neurons in control vs. MI hearts. C, cardiac cycle‐related periodicity of neurons in control vs. MI hearts. Note that subpopulations of neurons displayed diastolic‐related activity, systolic‐related activity, diastolic‐ and systolic‐related activity, or stochastic behaviour. MI did not significantly alter the functional or temporal characteristics of the neurons. A χ2 test was used in B and C to determine significance between groups.
Based on their response characteristics to the cardiovascular stimuli, IC neurons were functionally classified as afferent, efferent, or convergent (Figs 2B and C, and 5B). In control and MI animals, convergent neurons represented the largest subpopulation (47% vs. 48%, respectively), followed by fewer afferent (27% vs. 18%, respectively) and efferent (13% vs. 15%, respectively) neurons (Fig. 5 B). In control animals 13% of neurons and in MI animals 20% did not respond to any of the stimuli. There was no significant difference in the overall classifications in either state (P = 0.26).
The activity of IC neurons was compared with the cardiac cycle in order to determine if they exhibited cardiac cycle‐related periodicity (Figs 1 D and 5 C). Based on an activity histogram, neurons that generated at least 100 action potentials at baseline were classified as being related to a specific phase of the cardiac cycle if more than 30% of their activity occurred during the given phase. Forty‐six neurons (39%) in control animals and 30 neurons (29%) in MI animals that satisfied this criterion were analysed for cardiac cycle‐related periodicity (Fig. 5 C). In control animals, 52% of neurons displayed diastolic‐related activity, 28% displayed systolic‐related activity, 17% displayed dual diastolic‐ and systolic‐related activity, and 2% displayed stochastic behaviour. In contrast, in MI animals, 43% of neurons displayed diastolic‐related activity, 50% displayed systolic‐related activity, 3% displayed dual diastolic‐ and systolic‐related activity, and 3% displayed stochastic behaviour.
Afferent remodelling of intrinsic cardiac neurons post‐myocardial infarction
MI differentially affected the capacity of IC neurons to transduce mechanosensitive afferent inputs arising from the infarct vs. border and remote zones of the heart, as assessed by applying mechanical stimuli to myocardial tissue overlying these regions (Fig. 6 A and B). The neuronal response to activation of mechanosensitive inputs arising from border and remote zones (RV outflow tract, RV apex and LV mid‐anterior wall) in MI animals was similar to that in controls (34% in MI vs. 24% in control; P = 0.12). In contrast, significantly fewer neurons responded to activation of mechanosensitive inputs arising from the infarct (LV apex) following MI (7% in MI vs. 19% in control; P = 0.03).
Figure 6. Myocardial infarction induces afferent and efferent remodelling of intrinsic cardiac neurons .
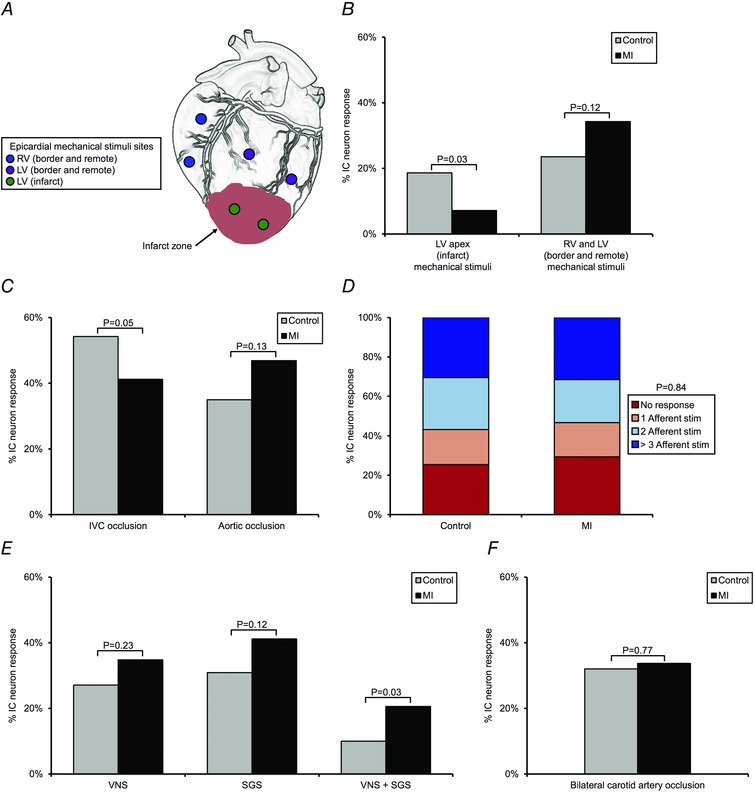
A, sites of epicardial mechanical stimuli that were used to assess the capacity of IC neurons to transduce mechanosensitive afferent inputs arising from the RV or LV. B, percentage of neurons receiving mechanosensitive inputs arising from the LV apex (infarct) vs. RV and LV (border and remote zones) in control vs. MI hearts. C, percentage of neurons responding to transient IVC or aortic occlusion in control vs. MI hearts. IVC and aortic occlusions were used to assess the capacity of neurons to transduce changes in preload and afterload, respectively. D, percentage of neurons transducing multiple afferent inputs in control vs. MI hearts. E, percentage of neurons receiving efferent inputs from parasympathetic and/or sympathetic nervous system, as assessed by cervical vagus nerve stimulation (VNS) and stellate ganglia stimulation (SGS), respectively, in control vs. MI hearts. F, percentage of neurons responding to transient bilateral carotid artery occlusion in control vs. MI hearts. Carotid artery occlusion was used to assess the capacity of the baroreflex to modulate efferent inputs to neurons. A χ2 test was used in B, C, D, E and F to determine significance between groups.
The capacity of IC neurons to transduce changes in cardiac loading conditions was also impacted following MI (Fig. 6 C). In MI animals, the neuronal response to a decrease in preload, induced by transient IVC occlusion, was significantly diminished (41% in MI vs. 54% in control; P = 0.05). There was no significant difference in the neuronal response to an increase in afterload induced by transient partial occlusion of the descending aorta (47% in MI vs. 35% in control; P = 0.13). MI likewise did not significantly alter the overall capacity of neurons to transduce multimodal afferent neural signals (Fig. 6 D; a similar percentage of neurons in both states received one, two, or greater than three afferent inputs (P = 0.84)). These afferent inputs included epicardial mechanical stimuli and transient occlusion of the IVC and aorta.
Efferent remodelling of intrinsic cardiac neurons post‐myocardial infarction
MI did not affect the capacity of IC neurons to individually transduce parasympathetic and sympathetic efferent inputs, as assessed by low frequency stimulation of the cervical vagi and stellate ganglia, respectively (Fig. 6 E). Stimulation of these autonomic efferent nerves was carried out at low frequencies to evaluate direct efferent inputs to the ICNS, rather than an indirect response resulting from changes in cardiac function. There was no significant difference in the percentage of neurons receiving inputs from either the left or the right cervical vagus nerve in MI animals compared with controls (35% vs. 27%, respectively; P = 0.23). A similar pattern was observed with regards to the percentage of neurons receiving inputs from either the left or right stellate ganglion (41% in MI vs. 31% in control; P = 0.12). Interestingly, there was a significant increase in the percentage of neurons that received efferent inputs from both the sympathetic and parasympathetic divisions of the ANS in MI animals relative to controls (21% vs. 10%, respectively; P = 0.03). Of these neurons that received both sympathetic and parasympathetic inputs, 90% also responded to stimulation of one or more afferent inputs in both control (10 of 11 neurons) and MI (19 of 21 neurons) animals. As such, these neurons were classified as convergent.
To assess the effects of MI on baroreflex modulation of efferent inputs to IC neurons, the bilateral carotid arteries were occluded caudal to carotid sinus (Fig. 6 F). There was no significant difference in the percentage of neurons responding to carotid artery occlusion in either state (34% in MI vs. 32% in control; P = 0.77).
Myocardial infarction induces changes in intrinsic cardiac neuronal response to pacing
MI differentially impacted the response of IC neurons to epicardial pacing (Figs 7 A and 8 A). Whereas the neuronal response to right atrial appendage pacing (with ventricular capture) was not altered (31% in MI vs. 36% in control; P = 0.46), the response to ventricular pacing was reduced (44% in MI vs. 63% in control; P < 0.01). Neurons that responded to pacing were classified functionally as afferent, efferent, or convergent (Figs 7B and C, and 8B). The neuronal response evoked from pacing at the RV outflow tract (remote zone) and LV apex (infarct) was most dramatically affected post‐MI (P = 0.05). This alteration was primarily reflected as an upregulation of pacing‐responsive convergent neurons and a corresponding downregulation in pacing‐responsive afferent neurons. It is also noteworthy that in all sites evaluated in control animals and most sites in MI animals, pacing engaged a unique subpopulation of neurons, which only responded to pacing and none of the other afferent or efferent stimuli (Fig. 8 B, red bars).
Figure 7. Analytics and functional classification of intrinsic cardiac neurons responsive to pacing .
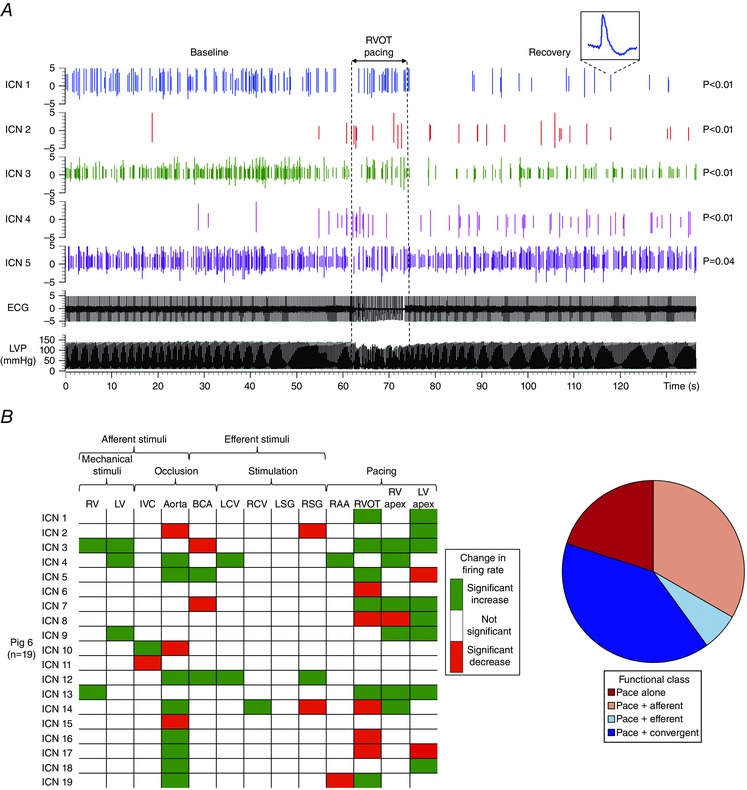
A, spiking activity recorded from 5 IC neurons in a control animal. Vertical dotted lines indicate the onset and offset of epicardial pacing at the right ventricular outflow tract (RVOT). The ICN 1 inset is an expanded version of an identified neuron. Note that subpopulations of neurons showed an increase, a decrease, or no change in activity from baseline. The significance levels of induced changes in activity for each neuron are shown to the right of the trace. P values were derived based on the analysis described in Methods. B, summary of evoked changes in neuronal activity in response to regional epicardial pacing in a MI animal, along with responses to other cardiovascular stimuli. Green indicates significant increases in activity (P < 0.05); red indicates significant decreases (P < 0.05). C, functional classification of pace‐responsive neurons depicted in panel B using the protocol outlined in Fig. 2. RAA, right atrial appendage.
Figure 8. Myocardial infarction alters response and characteristics of intrinsic cardiac neurons to pacing .
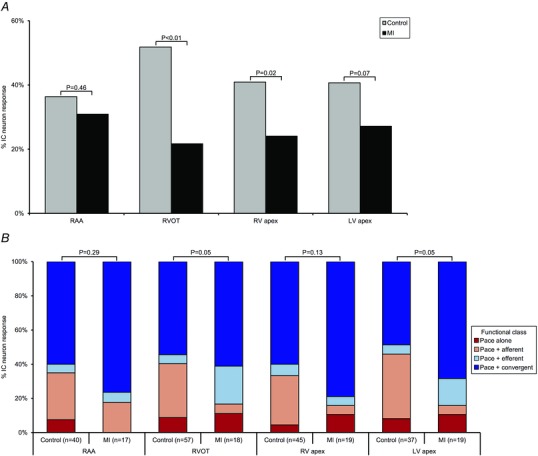
A, percentage of IC neurons responding to epicardial pacing at the RAA, RVOT, RV apex, or LV apex in control vs. MI hearts. MI induced a differential decrease in the neuronal response to ventricular vs. atrial pacing. B, functional classification of pace‐responsive neurons in control vs. MI animals. MI altered the response characteristics to pacing at both infarct (LV apex) and remote zones (RVOT). A χ2 test was used to determine significance between groups.
State dependence of intrinsic cardiac neurons: impact on evoked response
Basal activity impacts IC neuronal response to subsequent cardiovascular stimuli, including pacing (Fig. 9). In both control and MI animals, neurons with low basal activity tended to be activated by pacing (P < 0.01). Conversely, neurons with high basal activity tended to be suppressed by pacing (P < 0.01). These results indicate the state‐dependent nature of such neurons.
Figure 9. State dependence of intrinsic cardiac neurons .
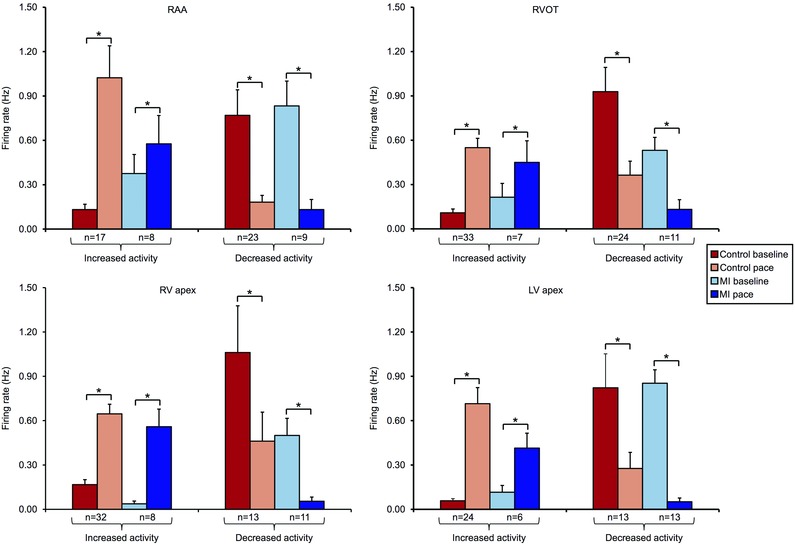
IC neuronal activity at baseline and in response to epicardial pacing at the RAA, RVOT, RV apex, or LV apex in control vs. MI animals. Neurons are subdivided based on evoked increases vs. decreases in activity in response to pacing. Neurons that had a low basal activity were activated by pacing, while those with a high basal activity were suppressed, suggesting a state‐dependent nature. Data are presented as means ± SEM. Wilcoxon's signed‐rank test was used to determine significance between groups. *P < 0.01.
Interdependence of intrinsic cardiac neuronal response to stimuli post‐myocardial infarction
The relationship between the IC neuronal responses to afferent and efferent stimuli, as well as pacing, was determined in both control and MI animals. The conditional probability of whether a neuron that responded to one stimulus also responded to another stimulus is represented in a matrix format (Fig. 10 A and C). These data are also depicted graphically as a network, with only links with conditional probabilities ≥ 0.6 displayed (Fig. 10 B and D). These relationships reflect the concordant behaviour among VIV GP neuronal populations induced by pairs of independent stimuli. MI reduces the overall functional network connectivity within the ICNS.
Figure 10. Myocardial infarction reduces functional network connectivity within the intrinsic cardiac nervous system .
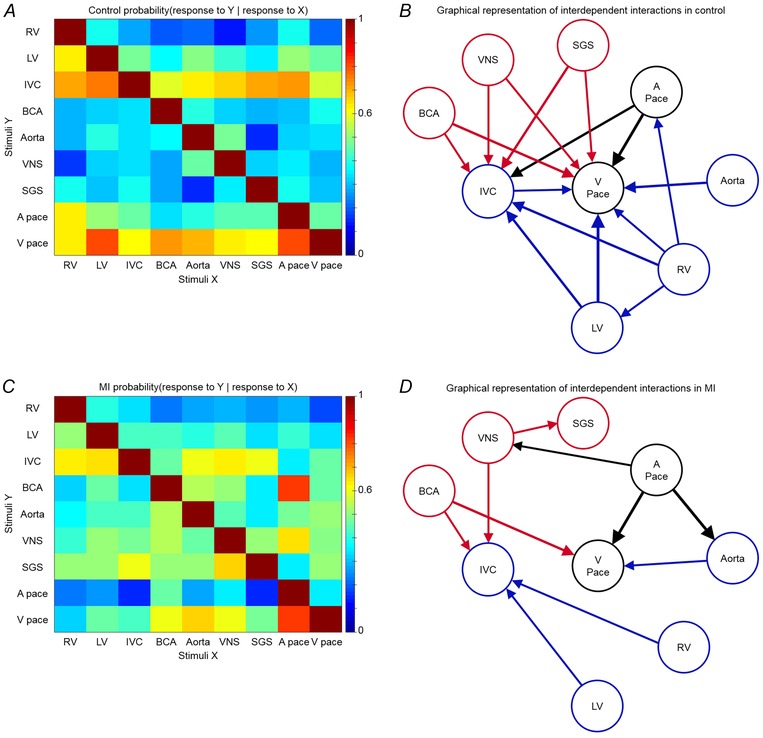
A, conditional probability that an IC neuron that responded to one stimulus (X, x‐axis) also responded to another stimulus (Y, y‐axis) in control animals. B, graphical representation of interdependent interactions between stimuli in control animals. C, conditional probability that a neuron that responded to one stimulus (X, x‐axis) also responded to another stimulus (Y, y‐axis) in MI animals. D, graphical representation of interdependent interactions between stimuli in MI animals. Colour scale in A and C indicates level of probability of each occurrence. Arrow thickness in B and D is proportional to the strength of conditional probability. Only links with probabilities ≥ 0.6 are displayed. Afferent and efferent stimuli are represented by blue and red, respectively. Atrial (A pace) and ventricular pacing (V pace) are represented by black.
Discussion
The present study characterized structural and in vivo functional remodelling of neuronal elements within the ICNS following MI, and represents the first ‘cardiac electroneurogram’ of the chronic infarcted heart. Network function of the ICNS was assessed in the VIV GP, a plexus primarily associated with control of ventricular function (Cardinal et al. 2009). There are several major findings from this study. First, IC ganglia undergo morphological and phenotypic remodelling post‐MI. The site of injury determines which ganglia remodel. Second, afferent neural signals from the infarcted region to IC neurons are attenuated, while those from border and remote regions are preserved following MI, giving rise to a ‘neural sensory border zone’, or heterogeneity in afferent information from injured vs. adjacent non‐injured myocardial tissue (Fig. 11). Alteration in afferent neural signals is also manifested by a reduced capacity of IC neurons to transduce changes in preload. Third, autonomic efferent inputs to the ICNS are maintained post‐MI (Fig. 11). Fourth, convergent IC LCNs, those receiving both afferent and efferent inputs, have enhanced transduction capacity following MI (Fig. 11). Fifth, functional network connectivity within the ICNS is reduced post‐MI. Finally, MI reduces the response and alters the characteristics of IC neurons to ventricular pacing.
Figure 11. Functional remodelling of the intrinsic cardiac nervous system post‐myocardial infarction .
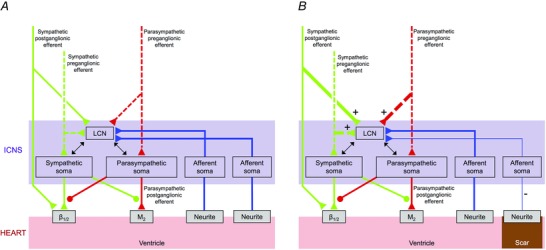
A, schematic diagram showing neural connections between the intrinsic cardiac nervous system (ICNS) and the heart, as well as inputs from higher centres of the cardiac neuraxis, in health. B, schematic diagram showing the alterations in neural connections between ICNS and heart that occur following MI. There is an increase in sympathetic and parasympathetic inputs to convergent local circuit neurons (LCNs), while there is a decrease in afferent inputs from the infarct compared with border and remote regions of the heart. Green and red dashed and continuous lines represent pre‐ and postganglionic fibres, respectively.
The only difference between control and MI animals was the presence of an infarct scar. Therefore, the structural and functional changes we noted are likely to be attributable to the MI. We studied the remodelling of the ICNS 6 weeks after the creation of the MI. This represents a stable phase for autonomic adaptations and is beyond the acute phase remodelling, characterized by myocyte death and neural degeneration (Hardwick et al. 2014). Based on haemodynamic indices such as LV end‐diastolic pressure and contractility, the animals were in a chronic compensated state and had not transitioned into overt heart failure. We recorded neuronal activity from the VIV GP because it is primarily involved in control of ventricular function (Cardinal et al. 2009) and its neuronal somata are located upstream from the infarct zone (Arora et al. 2003). Thus, the neural remodelling we observed is not due to direct ischaemic injury to the neurons.
We evaluated VT inducibility and demonstrated that MI animals in this model have a significantly higher inducibility. Since ventricular fibrillation is a terminal event, inducibility was evaluated in a separate set of animals. Specifically, the cardioversion shocks required to resuscitate the animals would have disrupted and/or dislodged our neuronal recording interface and precluded our detailed characterization of the ICNS. Moreover, the shocks by themselves have the potential to alter subsequent neural activity. We have also previously demonstrated that heterogeneous pharmacological or electric activation of the ICNS is arrhythmogenic (Armour et al. 2005; Beaumont et al. 2013), and that blunting of the neural response reduces the potential for arrhythmias (Richer et al. 2008; Gibbons et al. 2012; Ardell et al. 2014). Taken together, we can now hypothesize that the altered neural signature we observed following MI could be relevant to ventricular arrhythmogenesis.
Differential morphological and neurochemical remodelling of neurons within the intrinsic cardiac nervous system
MI induced morphological changes in neurons within the VIV GP. IC neuronal enlargement was observed in the GPs that exert preferential influence over the ventricles, and not the GP that exerts preferential influence over the atria (Cardinal et al. 2009). Neurons have been reported to hypertrophy in response injury (Barr & Hamilton, 1948; Hendrickson & Dineen, 1982; Geuna et al. 1998) and chronic signalling (Zhou et al. 2004). However, since the GPs that we studied were located upstream from the infarct, the latter mechanism is likely to be the case here. The differential enlargement in neuronal size suggests that there is heterogeneity in afferent and efferent neural signals following MI, with neurons directly involved in control of the infarcted regions most impacted. Further, the site of injury determines which ganglia remodel. This heterogeneity, observed in both our structural and our functional data, potentially has an important role in arrhythmogenesis (Chen et al. 2014). These structural changes are similar to those that have been reported in intrathoracic extracardiac ganglia in both humans and animal models of ischaemic cardiomyopathy (Zhou et al. 2004; Ajijola et al. 2012; Han et al. 2012; Ajijola et al. 2015).
MI induced a decrease in the cholinergic phenotype within the VIV GP. The decrease in cholinergic neurons may correlate with the centrally mediated parasympathetic withdrawal seen post‐MI (Vanoli et al. 1991; Billman, 2006). While we did not evaluate adrenergic–cholinergic transdifferentiation, there is strong evidence indicating that this occurs within other ganglia of the cardiac neuraxis in disease states (Kanazawa et al. 2010; Fukuda et al. 2015). There was also increased expression of VIP in all of the GPs following MI (Fig. 4 D and E). VIP is known to act as a coronary vasodilator and have inotropic and chronotropic effects (Weihe & Reinecke, 1981; Brum et al. 1986; Frase et al. 1987). The increased expression of VIP within the ICNS following MI may represent a compensatory mechanism to maintain cardiac function by vasodilatation to improve coronary blood flow, as well as enhanced inotropy and chronotropy. VIP has also been implicated in nociception (Rytel et al. 2015). The increase in VIP‐positive neurons may also be a result of enhanced afferent signalling post‐MI.
Neural sensory inputs from infarct border zones
While alterations in efferent neural signals post‐MI has been extensively documented, little attention has been given to afferent neural signals originating from the injured and adjacent non‐injured myocardial tissue. Anatomical and functional studies have identified unipolar neurons in IC ganglia with sensory neurites located in atrial and ventricular tissues (Armour, 2008). These afferent neurons transduce the local mechanical and chemical milieu of the heart (Armour, 2008). Afferent neurons contained within each GP have spatially divergent receptive fields (Waldmann et al. 2006; Cardinal et al. 2009), allowing for transduction of sensory information from widespread cardiac regions. We show that VIV GP neurons, located adjacent the origin of the left anterior descending coronary artery from the left main coronary artery (Arora et al. 2003), transduce sensory inputs arising from diverse cardiac regions overlying the RV and LV.
Following MI, myocardial necrosis occurs in the infarct zone secondary to ischaemia. Further, a lack of energy substrates and a build‐up of molecules such as reactive oxygen species trigger a cascade of intracellular signalling processes that result in remodelling of myocytes in the infarct border zone (Sutton & Sharpe, 2000). Concurrent with myocyte remodelling, there are adaptive and maladaptive changes that occur at multiple levels of the cardiac neuraxis including the ICNS (Armour, 1999). In the acute state, there is excessive and aberrant activation of IC neurons transducing afferent signals from the injured myocardial tissue (Huang et al. 1993; Armour, 1999). Our data demonstrate that in the chronic state, afferent signals from the infarct to the ICNS are reduced but not completely abolished, while those from border and remote regions are preserved. This heterogeneity in afferent neural signals gives rise to boundary conditions and a ‘neural sensory border zone’ analogous to the myocardial border zone induced by scar formation. The importance of sensory boundary conditions has already been shown in other neural circuits, such as the visual system where there is an enhanced ability for retinal ganglion cells to detect non‐uniform light fields (Tessier‐Lavigne, 2000). We hypothesize that the MI‐induced asymmetry in afferent inputs to the ICNS may underlie reflex activation of the ANS, including sympatho‐excitation. In this regard, application of resiniferatoxin, a potent agonist of transient receptor potential vanilloid 1, post‐MI decreases cardiac afferent nociceptive signalling, reduces sympatho‐excitation, and is associated with preserved cardiac function (Wang et al. 2014). These data point to the fundamental importance of afferent neural signals in the progression of cardiac disease and their role as a therapeutic target to manage the disease process.
Convergent local circuit neurons: information processing within the intrinsic cardiac nervous system
In the present study, we show that a subpopulation of IC neurons, termed convergent LCNs, receive both afferent and efferent inputs. Convergent LCNs, together with afferent and efferent neurons, form the basic constituents of the IC neural circuitry (Armour, 2008; Beaumont et al. 2013). Within this circuit, convergent LCNs integrate and process information, and the presence of a large subpopulation of these neurons even in the MI state demonstrates that the capacity for local information processing is maintained. While there was no difference in the overall functional classification of neurons post‐MI (afferent, efferent and convergent), the integrated neural network response to cardiovascular stimuli adapted and/or remodelled. This was evident in the neuronal response to changes in preload and to regional pacing, as well as the altered functional neural network connectivity as assessed by the conditional probability analysis. Although most neurons that responded to pacing were also found to transduce dynamic cardiovascular changes, there was a unique subset that solely responded to pacing. The altered ICNS response to pacing at not only the infarct but also remote zones of the heart indicates that pacing may place additional stress on the ICNS on top of that imposed by the MI. In the clinical setting, ventricular pacing has been shown to have detrimental effects on cardiac function, causing dys‐synchrony of the ventricles (Tops et al. 2009). Pacing in the presence of an infarct scar has also been reported to cause an electrical storm (Roque et al. 2014). Further, it can accelerate the progression of heart failure and increase mortality (Wilkoff et al. 2002; Sweeney et al. 2003). A potential mechanism underlying these adverse events relates to activation of the neuroendocrine system (Zhang et al. 2013). It is intriguing to hypothesize that modulation of ICNS activity, in conjunction with MI‐induced remodelling of afferent neural signals, could be a fundamental link between pacing and neuroendocrine system activation.
Efferent neural control
The ICNS was classically viewed as a simple relay station for parasympathetic preganglionic efferent projections to the heart (Langley, 1921). Contrary to this view and in support of data obtained in a canine model (Beaumont et al. 2013), we show that a large percentage of porcine IC neurons received inputs from the sympathetic or parasympathetic nervous system, as well as complex cardiovascular afferent inputs. The fact that a subset of IC neurons received a confluence of efferent inputs (inputs from both a stellate ganglia and vagus nerve) implies that a significant degree of sympathetic–parasympathetic interactions occur within the ICNS (McGuirt et al. 1997; Randall et al. 2003).
Alterations in efferent neural signals following MI occur at multiple levels of the cardiac neuraxis (Vracko et al. 1991; Armour, 1999; Cao et al. 2000; Zhou et al. 2008; Ajijola et al. 2012, 2013, 2015; Han et al. 2012; Hardwick et al. 2014). At the organ level, sympathetic denervation of the infarcted myocardium and hyperinnervation of the border zones has been observed (Vracko et al. 1991; Cao et al. 2000). Morphological and neurochemical changes have also been noted in neurons contained within sympathetic ganglia such as the stellate (Ajijola et al. 2012, 2015). The increases in sympathetic influences are accompanied by a withdrawal in centrally mediated parasympathetic influences (Vanoli et al. 1991; Billman, 2006). Despite these alterations in sympatho‐vagal balance, our data demonstrate sympathetic and parasympathetic inputs to the ICNS are maintained post‐MI. In fact, the percentage of IC neurons receiving convergent efferent inputs doubled following MI. The vast majority of these IC neurons, in turn, were convergent local circuit in nature as evidenced by the fact that 90% of them were impacted by afferent stimuli. This adaptation may be an attempt to maintain peripheral neural network stability in the face of the destabilizing effects imposed by sympatho‐vagal imbalance and the disparate afferent inputs arising from the infarct vs. border and remote regions of the heart.
Limitations
General anaesthetics can suppress the activity of the nervous system. To reduce the effects of inhaled anaesthetics, we switched to α‐chloralose infusion immediately after completing the surgical preparation. We used gentle touch as a mechanical stimulus to determine the sensory fields of afferent neurons contained within the VIV GP. While there are methods to record and label neurons, we were recording neurons from a beating heart, which necessitated the use of a floating microelectrode array. Therefore, we focused on characterizing the in vivo function of the neurons with post hoc evaluation for structural changes. We studied the functional neural remodelling that occurred in only one of the seven GPs in the porcine heart (Arora et al. 2003); however, this GP is primarily associated with control of ventricular function (Cardinal et al. 2009) and was likely to be most impacted by the infarction, as supported by our histological data. We evaluated structural and functional changes in the ICNS at a fixed point in time following MI. There are dynamic changes that occur in the cardiac neuraxis during the evolution of cardiac disease (Hardwick et al. 2014; Fukuda et al. 2015), and therefore, the neural signature may show different features as disease progresses. Finally, while we demonstrated that MI animals in this model have enhanced arrhythmogenic potential, we did not obtain neuronal recordings in the same animals that underwent VT inducibility and attempt to characterize the ICNS neural signature during VT. The cardioversion shocks required to resuscitate the animals would have disrupted and/or dislodged our neuronal recording interface and precluded detailed characterization of the ICNS.
Additional information
Competing interests
None.
Author contributions
P.S.R., J.L.A. and K.S. designed all experiments; P.S.R. performed all neuronal recording experiments and related analyses; K.N. and O.A.A. performed all immunohistochemical experiments and related analyses; P.S.R. and M.V. performed all VT inducibility studies; all authors contributed to writing the paper. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Grants (R01HL071830 to J.L.A.; R01HL084261 to K.S.). P.S.R. was supported by a NIH National Institute of General Medical Sciences Grant (2T32GM065823), an American Heart Association Grant (15PRE22230011), and a NIH NHLBI Grant (F31HL127974). O.A.A. was supported by an NIH NHLBI Grant (K08HL125730). M.V. was supported by an American Heart Association Grant (11FTF7550004).
Translational perspective
The present study provides direct evidence that the functional neural signature of the intrinsic cardiac nervous system (ICNS) is altered in myocardial infarction in a clinically relevant large animal model, and highlights the utility of neuronal recordings in elucidating the neural adaptation and/or maladaptation to cardiac disease. The heterogeneity of afferent neural signals is likely to be fundamental to reflex activation of the autonomic nervous system, thereby impacting the potential for arrhythmias (Chen et al. 2014; Fukuda et al. 2015) and progression to heart failure (Zucker et al. 2012). Modulation of afferent neural signals from the diseased myocardium to the ICNS, intrathoracic extracardiac ganglia, and higher centres of the cardiac neuraxis should be investigated as a novel therapeutic approach to mitigating ischaemic heart disease. These findings also raise the possibility that ‘cardiac electroneurography’ could serve as a modality for studying cardiac physiology and pathophysiology by providing a potential way to monitor disease progression and the effects of interventions.
References
- Ajijola OA, Wisco JJ, Lambert HW, Mahajan A, Stark E, Fishbein MC & Shivkumar K (2012). Extracardiac neural remodeling in humans with cardiomyopathy. Circ Arrhythm Electrophysiol 5, 1010–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A & Shivkumar K (2013). Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol 305, H1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL & Shivkumar K (2015). Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: Neuropeptide and morphologic changes. Heart Rhythm 12, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell J (2004). Intrathoracic neuronal regulation of cardiac function In Basic and Clinical Neurocardiology, ed. Armour J. & Ardell J, pp. 187–219. Oxford University Press, New York. [Google Scholar]
- Ardell JL, Cardinal R, Beaumont E, Vermeulen M, Smith FM & Armour JA (2014). Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Auton Neurosci 186, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour JA (1999). Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res 41, 41–54. [DOI] [PubMed] [Google Scholar]
- Armour JA (2004). Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 287, R262–271. [DOI] [PubMed] [Google Scholar]
- Armour JA (2008). Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93, 165–176. [DOI] [PubMed] [Google Scholar]
- Armour JA, Richer LP, Pagé P, Vinet A, Kus T, Vermeulen M, Nadeau R & Cardinal R (2005). Origin and pharmacological response of atrial tachyarrhythmias induced by activation of mediastinal nerves in canines. Auton Neurosci 118, 68–78. [DOI] [PubMed] [Google Scholar]
- Arora RC, Waldmann M, Hopkins DA & Armour JA (2003). Porcine intrinsic cardiac ganglia. Anat Rec A Discov Mol Cell Evol Biol 271, 249–258. [DOI] [PubMed] [Google Scholar]
- Barr ML & Hamilton JD (1948). A quantitative study of certain morphological changes in spinal motor neurons during axon reaction. J Comp Neurol 89, 93–121. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA & Ardell JL (2013). Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591, 4515–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE (2006). A comprehensive review and analysis of 25 years of data from in vivo canine model of sudden cardiac death: implications for future of anti‐arrhythmic drug development. Pharmacol Ther 111, 808–835. [DOI] [PubMed] [Google Scholar]
- Brum JM, Bove AA, Sufan Q, Reilly W & Go VL (1986). Action and localization of vasoactive intestinal peptide in the coronary circulation: evidence for nonadrenergic, noncholinergic coronary regulation. J Am Coll Cardiol 7, 406–413. [DOI] [PubMed] [Google Scholar]
- Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS & Chen LS (2000). Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101, 1960–1969. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Pagé P, Vermeulen M, Ardell JL & Armour JA (2009). Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci 145, 55–62. [DOI] [PubMed] [Google Scholar]
- Chen PS, Chen LS, Fishbein MC, Lin SF & Nattel S (2014). Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 114, 1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K & Jui J (2008). Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 51, 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frase LL, Gaffney FA, Lane LD, Buckey JC, Said SI, Blomqvist CG & Krejs GJ (1987). Cardiovascular effects of vasoactive intestinal peptide in healthy subjects. Am J Cardiol 60, 1356–1361. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kanazawa H, Aizawa Y, Ardell JL & Shivkumar K (2015). Cardiac innervation and sudden cardiac death. Circ Res 116, 2005–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuna S, Borrione P, Poncino A & Giacobini‐Robecchi MG (1998). Morphological and morphometrical changes in dorsal root ganglion neurons innervating the regenerated lizard tail. Int J Dev Neurosci 16, 85–95. [DOI] [PubMed] [Google Scholar]
- Gibbons DD, Southerland EM, Hoover DB, Beaumont E, Armour JA & Ardell JL (2012). Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 302, R357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Taggart P, Sutton PM, Groves D, Holdright DR, Bradbury D, Brull D & Critchley HD (2007). A cortical potential reflecting cardiac function. Proc Natl Acad Sci USA 104, 6818–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Kobayashi K, Joung B, Piccirillo G, Maruyama M, Vinters HV, March K, Lin SF, Shen C, Fishbein MC, Chen PS & Chen LS (2012). Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol 59, 954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JC, Ryan SE, Beaumont E, Ardell JL & Southerland EM (2014). Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci 181, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A & Dineen JT (1982). Hypertrophy of neurons in dorsal lateral geniculate nucleus following striate cortex lesions in infant monkeys. Neurosci Lett 30, 217–222. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Macdonald SE, Murphy DA & Armour JA (2000). Pathology of intrinsic cardiac neurons from ischemic human hearts. Anat Rec 259, 424–436. [DOI] [PubMed] [Google Scholar]
- Huang MH, Ardell JL, Hanna BD, Wolf SG & Armour JA (1993). Effects of transient coronary artery occlusion on canine intrinsic cardiac neuronal activity. Integr Physiol Behav Sci 28, 5–21. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi‐Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, Monkawa T, Hayashi M, Iwanami A, Okano H, Okada Y, Ishibashi‐Ueda H, Ogawa S & Fukuda K (2010). Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130‐signaling cytokines in rodents. J Clin Invest 120, 408–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kember G, Armour JA & Zamir M (2013). Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol Genomics 45, 638–644. [DOI] [PubMed] [Google Scholar]
- Langley JN (1921). The Autonomic Nervous System, W. Heffer, Cambridge, UK. [Google Scholar]
- McGuirt AS, Schmacht DC & Ardell JL (1997). Autonomic interactions for control of atrial rate are maintained after SA nodal parasympathectomy. Am J Physiol 272, H2525–2533. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Thompson GW, Ardell JL, McCraty R, Stevenson RS, Sangalang VE, Cardinal R, Wilkinson M, Craig S, Smith FM, Kingma JG & Armour JA (2000). The heart reinnervates after transplantation. Ann Thorac Surg 69, 1769–1781. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Vaseghi M, Ramirez RJ, Fonseca CG, Lai CK, Finn JP, Mahajan A, Boyle NG & Shivkumar K (2011). Characterization of myocardial scars: electrophysiological imaging correlates in a porcine infarct model. Heart Rhythm 8, 1060–1067. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S & Hopkins D (1994). Suprabulbar neuronal regulation of the heart In Neurocardiology, ed. Armour J. & Ardell J, pp. 309–342. Oxford University Press, New York. [Google Scholar]
- Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA & Ardell JL (2003). Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol 285, R1066–1075. [DOI] [PubMed] [Google Scholar]
- Richer LP, Vinet A, Kus T, Cardinal R, Ardell JL & Armour JA (2008). α‐Adrenoceptor blockade modifies neurally induced atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 295, R1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque C, Trevisi N, Silberbauer J, Oloriz T, Mizuno H, Baratto F, Bisceglia C, Sora N, Marzi A, Radinovic A, Guarracini F, Vergara P, Sala S, Paglino G, Gulletta S, Mazzone P, Cireddu M, Maccabelli G & Della Bella P (2014). Electrical storm induced by cardiac resynchronization therapy is determined by pacing on epicardial scar and can be successfully managed by catheter ablation. Circ Arrhythm Electrophysiol 7, 1064–1069. [DOI] [PubMed] [Google Scholar]
- Rytel L, Palus K & Całka J (2015). Co‐expression of PACAP with VIP, SP and CGRP in the porcine nodose ganglion sensory neurons. Anat Histol Embryol 44, 86–91. [DOI] [PubMed] [Google Scholar]
- Shen MJ & Zipes DP (2014). Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114, 1004–1021. [DOI] [PubMed] [Google Scholar]
- Shin HC, Aggarwal V, Acharya S, Schieber MH & Thakor NV (2010). Neural decoding of finger movements using Skellam‐based maximum‐likelihood decoding. IEEE Trans Biomed Eng 57, 754–760. [DOI] [PubMed] [Google Scholar]
- Sutton MG & Sharpe N (2000). Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101, 2981–2988. [DOI] [PubMed] [Google Scholar]
- Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA & Investigators MST (2003). Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 107, 2932–2937. [DOI] [PubMed] [Google Scholar]
- Tessier‐Lavigne M (2000). Visual processing by the retina In Principles of Neural Science, 4th edn, ed. Kandel ER, Schwartz JH. & Jessell TM, pp. 507–522. McGraw‐Hill, New York. [Google Scholar]
- Tops LF, Schalij MJ & Bax JJ (2009). The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol 54, 764–776. [DOI] [PubMed] [Google Scholar]
- Vanoli E, De Ferrari GM, Stramba‐Badiale M, Hull SS, Foreman RD & Schwartz PJ (1991). Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 68, 1471–1481. [DOI] [PubMed] [Google Scholar]
- Vaseghi M & Shivkumar K (2008). The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50, 404–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R, Thorning D & Frederickson RG (1991). Nerve fibers in human myocardial scars. Hum Pathol 22, 138–146. [DOI] [PubMed] [Google Scholar]
- Waldmann M, Thompson GW, Kember GC, Ardell JL & Armour JA (2006). Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol 101, 413–419. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Wang W, Cornish KG, Rozanski GJ & Zucker IH (2014). Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E & Reinecke M (1981). Peptidergic innervation of the mammalian sinus nodes: vasoactive intestinal polypeptide, neurotensin, substance P. Neurosci Lett 26, 283–288. [DOI] [PubMed] [Google Scholar]
- Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP & Sharma A; Dual Chamber and VVI Implantable Defibrillator Trial Investigators (2002). Dual‐chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 288, 3115–3123. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Wu DY, Fu NK, Lu FM & Xu J (2013). Neuroendocrine and haemodynamic changes in single‐lead atrial pacing and dual‐chamber pacing modes. J Int Med Res 41, 1057–1066. [DOI] [PubMed] [Google Scholar]
- Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B & Chen PS (2004). Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95, 76–83. [DOI] [PubMed] [Google Scholar]
- Zhou S, Jung BC, Tan AY, Trang VQ, Gholmieh G, Han SW, Lin SF, Fishbein MC, Chen PS & Chen LS (2008). Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm 5, 131–139. [DOI] [PubMed] [Google Scholar]
- Zipes DP & Wellens HJ (1998). Sudden cardiac death. Circulation 98, 2334–2351. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Patel KP & Schultz HD (2012). Neurohumoral stimulation. Heart Fail Clin 8, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


