Key points
The amount of Ca2+ stored in the sarcoplasmic reticulum (SR) of muscle fibres is decreased in aged individuals, and an important question is whether this results from increased Ca2+ leakage out through the Ca2+ release channels (ryanodine receptors; RyRs).
The present study examined the effects of blocking the RyRs with Mg2+, or applying a strong reducing treatment, on net Ca2+ accumulation by the SR in skinned muscle fibres from Old (∼70 years) and Young (∼24 years) adults.
Raising cytoplasmic [Mg2+] and reducing treatment increased net SR Ca2+ accumulation in type I fibres of Old subjects relative to that in Young.
The densities of RyRs and dihydropyridine receptors were not significantly changed in the muscle of Old subjects.
These findings indicate that oxidative modification of the RyRs causes increased Ca2+ leakage from the SR in muscle fibres in Old subjects, which probably deleteriously affects normal muscle function both directly and indirectly.
Abstract
The present study examined whether the lower Ca2+ storage levels in the sarcoplasmic reticulum (SR) in vastus lateralis muscle fibres in Old (70 ± 4 years) relative to Young (24 ± 4 years) human subjects is the result of increased leakage of Ca2+ out of the SR through the Ca2+ release channels/ryanodine receptors (RyRs) and due to oxidative modification of the RyRs. SR Ca2+ accumulation in mechanically skinned muscle fibres was examined in the presence of 1, 3 or 10 mm cytoplasmic Mg2+ because raising [Mg2+] strongly inhibits Ca2+ efflux through the RyRs. In type I fibres of Old subjects, SR Ca2+ accumulation in the presence of 1 mm Mg2+ approached saturation at shorter loading times than in Young subjects, consistent with Ca2+ leakage limiting net uptake, and raising [Mg2+] to 10 mm in such fibres increased maximal SR Ca2+ accumulation. No significant differences were seen in type II fibres. Treatment with dithiothreitol (10 mm for 5 min), a strong reducing agent, also increased maximal SR Ca2+ accumulation at 1 mm Mg2+ in type I fibres of Old subjects but not in other fibres. The densities of dihydropyridine receptors and RyRs were not significantly different in muscles of Old relative to Young subjects. These findings indicate that Ca2+ leakage from the SR is increased in type I fibres in Old subjects by reversible oxidative modification of the RyRs; this increased SR Ca2+ leak is expected to have both direct and indirect deleterious effects on Ca2+ movements and muscle function.
Key points
The amount of Ca2+ stored in the sarcoplasmic reticulum (SR) of muscle fibres is decreased in aged individuals, and an important question is whether this results from increased Ca2+ leakage out through the Ca2+ release channels (ryanodine receptors; RyRs).
The present study examined the effects of blocking the RyRs with Mg2+, or applying a strong reducing treatment, on net Ca2+ accumulation by the SR in skinned muscle fibres from Old (∼70 years) and Young (∼24 years) adults.
Raising cytoplasmic [Mg2+] and reducing treatment increased net SR Ca2+ accumulation in type I fibres of Old subjects relative to that in Young.
The densities of RyRs and dihydropyridine receptors were not significantly changed in the muscle of Old subjects.
These findings indicate that oxidative modification of the RyRs causes increased Ca2+ leakage from the SR in muscle fibres in Old subjects, which probably deleteriously affects normal muscle function both directly and indirectly.
Abbreviations
- DHPR
dihydropyridine receptor
- GSH
glutathione
- HDTA
hexa‐methylene‐diamine‐tetraacetate
- MHC
myosin heavy chain
- pCa
–log10 [Ca2+]
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarco(endo)plasmic reticulum Ca2+‐ATPase
Introduction
Skeletal muscle performance declines in old age in humans and other species, even in active individuals (Ballak et al. 2014; Miller et al. 2014). This decline in performance is the result of a loss of total muscle mass, as well as changes within the muscle fibres themselves. The decline in single muscle fibre performance in old age may be the result of some level of dysfunction in one or more of the steps in the excitation–contraction coupling sequence (Bottinelli & Reggiani, 2000; Rebbeck et al. 2014), including disruption of the coupling between the dihydropyridine receptors (DHPRs) and the ryanodine receptor (RyR)/Ca2+ release channels (Delbono et al. 1995; Wang et al. 2000), reduced storage and release of sarcoplasmic reticulum (SR) Ca2+ (Jimenez‐Moreno et al. 2008; Andersson et al. 2011; Lamboley et al. 2015) or a reduction in both the Ca2+‐sensitivity and maximal force production by the contractile apparatus (D'Antona et al. 2003; Yu et al. 2007; Hvid et al. 2011; Lamboley et al. 2015). It has been reported that, in the skeletal muscle of aged (24‐month‐old) mice, there is increased ‘leakage’ of Ca2+ out of the SR into the cytoplasm through the RyRs, stemming from a self‐reinforcing cycle in which oxidation and/or nitrosylation of the RyRs increases Ca2+ efflux from the SR, resulting in increased Ca2+ uptake by mitochondria, which in turn leads to increased production of reactive oxygen species and further oxidation of the RyRs (Andersson et al. 2011; Umanskaya et al. 2014). This Ca2+ leakage through the RyRs causes a decreased level of Ca2+ accumulation in the SR, and could be prevented by overexpression of catalase in the mitochondria or by in vitro treatment of the muscle fibres with the reducing agent dithiothreitol (DTT) (Umanskaya et al. 2014).
In the present study, we examined whether there is increased Ca2+ leakage through the RyRs in the skeletal muscle fibres of aged humans, which could help account for the reduced SR Ca2+ content in such fibres (Lamboley et al. 2015). This was investigated by examining the SR Ca2+ accumulation properties in mechanically skinned vastus lateralis muscle fibres from Old (70 ± 4 years) and Young (24 ± 4 years) subjects and, in particular, the ability of raised cytoplasmic [Mg2+] to increase SR Ca2+ accumulation. Ca2+ efflux through the RyRs is strongly inhibited by cytoplasmic Mg2+ (Meissner et al. 1986; Laver et al. 2004). In the presence of 1 mm Mg2+, which represents the normal cytoplasmic concentration, the resting Ca2+ efflux rate through the RyRs is already very low (<0.05% of the maximum efflux rate) in both SR vesicles (Meissner et al. 1986) and mechanically skinned muscle fibres (Lamb et al. 2001). Raising the [Mg2+] from 1 to 3 mm decreases Ca2+ efflux by a further 5‐fold in heavily Ca2+ loaded SR vesicles (Meissner et al. 1986) and strongly inhibits the ability of caffeine and Ca2+ to induce Ca2+ release in skinned fibres and single RyRs (Lamb et al. 2001; Laver et al. 2004). We hypothesized that SR Ca2+ leakage is elevated in muscle fibres in Old subjects and would be decreased by raising the cytoplasmic [Mg2+] from 1 mm to 10 mm, thereby producing an appreciable net increase in Ca2+ accumulation in fibres of Old subjects but not Young subjects. We further hypothesized that the increased SR Ca2+ leakage was the result of oxidation and/or nitrosylation of the RyRs and would be decreased by treating the fibres with DTT, as observed in muscle fibres of aged mice (Umanskaya et al. 2014). Finally, we also examined whether there was any decrease in the density of the DHPRs in the muscle of Old human subjects because decreased DHPR density has been observed in muscle of aged mice (Renganathan et al. 1997) and was proposed to be the cause of the reduced SR Ca2+ release seen in both mouse (Wang et al. 2000) and human muscle fibres (Delbono et al. 1995). We hypothesized that the density of the DHPRs, but not the RyRs, would be lower in muscle fibres of Old subjects relative to that in Young subjects.
Methods
Participants
The present study was approved by the Human Research Ethics Committees of Victoria University and La Trobe University, and conformed with the Declaration of Helsinki. The muscle tissue used was obtained as part of the study described in detail by Lamboley et al. (2015), supplemented with tissue from three additional Young subjects. A total of nineteen Young (12 males and seven females) and nineteen Old adult subjects (13 males and six females) provided their written informed consent. The mean ± SD age was 70 ± 4 years in the Old group and 24 ± 3 years in the Young group. All participants were healthy and recreationally active but were not specifically trained in any sport and the physical activity level was similar between the Old and Young cohorts, as described in detail in Lamboley et al. (2015). The muscle tissue was obtained from a thigh muscle biopsy in rested conditions as performed by an experienced medical practitioner. After injection of a local anaesthetic (1% lidocaine) into the skin and fascia, a small incision was made in the middle third of the vastus lateralis muscle of each subject and a muscle sample was taken using a Bergstrom biopsy needle. The excised muscle sample was rapidly blotted on filter paper to remove excess blood, with one part placed in paraffin oil (Ajax Chemicals, Sydney, Australia) for fibre dissection (see below) and the remaining part stored in liquid nitrogen for subsequent analysis. Physiological measurements were made on single fibres from a total of 13 Young (nine males and four females) and 14 Old subjects (10 males and four females), who were all fibre‐typed by western blotting (see below). Western blotting of muscle homogenates was performed on tissue from a total of 10 Young (four males and six females) and 11 Old subjects (seven males and four females), using the same homogenate tissue as that reported in Lamboley et al. (2015), with fibres from four of these Young subjects and six of these Old subjects being used in the physiological fibre measurements.
Skinned fibre preparation and force recording
The muscle biopsy was pinned at resting length in a petri dish containing paraffin oil and kept cool (∼10°C) on an icepack. Individual fibre segments were mechanically skinned under the paraffin oil, as described previously (Murphy et al. 2009; Lamboley et al. 2013). The skinned fibre segment was then mounted at 120% of resting length on a force transducer (AME801; SensoNor, Horten, Norway) and placed in a Perspex bath containing 2 ml of the standard K+‐based solution broadly mimicking the intracellular milieu (see below). Force responses were recorded using a Bioamp pod and Powerlab 4/20 series hardware (ADInstruments, Sydney, NSW, Australia). All experiments were performed at room temperature (∼23 ± 2°C).
Skinned fibre solutions
All chemicals were purchased from Sigma‐Aldrich (St Louis, MO, USA) unless specified otherwise. The standard K‐HDTA solution contained (in mm): 50 hexa‐methylene‐diamine‐tetraacetate (HDTA2−) (Fluka, Buchs, Switzerland); 8 total ATP, 36 Na+, 126 K+, 8.5 total Mg2+ (giving 1 mm free [Mg2+]), 10 creatine phosphate, 0.05 total EGTA and 90 Hepes; pH 7.1 and pCa (–log10 [Ca2+]) ∼7.1, except where stated. Where required, the SR of the skinned fibre was totally depleted of all releasable Ca2+ by exposure to the ‘full release solution’, which was similar to the K‐HDTA solution but with 30 mm caffeine, 0.05 mm free Mg2+ (total Mg2+ of 2.1 mm) and 0.5 mm free EGTA (pCa 8.5) present to chelate released Ca2+. The SR was reloaded with Ca2+ by exposing the fibre for a set time (10–180 s) to a load solution similar to the standard K‐HDTA solution but with 1 mm total EGTA and the pCa buffered at 6.7 (i.e. 0.5 mm CaEGTA and 0.5 mm free EGTA), and with the standard 1 mm free Mg2+ or with 12 or 22.7 mm total Mg2+ to give 3 and 10 mm free Mg2+, respectively (Lamb & Stephenson, 1991). The maximum force was determined in a solution similar to HDTA solution but with 50 mm EGTA and 49.5 mm Ca2+ (pCa 4.7) and 8.1 mm total Mg2+ to maintain the free [Mg2+] at 1 mm.
Ca2+ uptake and release protocol
The examination of Ca2+ accumulation by the SR in the skinned fibres was similar to that described previously (Lamboley et al. 2013). Because the muscle fibres were kept and skinned under paraffin oil, the SR initially retained its endogenous level of Ca2+. Each skinned fibre segment was first bathed for 2 min in the standard K‐HDTA solution to wash out all diffusible Ca2+ buffers present endogenously in the cytoplasm. The SR was then depleted of all of its releasable Ca2+ by exposing the fibre to the full release solution (see above). The fibre was then washed for 1 min in standard K‐HDTA solution (with 0.5 mm free EGTA present to prevent any Ca2+ reuptake) and subjected to repeated load–release cycles as follows:
Step 1: Load SR for a set time (10–180 s) in a load solution (pCa 6.7 with 0.5 mm CaEGTA and 0.5 mm free EGTA) with a free [Mg2+] of 1, 3 or 10 mm as indicated.
Step 2: Pre‐equilibrate skinned fibre for 15 s in standard K‐HDTA solution with 0.5 mm EGTA.
Step 3: Empty SR of all releasable Ca2+ by exposing fibre for 1 min to full release solution.
Step 4: Wash skinned fibre for 1 min in standard K‐HDTA solution with 0.5 mm EGTA.
The time integral (area) of the force response to the initial exposure to the full release solution was indicative of the endogenous SR Ca2+ content initially present in the fibre, and the responses on subsequent load–release cycles indicated the SR content for the respective loading time (Fig. 1). The relationship between the time integral of the force response and loading time was well fitted by an exponential fit approaching saturation after ∼180 s (Fig. 2). The parameters were adjusted to take into account the amount of SR Ca2+ that had to be released to elicit any detectable force in the presence of 0.5 mm EGTA, determined by back‐extrapolation of the response area–load time curve to zero loading time (Lamboley et al. 2013).
Figure 1. Effect of cytoplasmic [Mg2+] on SR Ca2+ accumulation in a type I muscle fibre from an Old and a Young subject .
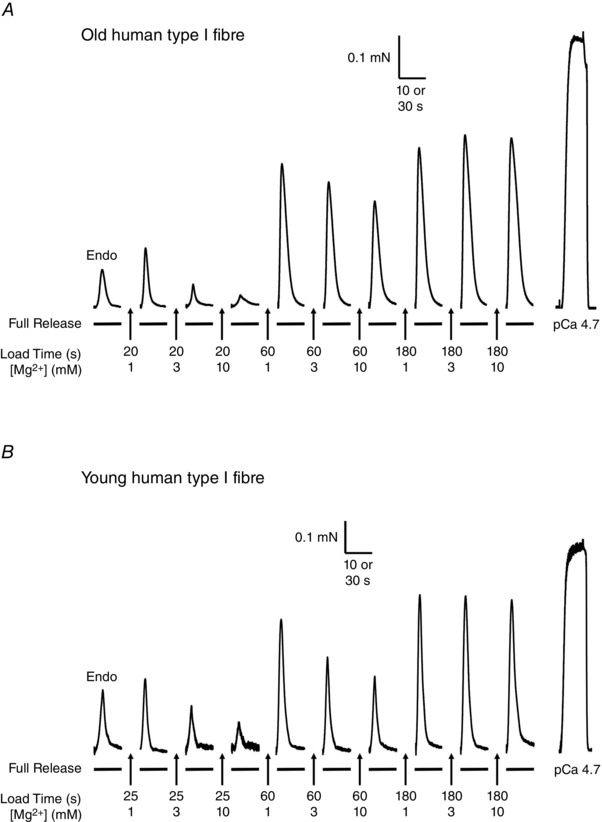
Force responses in type I fibres from Old (A) and Young (B) subjects. In each case, the skinned fibre was subjected to repeated load–release cycles in which the SR was loaded with Ca2+ for the indicated time in a load solution at pCa 6.7 with 1, 3 or 10 mm cytoplasmic free Mg2+ and then the SR Ca2+ released by exposing the fibre to ‘full release solution’ (see Methods). The first response in each sequence was that elicited upon releasing the endogenous SR Ca2+ (‘Endo’). The final response in each sequence shows the maximum force elicited by directly activating the contractile apparatus in a heavily Ca2+‐buffered solution at pCa 4.7 (see Methods). Time scale: 10 s during SR Ca2+ release; 30 s for maximum Ca2+‐activated force.
Figure 2. SR Ca2+ loading characteristics in type I fibre from an Old and a Young subject .
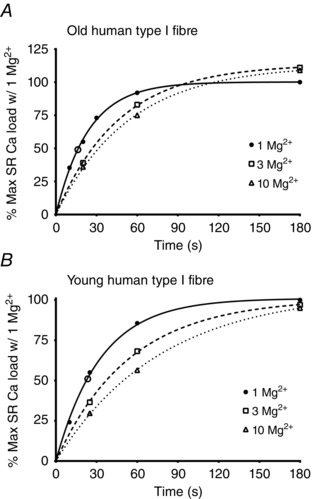
Plots of relative SR Ca2+ content versus loading time when Ca2+ loading a type I fibre from an Old (A) or Young (B) subjects in the presence of 1, 3 or 10 mm Mg2+, derived from records in Figs 1 A and 1 B, respectively. Each value is expressed as a percentage of maximal SR Ca2+ capacity found with 180 s of loading in 1 mm Mg2+. Data sets fitted with best‐fit single exponential function. Data have been adjusted for the negative ordinate intercept resulting from releasing SR Ca2+ in the presence of 0.5 mm EGTA (see Methods). Half‐time for Ca2+ loading with 1 mm Mg2+ present was ∼16 s and ∼23 s in (A) and (B), respectively. Open circle symbols on fitted functions for 1 mm Mg2+ indicate the relative force–time integral found upon releasing the endogenous Ca2+ content, and correspond to ∼49% and 51% of the maximum content in fibres in (A) and (B), respectively.
Western blotting
Whole muscle homogenates from Young (n = 10) and Old (n = 11) subjects comprised those used previously (Lamboley et al. 2015). Single fibres were dissected from tissue under paraffin oil from a subset of those individuals, and placed directly into 10 μl of 1 x solubilizing buffer, which contained 0.125 m Tris‐HCl, 10% glycerol, 4% SDS, 4 m urea, 1% mercaptoethanol and ∼0.001% bromophenol blue (pH 6.8) diluted (2:1 v/v) with double distilled water, as described previously (Murphy et al. 2011; Lamboley et al. 2013). Fibres were stored at −80°C until analysed by western blotting. For both whole muscle homogenates or isolated individual fibres, a four‐ to five‐point calibration curve consisting of the same mixed muscle homogenate was loaded onto each gel, as previously described (Lamboley et al. 2013; Murphy & Lamb, 2013). Total protein was separated on 4–12% Bis‐Tris Criterion gels (Bio‐Rad, Hercules, CA, USA) for 1 h at 200 V with a Mops running buffer (1 m Mops, 1 m Tris base, 69 mm SDS and 20.5 mm EDTA), and with 150 μl of β‐mercaptoethanol added to the top chamber of the electrophoretic tank after the samples were loaded in the gel. Protein was wet‐transferred onto nitrocellulose membrane (100 V for 30 min). Following transfer, the gel was stained with Biosafe Coomassie stain (Bio‐Rad), de‐stained with water overnight and an image collected using a Chemidoc MP with ImageLab software (BioRad). Membranes were treated with Miser antibody extender solution (ThermoFisher Scientific, Scoresby, VIC, Australia), and then blocked in 5% skim milk in Tris‐buffered saline with Tween for 1–2 h at room temperature. Membranes were exposed to primary antibodies overnight at 4°C and 2 h at room temperature. The antibodies used and their dilutions were: myosin heavy chain IIa [MHCIIa, mouse IgG; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA: A4.74, dilution 1:400], myosin heavy chain 1 (MHCI, mouse IgM; DSHB: A4.840, dilution 1:100), ryanodine receptor 1 (RyR1, mouse; DSHB: 34C, dilution 1:100), dihydropyridine receptor (DHPR, mouse; DSHB: IIID5EI, dilution 1:400), SERCA1 (mouse; DSHB: CaF2‐5D2, dilution 1:1000) and SERCA2a (rabbit; Badrilla, Leeds, UK, dilution 1:5000). The HRP secondary antibodies used were goat anti‐mouse HRP (ThermoFisher Scientific: PIE31430, dilution 1:20,000), goat anti‐rabbit HRP (ThermoFisher Scientific: PIE31460, dilution 1:60,000) or goat anti‐mouse IgM (Santa Cruz Biotechnology, Dallas, TX, USA: sc‐2064, dilution 1:20,000). Specific immunoreactive bands were visualized by means of West Femto chemiluminescent reagents (ThermoFisher Scientific) using a Chemidoc MP with ImageLab software.
Statistical analysis
Data are given as the mean ± SD unless otherwise stated. A Student's unpaired t test was used for comparison of two samples where data were normally distributed (Figs 3 and 6) and the Wilcoxin–Mann–Whitney test was used where data were not normally distributed (Fig. 4). Linear dependence between two variables examined by Pearson's correlation coefficient analysis (Fig. 5). P < 0.05 was considered statistically significant.
Figure 3. Measure of maximum SR Ca2+ loading in 1 mm Mg2+ in fibres from Young (Y) and Old (O) subjects .
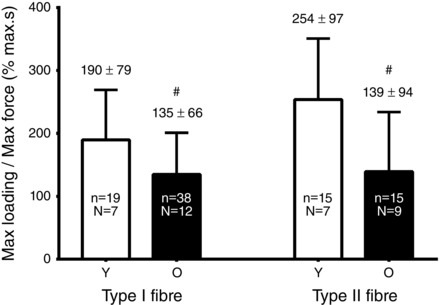
Mean ± SD of the time integral of the force response upon full release of SR Ca2+ after loading fibres for 180 s in the presence of 1 mm Mg2+ (Fig. 1); values normalized to maximum Ca2+‐activated force measured in same fibre. n, number of fibres; N, number of subjects. #Value in the Old group significantly different from the matching value in the Young group (Student's paired two‐tailed t test).
Figure 6. Effect of reducing treatment (10 mm DTT) on SR Ca2+ accumulation .
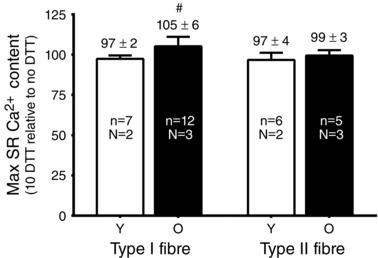
Mean ± SD of maximum SR Ca2+ accumulation (180 s of loading time) in 1 mm Mg2+ in type I and type II fibres from Young (Y) and Old (O) subjects following DTT treatment (10 mm for 5 min), expressed as a percentage of that accumulated in the same fibre before DTT treatment. n, number of fibres; N, number of subjects. #Value significantly greater than 100% (P < 0.025, Student's one‐tailed t test). Note that a small decrease in the amount of Ca2+ accumulation is observed upon successive measurements even without DTT treatment.
Figure 4. Effect of raised [Mg2+] on maximal SR Ca2+ accumulation in individual fibres .
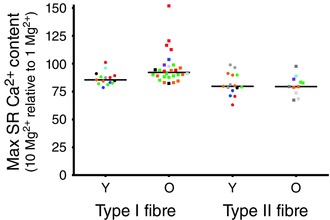
Values denote SR Ca2+ content accumulated after 180 s of loading in the presence of 10 mm cytoplasmic Mg2+, expressed as a percentage of that accumulated in the same fibre in 1 mm Mg2+, measured as in Figs 1 and 2. Each particular symbol labels fibres obtained from a given subject: nine Old (O) and nine Young (Y) subjects. Horizontal lines indicate median value in each case. The presence of 10 mm Mg2+ resulted in greater SR Ca2+ accumulation in type I fibres of Old subjects compared to that in type I fibres of Young subjects.
Figure 5. Inverse relationship between SR Ca2+ leak measure and half‐time for SR Ca2+ accumulation in type I fibres of Old subjects .
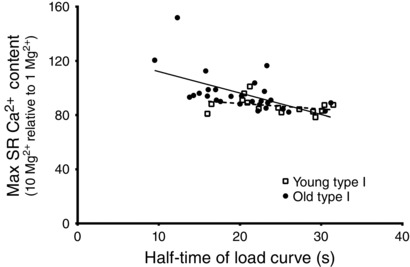
Relative SR Ca2+ accumulation in presence of 10 mm cytoplasmic Mg2+ (expressed as a percentage of that accumulated in 1 mm Mg2+) in individual type I fibres from Young and Old subjects versus half‐time of SR Ca2+ loading in presence of 1 mm Mg2+ (measured as in Fig 2). Pearson's correlation coefficient (r) was –0.56 for the fibres from the Old subjects (P < 0.05) indicating a negative correlation, and −0.35 for the Young type I fibres (P > 0.05, no significant correlation). The line of best fit is shown for each case.
Results
Segments of single muscle fibres from the vastus lateralis muscle of Old and Young subjects were subjected to repeated load–release cycles in which the SR was depleted of all releasable Ca2+ by exposure to the ‘full release solution’, containing 30 mm caffeine with low (0.05 mm) cytoplasmic Mg2+, and then reloaded with Ca2+ by exposure for a set time (10–180 s) to a load solution at pCa 6.7 before again emptying the SR (Fig. 1). The relative amount of Ca2+ accumulated by the SR could be gauged from the time‐integral of the force response elicited in the full release solution, as previously described in detail (Lamboley et al. 2013). In each skinned fibre, successive load–release cycles were carried out using load solutions containing 1, 3 and 10 mm free Mg2+. When the Ca2+ loading of the SR was carried out in the presence of 1 mm free Mg2+, which is close to the normal physiological level (Westerblad & Allen, 1992), the fibres from the Young subjects showed SR Ca2+ loading characteristics very similar to those reported previously for a similar cohort of young subjects (Lamboley et al. 2013), with the half‐time for maximal loading being ∼22 s and 27 s in type I and type II fibres, respectively (Fig. 2 B and Table 1). The relative amount of endogenous Ca2+ initially present in the SR, indicated by the force response upon first emptying the SR (Fig. 1) was ∼52% and 40% of the maximal SR load level in those fibre types, respectively (Fig. 2 B and Table 1), which also was very similar to that found previously in young subjects (Lamboley et al. 2013).
Table 1.
SR Ca2+ loading properties of skinned muscle fibres from Young and Old subjects
| Type I fibres | Type II fibres | |||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Caendo (% maximum) | 52 ± 9 | 55 ± 9 | 40 ± 7* | 40 ± 9* |
| (n = 25; N = 10) | (n = 28; N = 7) | (n = 30; N = 10) | (n = 14; N = 8) | |
| Half‐time (s) | 21.9 ± 7.9 | 18.7 ± 4.7# | 26.8 ± 10.6* | 25.2 ± 6.6* |
| (n = 34; N = 12) | (n = 39; N = 12) | (n = 41; N = 13) | (n = 14; N = 8) | |
| % Full at 60 s of loading | 81.3 ± 6.1 | 85.0 ± 6.1# | 77.5 ± 11.7* | 78.8 ± 7.7* |
| (n = 22; N = 9) | (n = 31; N = 7) | (n = 20; N = 7) | (n = 10; N = 6) | |
Mean ± SD of endogenous SR Ca2+ load level (Caendo) in mechanically skinned muscle fibres from Young and Old subjects, and half‐time for SR Ca2+ loading in presence of 1 mm cytoplasmic Mg2+ (derived as in Figs 1 and 2). n, number of fibres; N, number of subjects. #Value in the Old group significantly different from the matching value in the Young group. *Value for type II fibres significantly different from that in type I fibres in same age group (Student's two‐tailed t tests). Note that 60 s of loading time measurements were not made in some of the fibres for which loading curves were determined.
Although the characteristics of SR Ca2+ accumulation in fibres of the Old subjects were generally similar to those in Young subjects, one key difference was that Ca2+ loading in the type I fibres in the presence of 1 mm Mg2+ started to show saturation at relatively earlier loading times (e.g. compare the continuous line curves in Fig. 2 A and B). This could be seen both from the decrease in the half‐loading time (mean ± SD: 18.7 ± 4.7 s versus 21.9 ± 7.9 s in Old and Young type fibres, respectively) and the increase in the percentage filling of the SR with 60 s of loading (85.0 ± 6.1% versus 81.3 ± 6.1%, respectively, of that reached with 180 s of loading) (Table 1). Furthermore, it was apparent that the total amount of Ca2+ accumulated by the SR after 180 s of loading was significantly lower in the type I fibres of Old subjects than in the type I fibres of Young subjects, as shown by the smaller size of the time integral of the force response elicited when releasing all the SR Ca2+ (Fig. 3). Note that, in this assay, the time integral of the force response in each fibre was normalized to the maximal Ca2+‐activated force measured in that fibre (Fig. 1), thereby taking into account the small difference in maximum force generation in the fibres of Young and Old subjects (Lamboley et al. 2015). This latter finding is in accordance with our previous absolute measurements of SR Ca2+ content showing that the amount of releasable Ca2+ in the SR after maximal loading was significantly lower in type I fibres of Old subjects than in type I fibres of Young subjects [1.24 ± 0.12 versus 1.36 ± 0.15 mmol Ca2+ (litre fibre volume)–1, respectively] (Lamboley et al. 2015).
In type II fibres, there was no significant difference in either the half‐loading time or the percentage SR filling at 60 s between Old and Young subjects (Table 1). Nevertheless, the time integral of the force response elicited upon releasing all SR Ca2+ in the type II fibres was substantially smaller (∼55%) in the Old subjects compared to that in the Young subjects (Fig. 3). Some of this difference would have been caused by the lower Ca2+‐sensitivity of the contractile apparatus in type II fibres in Old subjects relative to that in Young subjects (Lamboley et al. 2015), although it is nevertheless clear from our previous absolute measurements that the maximal SR Ca2+ content of the type II fibres is significantly lower in Old compared to Young subjects [1.45 ± 0.11 versus 1.71 ± 0.11 mmol Ca2+ (litre fibre volume)–1, respectively] (Lamboley et al. 2015).
Effect of raised cytosolic [Mg2+]
The relatively rapid saturation of the SR Ca2+ loading curve in the presence of 1 mm Mg2+, as well as the lower total SR Ca2+ accumulation, observed in the type I fibres of the Old subjects might be explained by increased leakage of Ca2+ out of the SR occurring in parallel with the uptake. To investigate whether there was increased Ca2+ leakage through the RyRs, we examined the effects of raising cytoplasmic [Mg2+] on the ability of the SR to accumulate Ca2+. Raising the free [Mg2+] from 1 mm to 3 or 10 mm slowed the rate of SR Ca2+ accumulation in fibres of both Young and Old subjects (Fig. 2), as expected from the known competitive effects of Mg2+ on Ca2+ pumping by sarco(endo)plasmic reticulum Ca2+‐ATPase (SERCA) (Chiesi & Inesi, 1981; Kabbara & Stephenson, 1994), although the fibres were still able to accumulate substantial amounts of Ca2+ provided that the loading time was sufficiently long. In the type I fibres of Young subjects, the amount of Ca2+ accumulated by the SR after 180 s of loading in the presence of 10 mm Mg2+ reached an average of ∼87% of that accumulated in 1 mm Mg2+ (Fig. 2); the individual values found in 15 type I fibres from seven Young subjects are shown in Fig. 4. In comparison, the type I fibres from Old subjects in the presence of 10 mm Mg2+ accumulated an average of ∼96% of that accumulated in 1 mm Mg2+ and, strikingly, a number of the fibres accumulated more total Ca2+ in the SR when loaded in 10 mm Mg2+ than in 1 mm Mg2+ (e.g. Figs 2 A and 4, showing 28 type I fibres from seven Old subjects). The values for SR Ca2+ accumulation in 10 mm Mg2+ relative to 1 mm Mg2+ in the type I fibres of the Old subjects were significantly greater than in the Young subjects (Z = 2.91, P < 0.002, Wilcoxin–Mann–Whitney test; a non‐parametric test is used because values in Old subjects were not normally distributed). Furthermore, analysing the data in terms of individual subjects (i.e. averaging the values found across all fibres in each given subject) also confirmed that the values for the type I fibres in the Old subjects were significantly greater than in the Young subjects (P = 0.013, seven Old versus seven Young subjects, Wilcoxin–Mann–Whitney test). It was also apparent that the type I fibres of the Old subjects with the fastest saturation of Ca2+ loading in 1 mm Mg2+ (i.e. shortest time for half‐maximal loading) displayed the greatest relative increase in Ca2+ accumulation in 10 mm Mg2+ (Fig. 5), which is consistent with the saturating behaviour of the Ca2+ loading being the result of increased SR Ca2+ leakage through the RyRs. Taken together, these data show that raising the cytosolic [Mg2+] increased net Ca2+ accumulation by the SR to a greater degree in type I fibres of Old subjects than in type I fibres of Young subjects. By contrast, in type II fibres, the effect of raising cytosolic [Mg2+] on SR Ca2+ accumulation was not noticeably different between Old and Young subjects (Fig. 4).
Effect of DTT treatment
We also examined whether SR Ca2+ accumulation was affected by treating fibres with the strong reducing agent DTT (10 mm for 5 min). In each fibre, the amount of Ca2+ accumulated by the SR with 180 s of loading in the presence of 1 mm Mg2+ was measured both before and after DTT treatment. DTT treatment resulted in a significant increase in SR Ca2+ accumulation in the type I fibres of Old subjects but not in any of the other cases (Fig. 6). Control experiments showed that, in the absence of DTT treatment, total SR Ca2+ accumulation normally declined slightly on the successive load–release cycle [percentage accumulation on second control trial relative to first: 98 ± 2% in Young type I (n = 7); 97 ± 5% Old type I (n = 23); 94 ± 6% in Young type II (n = 9); 99 ± 2% in Old type II fibres].
Densities of DHPRs and RyR1s
We used western blotting to examine the densities of the DHPRs (α1 subunit) and RyR1s in muscle of the Old and Young subjects. Unfractionated muscle homogenates from 11 Old and 10 Young subjects were run on each of two gels, together with mixed muscle samples for signal calibration (Fig. 7). The relative densities of both the DHPRs and the RyR1s were very similar in the muscle homogenates from the Young and Old subjects, irrespective of whether the signals were normalized to actin or myosin (Table 2).
Figure 7. DHPR and RyR density in muscle homogenates from Old and Young subjects .

Upper panels: western blots for DHPR α1 subunit and RyR1 in muscle homogenates from seven Old (O) and five Young (Y) subjects. Right‐hand lanes contained 1–8 μl of a mixed homogenate of muscle from all subjects, used for signal calibration. Bottom panel: myosin band on Coomassie‐stained post‐transfer gel. 4–12% BIS/TRIS Criterion precast gel.
Table 2.
DHPR and RyR densities in muscle homogenates of Young and Old subjects
| Old (n = 11) | Young (n = 10) | |
|---|---|---|
| DHPR density | ||
| Normalized to myosin | 0.98 ± 0.26 | 1.00 ± 0.22 |
| Normalized to actin | 0.97 ± 0.31 | 1.00 ± 0.31 |
| RyR1 density | ||
| Normalized to myosin | 0.94 ± 0.26 | 1.00 ± 0.25 |
| Normalized to actin | 0.97 ± 0.32 | 1.00 ± 0.32 |
Mean ± SD relative density of DHPRs and RyR1s, obtained by western blotting, as in Fig. 7. Values for each subject normalized to the average of that found in the samples of all Young subjects on the same gel. Values are the average of duplicate measurements from different gels.
We also examined the DHPR and RyR1 densities in individual muscle fibres (Fig. 8) using a subset of the skinned fibre segments examined in the Ca2+ accumulation experiments. In the fibres of the four Young subjects examined, the densities of both the DHPRs and the RyR1s were ∼50% higher in the type II fibres (n = 19) than in the type I fibres (n = 17) on average. In fibres from the three Old subjects examined (29 type I and 12 type II), there was more disparity in the relative densities of the DHPRs and RyRs than in the fibres from the Young subjects, with a small proportion of the fibres displaying relatively high DHPR and low RyR density. Overall, the ratio of DHPRs to RyRs in the fibres of the Old subjects was, if anything, higher than that in the Young fibre sample.
Figure 8. DHPR and RyR density in single muscle fibres from Old and Young subjects .
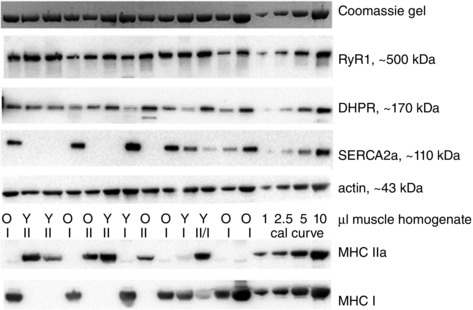
Western blots for DHPRα1, RyR1, SERCA2a, actin, MHCI and MHCIIa in individual fibres from Young (Y) and Old (O) subjects (single skinned fibre segment in each lane), with four right‐hand lanes containing 1–10 μl of a mixed homogenate of muscle from all subjects, used for signal calibration. Labels I and II denotes fibres containing predominantly MHCI and MHCIIa respectively; label II/I denotes a mixed fibre containing substantial amounts of both MHCIIa and MHCI. 4–12% Bis/Tris Criterion precast gel. Top panel: myosin band on Coomassie‐stained post‐transfer gel.
Finally, the SERCA1 and SERCA2 densities in the muscle homogenates from the Old subjects were on average 61 ± 19% and 114 ± 33%, respectively, of that found in the muscle of the Young subjects; these values primarily reflected the decreased proportion of type II fibres and the increased proportion of type I fibres in the muscle of the Old subjects (Lamboley et al. 2015). The relative density of SERCA2 found in six type I fibres from four Old subjects was an average of 94 ± 24% of that found in five type I fibres from three Young subjects run on same gel. These data indicate that the SERCA density in individual muscle fibres was broadly similar in Young and Old subjects.
Discussion
The present study examined the ability of the SR to accumulate Ca2+ in muscle fibres from active Old and Young adults, using mechanically skinned fibres in which the intracellular conditions could be set at a constant physiological level or varied similarly across all fibres. It was found that SR Ca2+ accumulation in the presence of 1 mm cytoplasmic Mg2+ saturated more rapidly (Fig. 2 and Table 1) and at a lower final level (Fig. 3) in type I fibres of Old subjects compared to similar fibres of Young subjects, indicative of increased SR Ca2+ leakage in the former. Importantly, raising the cytoplasmic [Mg2+] from 1 to 10 mm, aiming to specifically decrease Ca2+ leakage through the RyRs (Meissner et al. 1986; Lamb et al. 2001; Laver et al. 2004), was found to increase net SR Ca2+ accumulation to a significantly greater degree in the type I fibres of Old subjects than in the type I fibres of the Young subjects (Fig. 4), with some type I fibres from the Old subjects accumulating more total SR Ca2+ in 180 s of loading in 10 mm Mg2+ than in 1 mm Mg2+ despite the strong competitive effects of the increased [Mg2+] on SERCA Ca2+ pumping (Chiesi & Inesi, 1981; Kabbara & Stephenson, 1994). Furthermore, treatment with the reducing agent DTT also increased net Ca2+ accumulation in the type I fibres of Old subjects and not in the other groups (Fig. 6), with the magnitude of the increase being similar to that observed with raised [Mg2+] (Fig. 4). We have shown previously in rat muscle fibres that oxidative treatment with the sulphydryl‐specific oxidant 2,2’‐dithiodipyridine causes increased Ca2+ leakage from the SR and that reducing treatment with DTT reverses this effect (Posterino & Lamb, 1996). The above data demonstrate that: (i) there is increased Ca2+ leakage out of the SR in the type I fibres of Old human subjects relative to that in type I fibres of Young subjects; (ii) the leakage occurs primarily through the RyRs; and (iii) strong reducing treatment inhibits this RyR Ca2+ leakage, increasing net SR Ca2+ accumulation. These findings are analogous to those made in the muscle of aged mice (in muscles with predominantly type II fibres), where there was increased oxidation/nitrosylation of the RyRs, leading to greater SR Ca2+ leak and decreased SR Ca2+ content, which could be reversed by in vitro treatment with DTT (see Introduction) (Andersson et al. 2011; Umanskaya et al. 2014).
The present study did not find evidence of increased Ca2+ leakage in the type II fibres of the Old subjects; specifically, there was no significant difference from the type II fibres of Young subjects with regard to the time course of Ca2+ accumulation (Table 1), the effect of raising [Mg2+] to 10 mm (Fig. 4) or the effect of DTT treatment (Fig. 6). Nevertheless, we have previously observed that the maximal level of SR Ca2+ loading is lower in type II fibres of Old compared to Young subjects (Lamboley et al. 2015). One possible explanation for this apparent discrepancy is that the Ca2+ accumulation assay used in the present study is a relatively insensitive measure of SR Ca2+ leakage because the [Ca2+] within the skinned fibres during the loading was buffered with only a moderate [EGTA] (1 mm total at pCa 6.7) and hence Ca2+ leaking out of the SR would have increased the local [Ca2+] near the SR, in turn leading to increased uptake by the SERCA and recovery of some of the leaked Ca2+. Consequently, it is possible that SR Ca2+ leakage was increased also in the type II fibres in the Old subjects but that this local Ca2+ flux in the type II fibres was not detected with the whole fibre Ca2+ accumulation assay used, possibly in part because the SERCA density in the type II fibres (almost exclusively SERCA1a; Lamboley et al. 2014) is probably substantially higher than in type I fibres (almost exclusively SERCA2a), assuming that human fibres show a relative SERCA density distribution similar to that seen in rat and rabbit muscle (Leberer & Pette, 1986; Wu & Lytton, 1993). The level of Ca2+ buffering used in the assay of the present study was intentionally kept relatively low in order to study SR Ca2+ leakage at close to normal resting intracellular [Ca2+] without grossly perturbing local Ca2+ movements by dampening Ca2+‐activation or Ca2+‐inactivation of the RyRs.
Irrespective of whether there was some level of undetected SR Ca2+ leakage in the type II fibres, the present study clearly demonstrates that, at least in the type I fibres of the Old subjects, there is substantially increased Ca2+ leakage through the RyRs resulting from reversible oxidative modification of cysteine residues on the RyRs because this leakage can be prevented by treatment with DTT. It is noteworthy that our preceding study on the contractile apparatus properties in muscle of Old and Young subjects (Lamboley et al. 2015) found that maximum specific force, Ca2+ sensitivity and the effects of S‐glutathionylation on Ca2+‐sensitivity were all decreased in the type II fibres in the Old subjects, but not in the type I fibres, and that these particular effects in the type II fibres probably all reflected irreversible oxidative damage of the contractile apparatus. It was noted that type I fibres were probably less susceptible to such irreversible oxidative damage than type II fibres because type I fibres contain higher levels of glutathione (GSH) and superoxide dismutase (at least in rats) (Ji et al. 1992; Masuda et al. 2003). This may well also help explain the present findings. The higher GSH level in type I fibres would be expected to decrease the deleterious actions of superoxide, hydroxyl and peroxynitrite, all of which can potentially cause irreversible oxidation of cysteine (e.g. sulphonation) and other residues. However, the reaction of reactive oxygen or nitrogen species (ROS/RNS) with the GSH would be expected to result via various pathways not only in increased S‐glutathionylation of cysteine residues on the contractile apparatus (Klatt & Lamas, 2000; Lamb & Westerblad, 2011; Mollica et al. 2012), thereby protecting them from irreversible oxidative modification, but also in increased S‐glutathionylation of the RyRs, which has been shown to decrease the inhibitory effect of Mg2+ on the RyRs (Aracena et al. 2003), and which would increase resting Ca2+ leakage. Furthermore, there would probably also be increased generation of GSNO, which has been shown to lead to S‐nitrosylation of the RyRs, increasing their sensitivity to Ca2+ activation (Aracena et al. 2003), and this would also increase Ca2+ leakage. Similar reversible effects also occur with mild oxidation of the RyRs by hydrogen peroxide (by sulphenation or cross‐linking of cysteine residues) (Favero et al. 1995; Posterino et al. 2003; Aracena‐Parks et al. 2006). In summary, the increase in Ca2+ leakage through the RyRs could be a result of the single or combined effect of a variety of different types of reversible oxidative modifications of the RyRs. In view of this complexity, and the present lack of definitive information about which and how many of the many different cysteine residues on the RyR are involved in each of these types of oxidative modifications (Aracena‐Parks et al. 2006), we did not attempt to identify specific oxidative modifications of the RyRs, particularly given that the increased Ca2+ leakage could be a result of the modification of only a small proportion of the total pool of RyRs. We acknowledge that this is a limitation of the present study and note that a more complete understanding of the basis of the oxidation‐linked increase in SR Ca2+ leakage in ageing muscle will probably require a range of mass‐spectroscopic and labelling studies and further advances in knowledge about the effects of specific oxidative changes on the RyRs. Finally, we note that the importance of the specific cellular anti‐oxidant levels in influencing whether ROS/RNS affect contractile function or SR Ca2+ release is vividly illustrated by studies on single muscle fibres in which increased expression of superoxide dismutase or application of various different combinations of ROS/RNS‐neutralizing compounds was found to differentially alter the relative susceptibility of the contractile apparatus and the Ca2+ release process to oxidative modification (Bruton et al. 2008; Cheng et al. 2015).
DHPR and RyR densities
We also examined the densities of both the DHPR α1 subunits and the RyRs in the muscle of the Old relative to the Young subjects because it has been reported that there is a loss of DHPRs with age in rodent and rabbit muscle (Renganathan et al. 1997; Ryan et al. 2000). It has been suggested that such a loss of DHPRs underlies the decreased SR Ca2+ release seen in both rodent (Wang et al. 2000) and human muscle (Delbono et al. 1995) and it might also increase SR Ca2+ leakage because of a decrease in the resting inhibition exerted by the DHPRs on the RyRs (Zhou et al. 2006; Eltit et al. 2011; Robin & Allard, 2012). However, in the human tissue in the present study, we found no evidence of a differential loss of DHPRs or indeed of any overall loss of either DHPRs or RyRs in the muscle of the Old subjects; on average, the density of the DHPRs and RyRs in total muscle homogenates from the 11 Old subjects was ∼0.98 and ∼0.96 times, respectively, of that found in the muscle of the 10 Young subjects (Table 2). These values are similar to the ‘expected’ value of ∼0.94 derived taking into account the increase in the proportion of type I fibres in the Old subjects (ratio of type I to type II changing from ∼50:50 to ∼65:35; Lamboley et al. 2015) and the fact that the density of both DHPRs and RyRs is ∼50% lower in type I fibres compared to type II fibres. A previous study in SR vesicles also concluded that the DHPR density was unchanged in muscle of aged human subjects (Ryan et al. 2003). Furthermore, in the relatively small set of single fibres examined in the present study from a subset of the subjects, the DHPR density was, if anything, somewhat higher in the fibres of the Old subjects.
Conclusions
The findings obtained in the present study demonstrate that there is increased leakage of Ca2+ out of the SR through the RyRs in type I muscle fibres in aged humans as a result of the reversible oxidative modification of the RyRs, and that this Ca2+ leakage is probably the primary cause of the decreased SR Ca2+ content seen in such fibres (Lamboley et al. 2015). Such SR Ca2+ leakage and depletion in human fibres is analogous to that observed in muscle fibres of aged mice (Andersson et al. 2011; Umanskaya et al. 2014), and possibly may arise from a self‐reinforcing cycle in which Ca2+ leakage through the RyRs leads to increased ROS production by the mitochondria, which in turn further exacerbates RyR leakage. The increase in local [Ca2+] within the triad junction may also contribute to the decreased SR Ca2+ release observed in muscle of aged humans and rodents (Delbono et al. 1995; Wang et al. 2000) by increasing the activity of Ca2+‐dependent proteases (calpains), leading to disruption of the normal coupling between the DHPRs and the RyRs (Lamb et al. 1995; Murphy et al. 2013). Taken together with the direct effects of the SR Ca2+ depletion on Ca2+ release (Posterino & Lamb, 2003), this could be expected to have significant deleterious effects on the muscle performance in aged individuals, in addition to those arising from damage or modification of contractile apparatus properties (Lamboley et al. 2015).
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
Muscle biopsies were performed at Victoria University. Biochemical and physiological measurements on skinned fibres were conducted at La Trobe University. VLW, CRL and MJM were responsible for selection, care and testing of human subjects, and obtaining muscle biopsies from the subjects. Skinned fibre experiments were designed and analysed by GDL and CRL and carried out by CRL. RMM and GDL were responsible for western blotting procedures and analysis, with the assistance of VLW. CRL and GDL drafted the manuscript. All authors were involved in the conception of the project. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Supported by National Health & Medical Research Council of Australia (Grant number 1051460).
Acknowledgements
We thank Maria Cellini and Heidy Latchman for technical assistance. We also thank Dr Mitchell Anderson for performing muscle biopsies. The monoclonal antibodies directed against adult human MHC isoforms (A4.840 and A4.74) used in the present study were developed by Dr H. Blau; those directed against SERCA1 were developed by Dr D. Fambrough; those directed against RyR1 were developed by Drs J. Airey and J. Sutko; and those directed against DHPR were developed by Dr K. P. Campbell. All were obtained from the Development Studies Hybridoma Bank, under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA.
References
- Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A & Marks AR (2011). Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14, 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracena‐Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C & Hamilton SL (2006). Identification of cysteines involved in S‐nitrosylation, S‐glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem 281, 40354–40368. [DOI] [PubMed] [Google Scholar]
- Aracena P, Sanchez G, Donoso P, Hamilton SL & Hidalgo C (2003). S‐glutathionylation decreases Mg2+ inhibition and S‐nitrosylation enhances Ca2+ activation of RyR1 channels. J Biol Chem 278, 42927–42935. [DOI] [PubMed] [Google Scholar]
- Ballak SB, Degens H, de Haan A & Jaspers RT (2014). Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res Rev 14, 43–55. [DOI] [PubMed] [Google Scholar]
- Bottinelli R & Reggiani C (2000). Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73, 195–262. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG & Westerblad H (2008). Reactive oxygen species and fatigue‐induced prolonged low‐frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol 586, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ, Bruton JD, Lanner JT & Westerblad H (2015). Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J Physiol 593, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesi M & Inesi G (1981). Mg2+ and Mn2+ modulation of Ca2+ transport and ATPase activity in sarcoplasmic reticulum vesicles. Arch Biochem Biophys 208, 586–592. [DOI] [PubMed] [Google Scholar]
- D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B & Bottinelli R (2003). The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O, O'Rourke KS & Ettinger WH (1995). Excitation‐calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol 148, 211–222. [DOI] [PubMed] [Google Scholar]
- Eltit JM, Li H, Ward CW, Molinski T, Pessah IN, Allen PD & Lopez JR (2011). Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc Natl Acad Sci U S A 108, 7046–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero TG, Zable AC & Abramson JJ (1995). Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem 270, 25557–25563. [DOI] [PubMed] [Google Scholar]
- Hvid LG, Ortenblad N, Aagaard P, Kjaer M & Suetta C (2011). Effects of ageing on single muscle fibre contractile function following short‐term immobilisation. J Physiol 589, 4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL, Fu R & Mitchell EW (1992). Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol 73, 1854–1859. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Moreno R, Wang ZM, Gerring RC & Delbono O (2008). Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J 94, 3178–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara AA & Stephenson DG (1994). Effects of Mg2+ on Ca2+ handling by the sarcoplasmic reticulum in skinned skeletal and cardiac muscle fibres. Pflügers Arch 428, 331–339. [DOI] [PubMed] [Google Scholar]
- Klatt P & Lamas S (2000). Regulation of protein function by S‐glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267, 4928–4944. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA & Stephenson DG (2001). Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol 531, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR & Stephenson DG (1995). Raised intracellular [Ca2+] abolishes excitation‐contraction coupling in skeletal muscle fibres of rat and toad. J Physiol 489, 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD & Stephenson DG (1991). Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol 434, 507–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD & Westerblad H (2011). Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589, 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamboley CR, Murphy RM, McKenna MJ & Lamb GD (2013). Endogenous and maximal sarcoplasmic reticulum calcium content and calsequestrin expression in Type I and Type II human skeletal muscle fibres. J Physiol 591, 6053–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamboley CR, Murphy RM, McKenna MJ & Lamb GD (2014). Sarcoplasmic reticulum Ca2+ uptake and leak properties, and SERCA isoform expression, in type I and type II fibres of human skeletal muscle. J Physiol 592, 1381–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM & Lamb GD (2015). Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, O'Neill ER & Lamb GD (2004). Luminal Ca2+‐regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J Gen Physiol 124, 741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E & Pette D (1986). Immunochemical quantification of sarcoplasmic reticulum Ca‐ATPase, of calsequestrin and of parvalbumin in rabbit skeletal muscles of defined fiber composition. Eur J Biochem 156, 489–496. [DOI] [PubMed] [Google Scholar]
- Masuda K, Tanabe K, Kuno S, Hirayama A & Nagase S (2003). Antioxidant capacity in rat skeletal muscle tissues determined by electron spin resonance. Comp Biochem Physiol B Biochem Mol Biol. 134, 215–220. [DOI] [PubMed] [Google Scholar]
- Meissner G, Darling E & Eveleth J (1986). Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry 25, 236–244. [DOI] [PubMed] [Google Scholar]
- Miller MS, Callahan DM & Toth MJ (2014). Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol 5, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica JP, Dutka TL, Merry T, Lamboley C, McConell GK, McKenna MJ, Murphy RM & Lamb GD (2012). S‐glutathionylation of troponin I (fast) increases contractile apparatus Ca2+‐sensitivity in fast‐twitch muscle fibres of rats and humans. J Physiol 590, 1443–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM & Lamb GD (2013). Ca2+‐dependent proteolysis of junctophilin‐1 and junctophilin‐2 in skeletal and cardiac muscle. J Physiol 591, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM & Lamb GD (2013). Important considerations for protein analyses using antibody based techniques: down‐sizing western blotting up‐sizes outcomes. J Physiol 591, 5823–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Mollica JP & Lamb GD (2009). Plasma membrane removal in rat skeletal muscle fibers reveals caveolin‐3 hot‐spots at the necks of transverse tubules. Exp Cell Res 315, 1015–1028. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Vissing K, Latchman H, Lamboley C, McKenna MJ, Overgaard K & Lamb GD (2011). Activation of skeletal muscle calpain‐3 by eccentric exercise in humans does not result in its translocation to the nucleus or cytosol. J Appl Physiol 111, 1448–1458. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Cellini MA & Lamb GD (2003). Effects of oxidation and cytosolic redox conditions on excitation‐contraction coupling in rat skeletal muscle. J Physiol 547, 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS & Lamb GD (1996). Effects of reducing agents and oxidants on excitation‐contraction coupling in skeletal muscle fibres of rat and toad. J Physiol 496, 809–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS & Lamb GD (2003). Effect of sarcoplasmic reticulum Ca2+ content on action potential‐induced Ca2+ release in rat skeletal muscle fibres. J Physiol 551, 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck RT, Karunasekara Y, Board PG, Beard NA, Casarotto MG & Dulhunty AF (2014). Skeletal muscle excitation‐contraction coupling: who are the dancing partners? Int J Biochem Cell Biol 48, 28–38. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML & Delbono O (1997). Dihydropyridine receptor‐ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol 157, 247–253. [DOI] [PubMed] [Google Scholar]
- Robin G & Allard B (2012). Dihydropyridine receptors actively control gating of ryanodine receptors in resting mouse skeletal muscle fibres. J Physiol 590, 6027–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, Butler‐Browne G, Erzen I, Mouly V, Thornell LE, Wernig A & Ohlendieck K (2003). Persistent expression of the alpha1S‐dihydropyridine receptor in aged human skeletal muscle: implications for the excitation‐contraction uncoupling hypothesis of sarcopenia. Int J Mol Med 11, 425–434. [PubMed] [Google Scholar]
- Ryan M, Carlson BM & Ohlendieck K (2000). Oligomeric status of the dihydropyridine receptor in aged skeletal muscle. Mol Cell Biol Res Commun 4, 224–229. [DOI] [PubMed] [Google Scholar]
- Umanskaya A, Santulli G, Xie W, Andersson DC, Reiken SR & Marks AR (2014). Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci U S A 111, 15250–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Messi ML & Delbono O (2000). L‐Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys J 78, 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H & Allen DG (1992). Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 453, 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KD & Lytton J (1993). Molecular cloning and quantification of sarcoplasmic reticulum Ca(2+)‐ATPase isoforms in rat muscles. Am J Physiol Cell Physiol 264, C333–C341. [DOI] [PubMed] [Google Scholar]
- Yu F, Hedstrom M, Cristea A, Dalen N & Larsson L (2007). Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 190, 229–241. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yi J, Royer L, Launikonis BS, Gonzalez A, Garcia J & Rios E (2006). A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am J Physiol Cell Physiol 290, C539–C553. [DOI] [PubMed] [Google Scholar]


