Key points
Oral consumption of nitrate (NO3 −) in beetroot juice has been shown to decrease the oxygen cost of submaximal exercise; however, the mechanism of action remains unresolved.
We supplemented recreationally active males with beetroot juice to determine if this altered mitochondrial bioenergetics.
Despite reduced submaximal exercise oxygen consumption, measures of mitochondrial coupling and respiratory efficiency were not altered in muscle.
In contrast, rates of mitochondrial hydrogen peroxide (H2O2) emission were increased in the absence of markers of lipid or protein oxidative damage.
These results suggest that improvements in mitochondrial oxidative metabolism are not the cause of beetroot juice‐mediated improvements in whole body oxygen consumption.
Abstract
Ingestion of sodium nitrate (NO3 −) simultaneously reduces whole body oxygen consumption () during submaximal exercise while improving mitochondrial efficiency, suggesting a causal link. Consumption of beetroot juice (BRJ) elicits similar decreases in but potential effects on the mitochondria remain unknown. Therefore we examined the effects of 7‐day supplementation with BRJ (280 ml day−1, ∼26 mmol NO3 −) in young active males (n = 10) who had muscle biopsies taken before and after supplementation for assessments of mitochondrial bioenergetics. Subjects performed 20 min of cycling (10 min at 50% and 70% ) 48 h before ‘Pre’ (baseline) and ‘Post’ (day 5 of supplementation) biopsies. Whole body decreased (P < 0.05) by ∼3% at 70% following supplementation. Mitochondrial respiration in permeabilized muscle fibres showed no change in leak respiration, the content of proteins associated with uncoupling (UCP3, ANT1, ANT2), maximal substrate‐supported respiration, or ADP sensitivity (apparent K m). In addition, isolated subsarcolemmal and intermyofibrillar mitochondria showed unaltered assessments of mitochondrial efficiency, including ADP consumed/oxygen consumed (P/O ratio), respiratory control ratios and membrane potential determined fluorometrically using Safranine‐O. In contrast, rates of mitochondrial hydrogen peroxide (H2O2) emission were increased following BRJ. Therefore, in contrast to sodium nitrate, BRJ supplementation does not alter key parameters of mitochondrial efficiency. This occurred despite a decrease in exercise , suggesting that the ergogenic effects of BRJ ingestion are not due to a change in mitochondrial coupling or efficiency. It remains to be determined if increased mitochondrial H2O2 contributes to this response.
Key points
Oral consumption of nitrate (NO3 −) in beetroot juice has been shown to decrease the oxygen cost of submaximal exercise; however, the mechanism of action remains unresolved.
We supplemented recreationally active males with beetroot juice to determine if this altered mitochondrial bioenergetics.
Despite reduced submaximal exercise oxygen consumption, measures of mitochondrial coupling and respiratory efficiency were not altered in muscle.
In contrast, rates of mitochondrial hydrogen peroxide (H2O2) emission were increased in the absence of markers of lipid or protein oxidative damage.
These results suggest that improvements in mitochondrial oxidative metabolism are not the cause of beetroot juice‐mediated improvements in whole body oxygen consumption.
Abbreviations
- ANT
adenine nucleotide translocase
- BRJ
beetroot juice
- IMF
intermyofibrillar mitochondria
- PDH
pyruvate dehydrogenase
- PmFB
permeabilized muscle fibre bundles
- P/O ratio
ADP consumed/oxygen consumed
- RCR
respiratory control ratio
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SS
subsarcolemmal mitochondria
- UCP3
uncoupling protein 3
Introduction
Nitric oxide (NO) is an important signalling molecule; it has been implicated in the regulation of many biological processes, including blood flow (as reviewed by Joyner & Tschakovsky, 2003), skeletal muscle excitation–contraction coupling (Andrade et al. 1998) and mitochondrial bioenergetics (Clerc et al. 2007; Boushel et al. 2012). While NO was originally thought to be exclusively generated from nitric oxide synthase‐mediated oxidation of l‐arginine (Moncada & Higgs, 1993), it is now acknowledged that NO can also be generated from the sequential reduction of nitrate (NO3 −) and nitrite (NO2 −) in a process dependent on bacterial nitrate reductases produced by facultative anaerobes in the oral cavity (Spiegelhalder et al. 1976; Govoni et al. 2008).
Given the diverse biological effects of NO, the oral consumption of NO3 − has been investigated as a modality to alter metabolic responses to exercise. Indeed, amongst dietary supplements, nitrate is unique in that it decreases the oxygen cost of submaximal exercise in humans (Larsen et al. 2007, 2011; Bailey et al. 2009, 2010; Vanhatalo et al. 2010; Lansley et al. 2011; Cermak et al. 2012). While the mechanism(s) of action remain unresolved, one prominent working model is an improvement in mitochondrial coupling efficiency (Larsen et al. 2011). This possibility is supported by the in vitro observations that NO directly increases oxidative phosphorylation efficiency (Clerc et al. 2007), particularly when cytochrome oxidase is reduced (Bolaños et al. 1994; Brown & Cooper, 1994; Cleeter et al. 1994; Giuffrè et al. 2000). In addition, dietary supplementation with sodium nitrate for 3 days in humans improves various parameters within isolated mitochondria, including assessments of efficiency (P/O ratios and thermodynamic coupling) (Larsen et al. 2011). Altogether, sodium nitrate consumption and NO appear to have the capacity to improve mitochondrial bioenergetics through alterations in coupling efficiency.
Several vegetables have high NO3 − concentrations, creating the potential for a natural food product to exert similar beneficial effects to sodium nitrate. To date, beetroot juice (BRJ) has been shown to reduce blood pressure (Webb et al. 2008; Bailey et al. 2009, 2010; Vanhatalo et al. 2010; Lansley et al. 2011), oxygen consumption during exercise (Bailey et al. 2009, 2010; Vanhatalo et al. 2010; Wylie et al. 2013 a), and improve exercise performance (Lansley et al. 2011 a, b ; Bond et al. 2012; Cermak et al. 2012; Wylie et al. 2013 b; Peeling et al. 2015), vascular control (Ferguson et al. 2013), and muscle contractile function in humans (Fulford et al. 2013; Haider & Folland, 2014). Indirect assessments of muscle metabolism via 31P‐magnetic resonance spectroscopy suggest that BRJ consumption reduces ATP turnover during muscle contraction, which probably contributes to the observed attenuation in oxygen consumption during exercise (Bailey et al. 2010). It remains to be determined if BRJ, similar to sodium NO3 −, improves mitochondrial oxidative phosphorylation efficiency to further explain the observed reduction in whole body oxygen consumption.
In addition to exercise performance, NO3 − supplements are being investigated as potential therapeutic interventions for several clinical populations, including chronic obstructive pulmonary disease (Kerley et al. 2015), hypertension (Kapil et al. 2015), heart failure (Zamani et al. 2015; Ormerod et al. 2015) and insulin resistance (Nyström et al. 2012). Understanding the mechanism(s) by which BRJ alters excitation–contraction coupling/metabolism is therefore essential to ensure that undesirable ‘off‐target’ effects do not occur. For instance, the reported reduction in adenine nucleotide translocase (ANT) proteins and uncoupling protein 3 (UCP3), and the increase in coupling efficiency following sodium nitrate consumption (Larsen et al. 2011), may increase proton motive force and mitochondrial reactive oxygen species (ROS) emission. ANT has previously been shown to contribute between one‐half and two‐thirds of basal mitochondrial leak respiration (Brand et al. 2005) and a reduction in ANT protein has been associated with impairments in submaximal ADP sensitivity and oxidative stress (Smith et al. 2013).
Therefore, the primary aim of the current study was to determine if BRJ altered various indices of mitochondrial bioenergetics. The current data suggest that an improvement in mitochondrial oxidative efficiency is not required for BRJ‐mediated reductions in whole body . However, an increase in mitochondrial H2O2 emission was observed in both permeabilized muscle fibres (PmFB) and in isolated mitochondria following supplementation, suggesting BRJ can modify mitochondrial bioenergetics.
Methods
Ethics approval
Thirteen healthy, recreationally active males (23.6 ± 0.6 years, 79.4 ± 1.5 kg, 49.6 ± 1.4 ml−1 min−1 kg−1) were initially recruited for this study, of which 10 underwent biopsy trials both prior to, and following, supplementation. Prior to participation, written informed consent was obtained from all subjects. All procedures were approved by the Research Ethics Boards of the University of Guelph (Guelph, Ontario) and McMaster University (Hamilton, Ontario), and conform to the Declaration of Helsinki.
Pre‐experimental tests
Prior to supplementation, subjects underwent a continuous incremental cycling test to exhaustion on an electronically braked cycle ergometer (Lode, Groningen, the Netherlands) for determination of pulmonary , as well as a 20 min cycle test with 10 min at a workload corresponding to 50% followed by an immediate step increase to a workload corresponding to 70% for an additional 10 min. For familiarization, subjects performed a practice trial of the 20 min test, which also served to confirm subjects were at the correct workloads. Trials were separated by 48 h. Ventilation and expired oxygen and carbon dioxide concentrations were measured throughout all tests using a metabolic measurement system (MOXUS Modular VO2 System, AEI Technologies, Pittsburgh, PA, USA), and the last 5 min at each power output was used for analysis (Fig. 1).
Figure 1. Schematic overview of the experimental protocol .
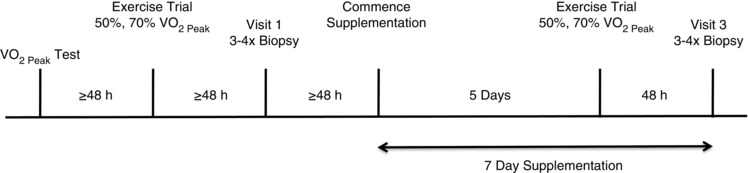
Subjects had biopsies taken at rest and on the morning of day 7 following the completion of 7 days of beetroot juice supplementation. On day 5 of the supplementation period, subjects performed submaximal cycling exercise at 50 and 70% of VO2.
Study design
Following the completion of pre‐experimental tests (≤48 h) and an overnight fast, 10 subjects had four biopsies taken from the vastus lateralis as previously described (Bergstrom, 1975). A small portion of the first biopsy was taken and immediately used for the preparation of permeabilized muscle fibres (PmFB; described below). The remaining portion was combined with the second and third biopsies for the isolation of mitochondrial subfractions. The fourth biopsy was flash‐frozen for Western blotting. Due to limited tissue yield, mitochondrial isolations and Western blotting could only be performed on a subset of five participants. Following the biopsies, subjects began supplementing with concentrated BRJ (2 × 6.5 mmol NO3 −/70 ml Beet It Sport; James White Drinks, Ipswich, UK; taken twice daily for a total daily intake of ∼26 mmol NO3 −) for 7 days in an effort to maintain elevated blood plasma NO3 − and NO2 − levels. On day 5 of the supplementation period, subjects repeated the submaximal cycling exercise at 50 and 70% of . On the morning of day 7 after an overnight fast, subjects consumed 2 × Beet It Sport shots 90 min prior to having four biopsies taken from the vastus lateralis of the contralateral leg for post‐supplementation measurements. Subjects were asked to refrain from consuming foods rich in nitrates for the duration of the study, and to abstain from the use of antibacterial mouthwash, as this has been shown to attenuate the conversion of nitrate to nitrite by commensal bacteria (Govoni et al. 2008).
Preparation of permeabilized fibres
A small portion of each biopsy was placed in ice‐cold BIOPS (50 mm MES, 7.23 mm K2EGTA, 2.77 mm CaK2EGTA, 20 mm imidazole, 0.5 mm DTT, 20 mm taurine, 5.77 mm ATP, 15 mm PCr, and 6.56 mm MgCl2.H2O; pH 7.1) and separated under a microscope into bundles using fine‐tipped forceps as described previously (Perry et al. 2012). Fibre bundles were then treated with 30 μg ml−1 saponin for 30 min at 4°C, then either washed for 15 min in MiR05 respiration buffer (0.5 mm EGTA, 10 mm KH2PO4, 110 mm sucrose, and 1 mg ml−1 fatty acid‐free bovine serum albumin (BSA); pH 7.1) for respiration analysis, or buffer Z (105 mm K‐Mes, 30 mm KCl, 1 mm EGTA, 10 mm K2HPO4, 5 mm MgCl2.H2O, 0.005 mm pyruvate, 0.002 mm malate with 5 mg ml−1 fatty acid‐free BSA; pH 7.4) for measurements of H2O2 emission as described below.
Mitochondrial isolation
Mitochondrial subsarcolemmal (SS) and intermyofibrillar (IMF) populations were isolated by differential centrifugation as described previously (Campbell et al. 2004) with minor modifications (Matravadia et al. 2014). Muscle was quickly minced and diluted in 1 ml mg−1 isolation buffer (100 mm sucrose, 100 mm KCl, 50 mm Tris HCl, 1 mm KH2PO4, 0.1 mm EGTA, 2 mg ml−1 fatty acid‐free BSA, 1 mm ATP; pH 7.4) and homogenized using a Polytron with a Teflon head. SS mitochondria underwent centrifugation at 800 g for 10 min and the supernatant was pelleted at 10,000 g for 10 min before being washed in Mg2+‐absent MiR05 buffer (0.5 mm EGTA, 60 mm potassium lactobionate, 10 mm KH2PO4, 20 mm HEPES, 110 mm sucrose, 1 mg ml−1 BSA; pH 7.1), and spun again at 10,000 g for 10 min. After the initial 800 g spin, the IMF mitochondrial pellet was resuspended 10‐fold in isolation buffer and treated with 0.025 ml g−1 protease for 5 min before being diluted 10‐fold in isolation buffer and spun again at 5000 g for 5 min. The IMF pellet was resuspended in isolation buffer and pelleted at 800 g for 10 min after which the supernatant was pelleted at 10,000 g for 10 min before being washed in Mg2+‐absent MiR05 buffer, and spun again at 10,000 g for 10 min. The final pellets for both SS and IMF isolations were resuspended in Mg2+‐absent MiR05 buffer prior to respiration, H2O2 emission and membrane potential measurements. Protein content was quantified spectrophotometrically using bicinchroninic acid.
Mitochondrial respiration
Measurements of O2 consumption were performed in MiR05 respiration medium on prepared permeabilized fibres using an Oxygraph‐2 K (Oroboros Instruments, Innsbruck, Austria) at 37°C in the presence of 25 μm blebbistatin as previously described (Perry et al. 2011). Mitochondrial kinetics in PmFB were analysed using three separate titration protocols. ADP kinetics were analysed in the presence and absence of 20 mm creatine. Both ADP titrations were initiated with 2 mm malate and 10 mm pyruvate, and ADP was titrated at various concentrations. Pyruvate kinetics were determined in the presence of 5 mm ADP, 5 mm malate, and 20 mm creatine while pyruvate was titrated in at various concentrations. Glutamate (10 mm) and 10 mm succinate were added following the pyruvate and ADP titrations to determine maximum mitochondrial respiration. Finally, 10 μm cytochrome c was added, with <10% increase in respiration in all measurements indicating outer mitochondrial membrane integrity. All fibres were recovered from the respirometer, freeze‐dried, and weighed for normalization to muscle bundle weight. The apparent K m for pyruvate and ADP were determined as previously described (Perry et al. 2012).
In isolated mitochondria, respiration experiments were performed at 25°C as described previously (Lally et al. 2013). State IV respiration was determined in the presence of 5 mm pyruvate and 2 mm malate. To determine P/O ratios, 50 μm ADP was added followed by two boluses of 100 μm ADP. The change in oxygen concentration in the chamber following each of the two 100 μm ADP boluses was averaged and used for the calculation of P/O ratios. Maximal state III respiration was determined by the addition of 5 mm ADP, with 10 mm glutamate and 10 mm succinate added to determine maximal complex I and maximal complex I + II respiration, respectively.
H2O2 emission
Mitochondrial H2O2 emission in PmFB was measured in buffer Z (105 mm K‐MES, 30 mm KCl, 1 mm EGTA, 10 mm K2HPO4, 5 mm MgCl2.H2O, 0.005 mm pyruvate, 0.002 mm malate with 5 mg ml−1 fatty acid‐free BSA; pH 7.4) fluorometrically (Lumina; Thermo Scientific, Waltham, MA, USA) at 37°C in the presence of 25 μm blebbistatin. Cuvettes contained 10 μg ml−1 oligomycin, 10 μm Amplex Red (Invitrogen, Carlsbad, CA, USA), 0.5 U ml−1 horseradish peroxidase, and in both the presence and absence of 40 U ml−1 superoxide dismutase (SOD). In isolated mitochondria, H2O2 emission was measured in MiR05 at 25°C in cuvettes containing 10 μg ml−1 oligomycin, 10 μm Amplex Red (Invitrogen), 0.5 U ml−1 horseradish peroxidase, and 40 U ml−1 SOD. The reaction for both PmFB and isolated mitochondria was initiated by the addition of 10 mm succinate. The rate of H2O2 emission was calculated from the slope (absorbance/minutes) after subtracting the background, from a standard curve established with the same reaction conditions. In PmFB, H2O2 rates were normalized to fibre dry weight as performed previously (Anderson et al. 2009), and in isolated mitochondria were normalized to protein content.
Membrane potential
Mitochondrial membrane potential was measured fluorometrically at 25°C in cuvettes filled with MiR05 and 2 μm Safranine‐O as described previously (Goo et al. 2013), with some modifications. Briefly, mitochondria were added to cuvettes followed by the addition of 10 mm pyruvate and 5 mm malate (state IV), 2.5 mm ADP (state III), 10 mm glutamate (maximal complex I), and 10 mm succinate (maximal complex I + II). Mitochondria were uncoupled by the addition of 2 μl 2,4‐dinitrophenol (DNP), followed by the addition of Safranine‐O (2 × 1 μm) for calibration of the fluorescence signal. Membrane potential was then estimated using the Nernst equation:
where R is the universal gas constant, F is Faraday's constant, T is absolute temperature, and z is the valency (+1) of Safranine.
Western blotting
Western blotting was performed on whole muscle homogenate using methods described previously (Herbst et al. 2014). Samples were loaded equally for α‐tubulin (Abcam, Cambridge, UK), UCP3 (Millipore, Billerica, MA, USA), ANT1 (Mitosciences, Eugene, OR, USA), ANT2 (Abcam), SOD2 (Abcam), catalase (Abcam), 4‐hydroxynonenal (4HNE; Alpha Diagnostics, Antonio, TX, USA), pyruvate dehydrogenase (PDH) subunit E1α (Molecular Probes, Eugene, OR, USA), oxidative phoshorylation (OXPHOS; Mitosciences), and for the Oxyblot protein oxidation detection kit (Millipore) and N3‐nitrosylation (Cayman Chemical, Ann Arbor, MI, USA). Proteins were separated by SDS‐polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and were incubated in blocking solution, primary antibody, and the corresponding secondary antibody as specified by the supplier. All samples were detected from the same Western blot by cutting gels and transferring onto a single membrane to limit variability, and all gels were loaded with 10 μg protein. Linearity of signal was confirmed from 5–15 μg of protein. Membrane proteins were detected by enhanced chemiluminesence (Perkin Elmer, Woodbridge, ON, Canada) and quantified by densitometry (Alpha Innotech Fluorchem HD2; Fisher Scientific, Ottawa, ON, Canada).
Plasma analyses
Five separate subjects repeated the supplementation protocol for the collection of blood plasma. Subjects reported to the lab following an overnight fast, and had blood samples taken immediately prior to consuming their first bolus of BRJ. Samples were then taken again 1.5 and 3 h after ingestion. This process was repeated again in the evening, and on day 6 of the supplementation period. Blood samples (8 ml) were collected in EDTA‐containing tubes and centrifuged at 1000 g for 10 min at 4°C. Plasma aliquots were frozen and stored at −80°C for subsequent analysis of plasma nitrate (NO3 −) and nitrite (NO2 −) by chemiluminescence using a Sievers gas‐phase chemiluminescence NO analyser (Sievers NOA 280i; Analytix, Durham, UK) (Lundberg & Govoni, 2004).
Statistics
A two‐way analysis of variance was used to detect differences between day 1, day 6, and sampling time‐points for blood plasma NO3 − and NO2 − measurements. If significance was detected, a Fischer LSD post hoc test was applied. A Student's paired t test (two‐way) was used to detect differences between pre‐ and post‐supplementation data in submaximal exercise trials, and in isolated mitochondria and permeabilized muscle fibre experiments. Significance was determined at P < 0.05 with confidence intervals ≥95%.
Results
Whole body oxygen consumption and energy expenditure
We first characterized the pulmonary responses during exercise to ensure that the supplementation with BRJ coincided with the expected metabolic effect. Oxygen consumption was unaltered while exercising at 50% (Fig. 2 A). In contrast, oxygen consumption while exercising at 70% decreased ∼100 ml following BRJ consumption, representing ∼3% of oxygen consumption (Fig. 2 B). It is worth noting that of the 13 participants tested in the exercise trials, 8 showed a decrease in pulmonary at 50% (Fig. 2 A), and 12 of 13 subjects decreased at 70% (Fig. 2 B). Despite the change in oxygen consumption at 70% , there was no change in respiratory exchange ratio (Pre, 0.99 ± 0.01; Post, 0.99 ± 0.01), indicating that there was no change in fuel preference following BRJ consumption.
Figure 2.
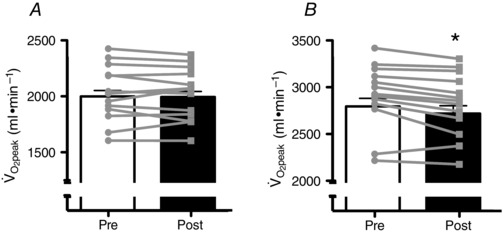
Inset represents the individual responses. Values are means ± SEM. *P < 0.05. n = 13.
Respiration in permeabilized fibres
Given the change in oxygen consumption, we next aimed to determine if BRJ supplementation altered various indices of mitochondrial bioenergetics. Supplementation did not alter leak respiration (non‐ADP‐stimulated respiration; Fig. 3 A) or maximal substrate‐supported respiration (Fig. 3 A). In support of a lack of change in maximal respiration, the content of various electron transport chain proteins and PDH was not altered (Fig. 3 B and C). A previous paper found that sodium nitrate supplementation decreased ANT protein content (Larsen et al. 2011), and therefore we next examined if BRJ altered mitochondrial ADP sensitivity. Titrating ADP in the presence of substrates revealed an expected Michaelis–Menten curve (insets in Fig. 4 A and D) that was utilized to estimate the ADP sensitivity (apparent K m). BRJ supplementation did not alter maximal respiration (Fig. 4 B and E) or the apparent K m (Fig. 4 C and E) in the presence or absence of creatine. In support of this, ANT1, ANT2 and UCP3 protein contents were unaltered following supplementation (Fig. 4 G and H). To further examine the potential effect of BRJ supplementation on electron transport function we determined the kinetic properties of pyruvate to stimulate respiration in the presence of saturating ADP concentrations. Unfortunately some of the permeabilized fibre bundles could not be recovered for dry weight assessments following respiration experiments, and therefore only kinetic properties were examined and not maximal respiration. Pyruvate displayed a typical Michaelis–Menten curve (Fig. 5 A); however, BRJ did not alter the apparent sensitivity of mitochondria to pyruvate (Fig. 5 B). Altogether these data suggest that BRJ, while decreasing oxygen consumption during exercise, does not improve mitochondrial respiratory measures in permeabilized muscle fibres.
Figure 3. Mitochondrial capacity in permeabilized fibres (A) and the expression of oxidative phosphorylation (OXPHOS) proteins (B and C) .
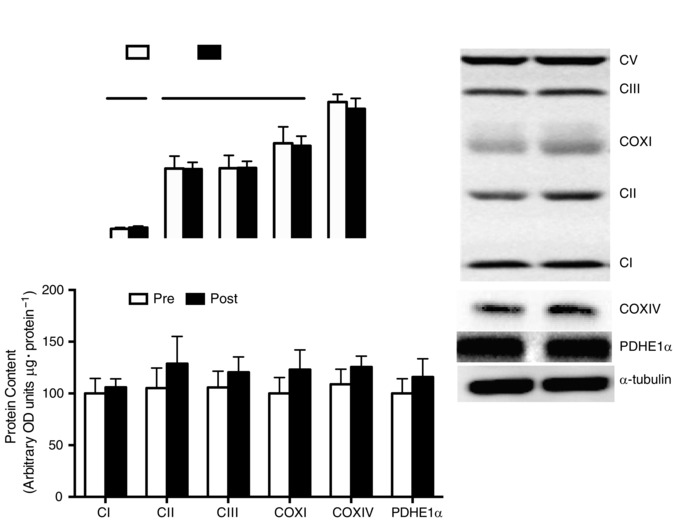
In A, represents mitochondrial O2 flux. In C, OXPHOS proteins are expressed in arbitrary optical density (OD) units (μg total protein)−1. PM: 10 mm pyruvate + 2 mm malate either without (leak, −ADP) or with (state III, +ADP) 5 mm ADP. PMG: state III maximal complex 1 linked, 10 mm pyruvate + 2 mm malate + 5 mm ADP + 10 mm glutamate. PMGS: max complex 1 and 2 linked, 10 mm pyruvate + 2 mm malate + 5 mm ADP + 10 mm glutamate + 10 mm succinate. Values are means ± SEM. n = 10 for PmFB, n = 6 for Western blot data.
Figure 4. ADP titrations either in the presence (A–C) or absence (D–F) of creatine with pyruvate and malate present in the respiration media, and the expression of protein targets contributing to mitochondrial membrane potential (G and H) .
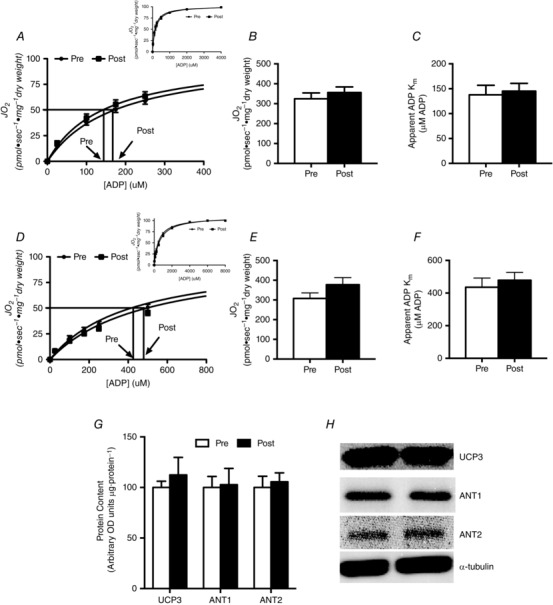
The corresponding V max (B and E) indicated there was no change in mitochondrial capacity, and no change in the apparent K m indicating no change in sensitivity to ADP (C and F). Values are means ± SEM. n = 10 for PmFB, n = 6 for Western blot data.
Figure 5. Pyruvate titrations with malate and ADP present in the respiration media .
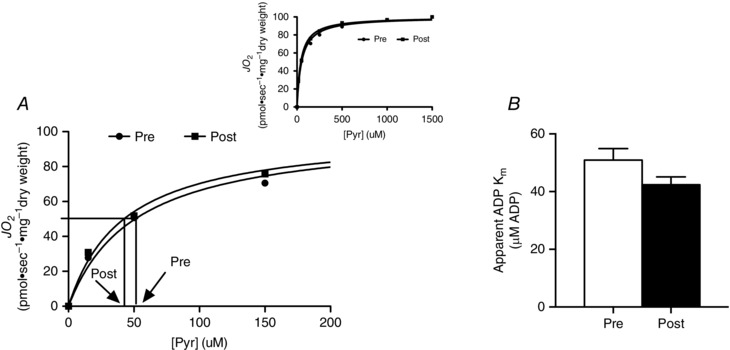
There was no change in the apparent K m indicating no change in sensitivity to pyruvate. Values are means ± SEM. n = 10.
Respiration and membrane potential in isolated mitochondria
Given the lack of change in mitochondrial respiration, in a subset of participants (n = 5) both subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondrial subpopulations were isolated to directly examine mitochondrial coupling efficiencies. BRJ supplementation did not alter the P/O ratio (Fig. 6 A and B), or the respiratory control ratio (RCR) (Fig. 6 C and D) in SS or IMF mitochondria. Furthermore, there was no correlation between the measured coupling ratios in either subpopulation and the change in whole body at either power output (50%: R 2 = 0.04 SS, 0.003 IMF; 70%; R 2 = 0.008 SS, 0.119 IMF). In addition, changes in membrane potential (ΔΨ) were estimated fluorometrically using Safranine‐O. In both SS and IMF mitochondria, ΔΨ decreased following the addition of substrates and ADP (Fig. 7 A and B); however, there were no differences in either fraction following supplementation. These data further support the interpretation that a reduction in whole body oxygen consumption during exercise is independent of improvements in mitochondrial respiration/coupling.
Figure 6. Measures of mitochondrial efficiency (P/O ratio, A and B) and coupling (RCR, C and D) in SS and IMF mitochondria .
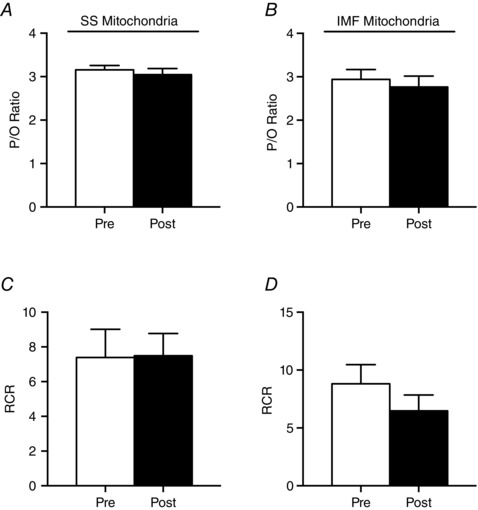
Values are means ± SEM. n = 5.
Figure 7. Membrane potential measured using Safranine‐O in SS (A) and IMF (B) mitochondrial subpopulations .
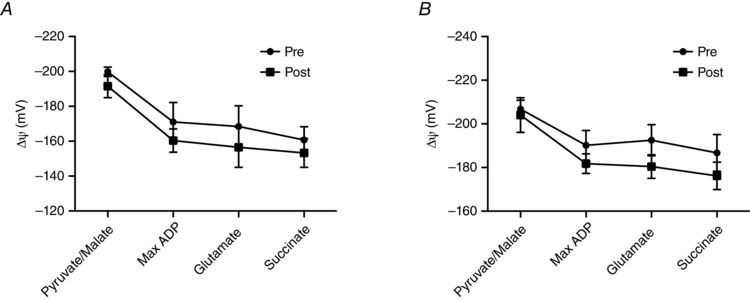
Pyruvate/malate: 10 mm pyruvate + 5 mm malate. ADP: 10 mm pyruvate + 5 mm malate + 2.5 mm ADP. Glutamate: 10 mm pyruvate + 5 mm malate + 2.5 mm ADP + 10 mm glutamate. Succinate: 10 mm pyruvate + 5 mm malate + 2.5 mm ADP + 10 mm glutamate + 10 mm succinate. Values are means ± SEM. n = 5.
H2O2 emission and oxidative stress
Given that NO can inhibit cytochrome oxidase (Bolaños et al. 1994; Brown & Cooper, 1994; Cleeter et al. 1994; Giuffrè et al. 2000), we also examined the possibility that BRJ could increase the propensity of mitochondria to produce ROS. In permeabilized fibres, maximal succinate‐induced mitochondrial H2O2 emission increased ∼50% following supplementation in the absence (Fig. 8 A) and presence (Fig. 8 B) of exogenous superoxide dismutase (SOD). The presence of exogenous SOD did not increase H2O2 emission (Fig. 8 A and B), suggesting the Qo site of complex III does not release superoxide into the intermembrane space or contribute to succinate‐induced ROS in humans. This supports the notion that reverse electron flow to complex I represents the majority of superoxide production in the presence of succinate. However, there was no correlation between the propensity to produce H2O2 and the change in whole body either with (R 2 = 0.03 at 50%, 0.115 at 70%) or without (R 2 = 0.08 at 50%, 0.209 at 70%) exogenous SOD. Within IMF mitochondria, which represent ∼80% of the mitochondria within muscle (Hoppeler et al. 1985; Ferreira et al. 2010), there was also a strong trend for an increase in H2O2 emission (P = 0.07). However, there was no increase within SS mitochondria (Fig. 8 C and D). The increased propensity to produce ROS within permeabilized fibres did not result from a reduction in either catalase or SOD2 (Fig. 8 E and F) and did not manifest with overt oxidative stress, as indicated by a lack of change in lipid peroxidation (4HNE), protein carbonylation or nitrotyrosine residues in muscle homogenates (Fig. 8 G and H).
Figure 8. Propensity for succinate‐supported mitochondrial ROS emission before (Pre) and after (Post) beetroot juice supplementation .
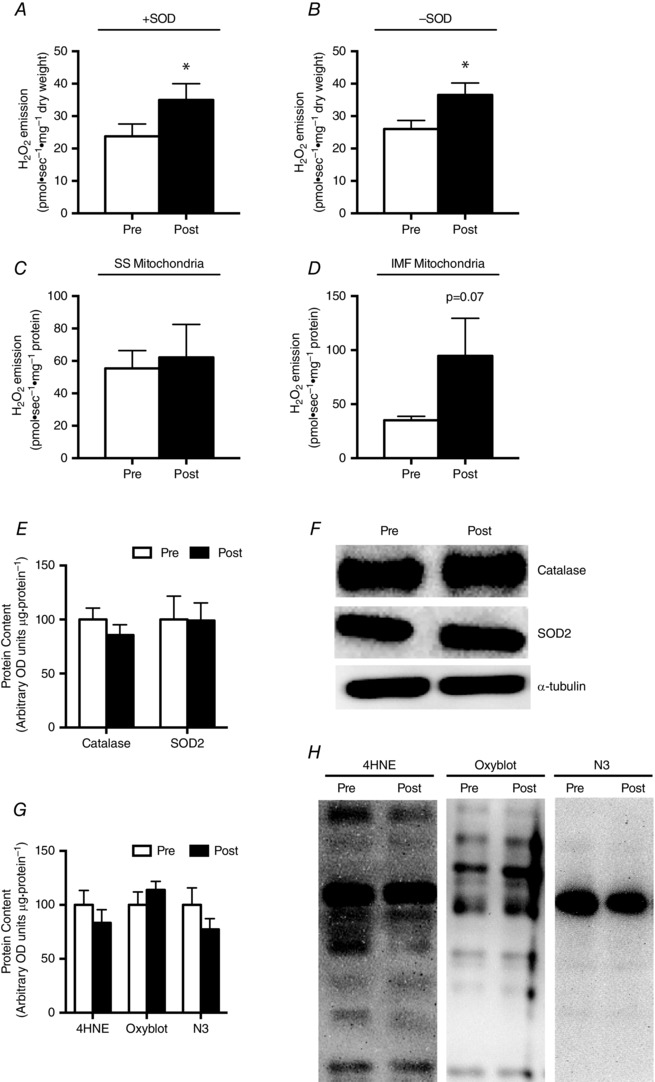
Permeabilized fibre traces were performed in the presence (A) and absence (B) of 40 U/ml exogenous SOD with 20 mm succinate and 25 μm blebbistatin present in the media. Isolated mitochondria traces for SS (C) and IMF (D) were performed in the presence of 40 U/ml of exogenous SOD and 8 mm succinate. Values are means ± SEM. *P < 0.05. n = 10 for PmFB, n = 5 for isolated mitochondria.
Temporal effects of beetroot juice consumption on blood nitrate and nitrite concentrations
Despite the observed decrease in whole body and increase in muscle mitochondrial ROS emission, given the lack of response with respect to mitochondrial coupling we wanted to confirm that the dosing protocol resulted in increases in plasma NO3 − and NO2 −. Therefore in separate participants we re‐ran the study to only measure plasma responses. In these individuals we characterized the blood NO3 − and NO2 − responses during the first and last full day of the supplementation period. In general, both NO3 − (Fig. 9 A) and NO2 − (Fig. 9 B) concentrations increased (P < 0.05) within 90 min, and remained elevated 2 h following ingestion in the morning and in the evening. The basic response to BRJ consumption was not dramatically altered when measured following 6 days of supplementation. However, NO3 − levels were higher in the morning on day 6 (Fig. 9 A), such that NO3 − was higher in the morning at all time points examined, in association with a strong trend (P = 0.07) for an increase in NO2 − (Fig. 9 B) 90 min following ingestion. In the evening, NO3 − was not dramatically different on day 6, while NO2 − was lower 120 min after the consumption of BRJ (Fig. 9 A and B). Therefore, the plasma responses indicate that the dosing protocol used in the current study was sufficient to maintain elevated plasma NO3 − and NO2 − concentrations throughout the experiment.
Figure 9. Plasma nitrate concentration ([NO3−)]; A) and nitrite concentration ([NO2−]); B) following consumption of beetroot juice (13 mmol NO3−) in the morning and in the evening .
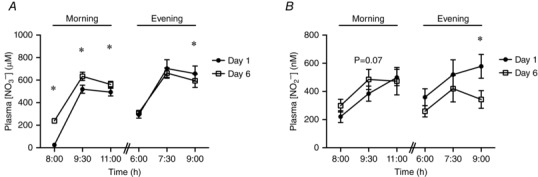
Baseline samples were taken prior to the morning and evening supplementation (8.00 am and 6.00 pm, respectively), and at 90 min and 3 h post‐consumption. Values are means ± SEM. *P < 0.05 vs. same time‐point on day 1. n = 6.
Discussion
In the current study we show that BRJ consumption, while decreasing the oxygen utilization of submaximal exercise, did not alter various indices of mitochondrial coupling/respiratory responses. Specifically, state IV and III respirations were not altered in either isolated SS and IMF mitochondria or permeabilized muscle fibres, and coupling (P/O) ratios and proton motive force were also unaffected by BRJ consumption. There was also no correlation between the measured coupling ratios in either subpopulation and the change in whole body at either power output. These data are in stark contrast to a previous publication utilizing sodium nitrate consumption, which reported improved coupling efficiency within pooled isolated mitochondria (Larsen et al. 2011). This previous paper argued that a reduction in UCP3 and ANT protein content accounted for the improvement in the observed P/O ratios. However, in the current study UCP3 and ANT proteins were not reduced and the apparent mitochondrial ADP respiratory sensitivity remained unaltered. Therefore, it appears that although sodium nitrate and BRJ both reduce whole body oxygen consumption during exercise, they may work through divergent mechanisms of action.
Previous work using 31P‐magnetic resonance spectroscopy (Bailey et al. 2010) suggested that BRJ reduces ATP turnover rate during exercise. Combined with the current observation that BRJ does not alter mitochondrial coupling, these results strongly suggest an improvement in excitation–contraction efficiency (i.e. increased work/ATP consumption) is responsible for the reduction in following BRJ. Considering NO has been shown to increase calcium ATPase (SERCA) efficiency (Ca2+ uptake/ATPase activity; Ishii et al. 1998), and oral NO3 − consumption increases intracellular calcium handling and force production in rodents (Hernández et al. 2012) as well as low‐frequency force generation and rates of relaxation in humans (Haider & Folland, 2014), future work should focus on the potential for BRJ to alter SERCA properties. While we have not provided a mechanism of action in the current study to account for the reduction in during exercise, the present data strongly suggest that alterations in mitochondrial efficiency are not required for the observed whole body responses.
While indices of mitochondrial oxygen consumption were not altered following BRJ consumption, we have provided evidence that BRJ supplementation increases the capacity for mitochondrial ROS emission. The functional consequence of an increased propensity for mitochondrial H2O2 emission following BRJ consumption is not currently known. Furthermore, in resting muscle neither BRJ (current study) nor sodium nitrate (Larsen et al. 2014) increased measures of overt oxidative stress within skeletal muscle, and therefore it remains unclear if the observed increase in the in vitro capacity for ROS emission translates to a functional effect in vivo. This is further emphasized by the fact that the change in whole body and the increase in H2O2 emission did not significantly correlate. However, the study of redox biology is complex and ROS‐induced signalling can occur in the absence of overt oxidative damage. As a result, it remains possible that an increase in mitochondrial ROS could contribute to the observed reduction in whole body following BRJ consumption. In support of this, H2O2 has been shown to directly interact with the contractile apparatus to increase force production in the absence of changes in cytosolic calcium concentrations (Andrade et al. 1998), and H2O2 can directly increase the mechanical efficiency of skeletal muscle (Andrade et al. 2001). However, H2O2 has also been shown to decrease myofibrillar calcium sensitivity and muscle force production (Moopanar & Allen, 2005), a process that appears to require thiol alterations (Moopanar & Allen, 2006). Therefore, while the potential mechanistic role of increased mitochondrial H2O2 in the observed reduction in whole body during exercise remains speculative, it is possible that the redox signalling initiated in resting muscle following BRJ supplementation may augment the response to exercise. Clearly, future work is required to elucidate how BRJ alters whole body during exercise; however, if ROS contributes as a signalling molecule in these responses this may explain the relative insensitivity to BRJ in elite athletes (Bescós et al. 2012; Christensen et al. 2013; Boorsma et al. 2014), as these individuals have increased skeletal muscle antioxidant enzyme content (Powers & Jackson 2008).
Previous literature has shown that acute BRJ consumption containing ∼16 mmol NO3 − is ideal with respect to peak blood NO3 − and NO2 −, responses that are normalized over a 12 h period (Wylie et al. 2013 a). Therefore, in the current study we used this dosing intervention every 12 h to maintain chronically elevated NO3 − and NO2 − plasma profiles over the entire 7 day study. The plasma profiles on the final day of the intervention show that while NO3 − was higher in the morning, the concentration of NO2 − was ∼50% lower in the evening. This is in contrast to previous work showing similar responses over 15 days with a lower dose of NO3 − (Vanhatalo et al. 2010). However, intriguingly, on day 8 plasma NO2 − was not significantly different from the pre‐supplementation baseline, suggesting the response observed in the current study on day 6 may be transient. Carlström and colleagues (2010, 2014) have shown that 8–10 weeks of supplementation with sodium nitrate is followed by a return to baseline in plasma [NO3 −]/[NO2 −] in rodents suggesting that it may theoretically be possible to become resistant to prolonged high plasma [NO3 −]/[NO2 −]. This may contribute to the relative insensitivity in elite athletes (Peacock et al. 2012; Christensen et al. 2013; Boorsma et al. 2014) to the otherwise consistent finding with recreationally active subjects that BRJ lowers during exercise, as elite athletes exhibit higher baseline plasma [NO3 −] when compared with untrained individuals (Poveda et al. 1997; Vassale et al. 2003). In the current study whole body measurements were made 48 h prior to biopsies, and therefore it remains possible that mitochondrial efficiency was altered 2 days earlier, especially since Larsen and colleagues (2011) examined mitochondrial efficiency after 3 days of sodium nitrate. However, this remains an unlikely explanation, since reductions in steady‐state pulmonary are seen 2.5 h after an acute dose of BRJ (Vanhatalo et al. 2010).
Limitations
A limitation of this study was the time delay between measurements, as whole body oxygen consumption, muscle biopsies and the collection of blood were taken on different days. While we could have performed the exercise trials on the same experimental day as the biopsies, out of necessity this would dictate that the exercise and biopsies occurred at different times following the consumption of the BRJ (i.e. biopsies at 90 min and exercise 120–150 min post‐consumption). Instead, we opted to standardize the time that all measurements were taken following the final morning's dose of BRJ, which dictated that measurements were taken on different days. This decision was made as we are unaware of data to indicate that the response to BRJ would be different between days 5 and 7; however, the acute response to beetroot juice is known to vary considerably across time. Nevertheless, the reader should be aware of the time delay between measures.
Unfortunately the subjects recruited for blood measurements represent a different cohort, and therefore should only be interpreted as proof‐of‐principle that the current dosing protocol resulted in the expected increase in plasma NO2 − and NO3 −. Given the results of our blood measures, we are confident our dosing protocol was sufficient to result in a prolonged elevation in plasma NO2 − and NO3 − in the first cohort of subjects, and therefore the lack of an effect is not the result of our supplementation regime. This is further corroborated by our results at 70% exhibiting the well‐documented decrease in whole body following NO3 − supplementation (Larsen et al. 2007, 2011; Bailey et al. 2009, 2010; Vanhatalo et al. 2010; Lansley et al. 2011; Cermak et al. 2012). This shows that our dosing protocol is still eliciting an ergogenic effect, but the underlying mechanism is not an alteration in mitochondrial coupling, and thus lies elsewhere.
Another possible perceived limitation was the small sample size, particularly for the isolated mitochondria experiments. However, in order to detect a 5% change in mitochondrial coupling ratios with 80% power, for SS mitochondria alone we would need to recruit an additional 50 subjects. Furthermore, the results of our mitochondrial measurements support what was seen within the larger group in PmFB experiments. We therefore felt we could not ethically justify recruiting more subjects and performing biopsies just to increase the sample size when we did not anticipate seeing an effect, as the tissue requirement for isolated mitochondria is significant.
Conclusions
Altogether, in the current study we provide evidence that improvements in mitochondrial coupling efficiency are not required for the reduction in whole body oxygen utilization during exercise following BRJ supplementation in recreationally active subjects. Instead, we provide evidence that mitochondrial H2O2 is increased; however, it remains to be determined if this contributes to the observed reduction in . This is in contrast to the results found following 3 days of sodium nitrate supplementation (Larsen et al. 2011). This is surprising given that nitrate appears to be the active ingredient in both supplements, as a nitrate‐depleted beetroot juice placebo has been shown to have no effect on pulmonary responses to exercise in both moderate‐ and severe‐intensity exercise, as well as blood pressure (Lansley et al. 2011). It is therefore possible that these two supplements act through different mechanisms, or elicit effects beyond simply increasing dietary nitrate intake. Future work should compare both BRJ and sodium nitrate directly with respect to mitochondrial adaptations and H2O2 emission to further our understanding of how these supplements may alter metabolism.
Additional information
Competing interests
None declared.
Author contributions
J.W. and G.P.H. designed experiments, interpreted the data, and wrote the manuscript. L.L.S. designed experiments and interpreted the data. J.W., A.L., G.J.F.H., J.M.G.S., L.B.V. and L.J.C.v.L. performed experiments. All authors edited the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was funded by The Natural Sciences and Engineering Research Council of Canada (G.P.H, 03656; L.L.S., 03996) and infrastructure was purchased with the assistance of the Canadian Foundation for Innovation/Ontario Research Fund (G.P.H., 25136).
References
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH & Neufer PD (2009). Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG & Westerblad H (1998). Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol 509, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB & Westerblad H (2001). Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox‐modulation. FASEB J 15, 309–311. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N & Jones AM (2010). Dietary nitrate supplementation enhances muscle contractile efficiency during knee‐extensor exercise in humans. J Appl Physiol (1985) 109, 135–148. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N & Jones AM (2009). Dietary nitrate supplementation reduces the O2 cost of low‐intensity exercise and enhances tolerance to high‐intensity exercise in humans. J Appl Physiol (1985) 107, 1144–1155. [DOI] [PubMed] [Google Scholar]
- Bergstrom J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Bescós R, Ferrer‐Roca V, Galilea PA, Roig A, Drobnic F, Sureda A, Martorell M, Cordova A, Tur JA & Pons A (2012). Sodium nitrate supplementation does not enhance performance of endurance athletes. Med Sci Sports Exerc 24, 2400–2409. [DOI] [PubMed] [Google Scholar]
- Bolaños JP, Peuchen S, Heales SJ, Land JM & Clark JB (1994). Nitric oxide‐mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem 63, 910–916. [DOI] [PubMed] [Google Scholar]
- Bond H, Morton L & Braakhuis AJ (2012). Dietary nitrate supplementation improves rowing performance in well‐trained rowers. Int J Sport Nutr Exerc Metab 22, 251–256. [DOI] [PubMed] [Google Scholar]
- Boorsma RK, Whitfield J & Spriet LL (2014). Beetroot juice supplementation does not improve performance in elite 1500‐m runners. Med Sci Sports Exerc 46, 2326–2334. [DOI] [PubMed] [Google Scholar]
- Boushel R, Fuentes T, Hellsten Y & Saltin B (2012). Opposing effects of nitric oxide and prostaglandin inhibition on muscle mitochondrial VO2 during exercise. Am J Physiol Regul Integr Comp Physiol 303, R94–R100. [DOI] [PubMed] [Google Scholar]
- Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS & Cornwall EJ (2005). The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC & Cooper CE (1994). Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356, 295–298. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF & Bonen A (2004). A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem 279, 36235–36241. [DOI] [PubMed] [Google Scholar]
- Carlström M, Larsen FJ, Nyström T, Hezel M, Borniquel S, Weitzberg E & Lundberg JO (2010). Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase‐deficient mice. Proc Natl Acad Sci USA 107, 17716–17720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlström M, Liu M, Yang T, Zollbrecht C, Huang L, Peleli M, Borniquel S, Kishikawa H, Hezel M, Persson AE, Weitzberg E & Lundberg JO (2014). Cross‐talk between nitrate‐nitrite‐NO and NO synthase pathways in control of vascular NO homeostasis. Antioxid Redox Signal 23, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak NM, Gibala MJ & van Loon LJ (2012). Nitrate supplementation's improvement of 10‐km time‐trial performance in trained cyclists. Int J Sport Nutr Exerc Metab 22, 64–71. [DOI] [PubMed] [Google Scholar]
- Christensen PM, Nyberg M & Bangsbo J (2013). Influence of nitrate supplementation on VO₂ kinetics and endurance of elite cyclists. Scand J Med Sci Sports 23, e21–31. [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Darley‐Usmar VM, Moncada S & Schapira AH (1994). Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345, 50–54. [DOI] [PubMed] [Google Scholar]
- Clerc P, Rigoulet M, Leverve X & Fontaine E (2007). Nitric oxide increases oxidative phosphorylation efficiency. J Bioenerg Biomembr 39, 158–166. [DOI] [PubMed] [Google Scholar]
- Fajardo VA, Bombardier E, Vigna C, Devji T, Bloemberg D, Gamu D, Gramolini AO, Quadrilatero J & Tupling AR (2013). Co‐expression of SERCA isoforms, phospholamban and sarcolipin in human skeletal muscle fibers. PLoS One 8, e84304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI & Poole DC (2013). Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir Physiol Neurobiol 187, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Vitorino R, Alves RM, Appell HJ, Powers SK, Duarte JA & Amado F (2010). Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10, 3142–3154. [DOI] [PubMed] [Google Scholar]
- Fulford J, Winyard PG, Vanhatalo A, Bailey SJ, Blackwell JR & Jones AM (2013). Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch 465, 517–528. [DOI] [PubMed] [Google Scholar]
- Giuffrè A, Barone MC, Mastronicola D, D'Itri E, Sarti P & Brunori M (2000). Reaction of nitric oxide with the turnover intermediates of cytochrome c oxidase: reaction pathway and functional effects. Biochemistry 39, 15446–15453. [DOI] [PubMed] [Google Scholar]
- Goo S, Pham T, Han JC, Nielsen P, Taberner A, Hickey A & Loiselle D (2013). Multiscale measurement of cardiac energetics. Clin Exp Pharmacol Physiol 40, 671–681. [DOI] [PubMed] [Google Scholar]
- Govoni M, Jansson EA, Weitzberg E & Lundberg JO (2008). The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19, 333–337. [DOI] [PubMed] [Google Scholar]
- Haider G & Folland JP (2014). Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med Sci Sports Exerc 46, 2234–2243. [DOI] [PubMed] [Google Scholar]
- Herbst EA, Paglialunga S, Gerling C, Whitfield J, Mukai K, Chabowski A, Heigenhauser GJ, Spriet LL & Holloway GP (2014). Omega‐3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol 592, 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, & Westerblad H (2012). Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast‐twitch muscle. J Physiol 590, 3575–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H, Howald H, Conley K, Lindstedt SL, Claassen H, Vock P & Weibel ER (1985). Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol (1985) 59, 320–327. [DOI] [PubMed] [Google Scholar]
- Ishii T, Sunami O, Saitoh N, Nishio H, Takeuchi T & Hata F (1998). Inhibition of skeletal muscle sarcoplasmic reticulum Ca2+‐ATPase by nitric oxide. FEBS Lett 440, 218–222. [DOI] [PubMed] [Google Scholar]
- Joyner MJ & Tschakovsky ME (2003). Nitric oxide and physiologic vasodilation in human limbs: where do we go from here? Can J Appl Physiol 28, 475–490. [DOI] [PubMed] [Google Scholar]
- Kapil V, Khambata RS, Robertson A, Caulfield MJ & Ahluwalia A (2015). Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double‐blind, placebo‐controlled study. Hypertension 65, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley CP, Cahill K, Bolger K, McGowan A, Burke C, Faul J & Cormican L (2015). Dietary nitrate supplementation in COPD: an acute, double‐blind, randomized, placebo‐controlled, crossover trial. Nitric Oxide 44, 105–111. [DOI] [PubMed] [Google Scholar]
- Lally JS, Herbst EA, Matravadia S, Maher AC, Perry CG, Ventura‐Clapier R & Holloway GP (2013). Over‐expressing mitofusin‐2 in healthy mature mammalian skeletal muscle does not alter mitochondrial bioenergetics. PLoS One 8, e55660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N and Jones AM (2011. b). Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc 43, 1125–1131. [DOI] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N & Jones AM (2011. a). Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo‐controlled study. J Appl Physiol (1985) 110, 591–600. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO & Weitzberg E (2011). Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13, 149–159. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Ekblom B, Mattsson MP, Checa A, Wheelock CE, Nyström T, Lundberg JO & Weitzberg E (2014). Dietary nitrate reduces resting metabolic rate: a randomized, crossover study in humans. Am J Clin Nutr 99, 843–850. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO & Ekblom B (2007). Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191, 59–66. [DOI] [PubMed] [Google Scholar]
- Lundberg JO & Govoni M (2004). Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37, 395–400. [DOI] [PubMed] [Google Scholar]
- Matravadia S, Herbst EA, Jain SS, Mutch DM & Holloway GP (2014). Both linoleic and α‐linolenic acid prevent insulin resistance but have divergent impacts on skeletal muscle mitochondrial bioenergetics in obese Zucker rats. Am J Physiol Endocrinol Metab 307, E102–E114. [DOI] [PubMed] [Google Scholar]
- Moncada S & Higgs A (1993). The L‐arginine‐nitric oxide pathway. N Engl J Med 329, 2002–2012. [DOI] [PubMed] [Google Scholar]
- Moopanar TR & Allen DG (2005). Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37°C. J Physiol 564, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moopanar TR & Allen DG (2006). The activity‐induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol 571, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T, Ortsäter H, Huang Z, Zhang F, Larsen FJ, Weitzberg E, Lundberg JO & Sjöholm Å (2012). Inorganic nitrite stimulates pancreatic islet blood flow and insulin secretion. Free Radic Biol Med 53, 1017–1023. [DOI] [PubMed] [Google Scholar]
- Ormerod JO, Arif S, Mukadam M, Evans JD, Beadle R, Fernandez BO, Bonser RS, Feelisch M, Madhani M & Frenneaux MP (2015). Short‐term intravenous sodium nitrite infusion improves cardiac and pulmonary hemodynamics in heart failure patients. Circ Heart Fail 8, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock O, Tjonna AE, James P, Wisloff U, Welde B, Bohlke N, Smith A, Stokes K, Cook C & Sandbakk O (2012). Dietary nitrate does not enhance running performance in elite cross‐country skiers. Med Sci Sports Exerc 44, 2213–2219. [DOI] [PubMed] [Google Scholar]
- Peeling P, Cox GR, Bullock N & Burke LM (2015). Beetroot juice improves on‐water 500 m time‐trial performance, and laboratory‐based paddling economy in national and international‐level kayak athletes. Int J Sport Nutr Exerc Metab 25, 278–284. [DOI] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Herbst EA, Mukai K, Lark DS, Wright DC, Heigenhauser GJ, Neufer PD, Spriet LL & Holloway GP (2012). Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol 590, 5475–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ & Neufer PD (2011). Inhibiting myosin‐ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J 437, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda JJ, Riestra A, Salas E, Cagigas ML, López‐Somoza C, Amado JA & Berrazueta JR (1997). Contribution of nitric oxide to exercise‐induced changes in healthy volunteers: effects of acute exercise and long‐term physical training. Eur J Clin Invest 27, 967–971. [DOI] [PubMed] [Google Scholar]
- Powers SK & Jackson MJ (2008). Exercise‐induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88, 1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Perry CG, Herbst EA, Ritchie IR, Beaudoin MS, Smith JC, Neufer PD, Wright DC & Holloway GP (2013). Submaximal ADP‐stimulated respiration is impaired in ZDF rats and recovered by resveratrol. J Physiol 591, 6089–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder B, Eisenbrand G & Preussmann R (1976). Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N‐nitroso compounds. Food Cosmet Toxicol 14, 545–548. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S & Matsushima S (2011). Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301, H2181–H2190. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG & Jones AM (2010). Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate‐ intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299, R1121–R1131. [DOI] [PubMed] [Google Scholar]
- Vassalle C, Lubrano V, Domenici C & L'Abbate A (2003). Influence of chronic aerobic exercise on microcirculatory flow and nitric oxide in humans. Int J Sports Med 24, 30–35. [DOI] [PubMed] [Google Scholar]
- Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ & Ahluwalia A (2008). Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51, 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A & Jones AM (2013. a). Beetroot juice and exercise: pharmacodynamic and dose‐response relationships. J Appl Physiol (1985) 115, 325–336. [DOI] [PubMed] [Google Scholar]
- Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermιdis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A & Jones AM (2013. b). Dietary nitrate supplementation improves team sport‐specific intense intermittent exercise performance. Eur J Appl Physiol 113, 1673–1684. [DOI] [PubMed] [Google Scholar]
- Zamani P, Rawat D, Shiva‐Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC & Chirinos JA (2015). Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 131, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]


