Abstract
[Purpose] The aim of this study was to investigate the changes in spinal motor neuron excitability and autonomic nervous system activity during motor imagery of isometric thenar muscle activity at 10% and 50% maximal voluntary contraction (MVC). [Methods] The F-waves and low frequency/high frequency (LF/HF) ratio were recorded at rest, during motor imagery, and post-trial. For motor imagery trials, subjects were instructed to imagine thenar muscle activity at 10% and 50% MVC while holding the sensor of a pinch meter for 5 min. [Results] The F-waves and LF/HF ratio during motor imagery at 50% MVC were significantly increased compared with those at rest, whereas those during motor imagery at 10% MVC were not significantly different from those at rest. The relative values of the F/M amplitude ratio during motor imagery at 50% MVC were significantly higher than those at 10% MVC. The relative values of persistence and the LF/HF ratio during motor imagery were similar during motor imagery at the two muscle contraction strengths. [Conclusion] Motor imagery can increase the spinal motor neuron excitability and cardiac sympathetic nerve activity. Motor imagery at 50% MVC may be more effective than motor imagery at 10% MVC.
Key words: Motor imagery, F-wave, Autonomic nervous system activity
INTRODUCTION
Motor imagery (MI) is defined as an active process during which the representation of a specific action is internally reproduced within working memory without overt movement or muscle contraction1). In recent years, the effectiveness of MI has been recognized in rehabilitation. MI can improve various motor functions such as muscle strength2,3,4) and range of motion5). When MI is used in rehabilitation to improve motor function, it has the potential to increase both central and spinal neural functions. In other words, improved spinal neural function can result in improved motor function. Neurophysiological studies investigating brain activity during MI have found activity in the primary motor area (M1), the supplementary motor area (SMA), the premotor area (PM), the primary somatosensory area (S1), the cingulate area (Cg), the cerebellum (Cb), and the basal ganglia (BG)6,7,8,9). Corticospinal excitability during MI may result from an increase in the motor evoked potential (MEP) amplitude as measured by transcranial magnetic stimulation (TMS)10). We previously reported that F-wave measurements demonstrate that the excitability of spinal motor neurons increases during MI11). These results suggest that MI may facilitate central nervous system and spinal motor neuron excitability. However, only a few studies have investigated spinal motor neuron excitability during MI under different imagined muscle contraction strengths, and they were unable to determine spinal motor neuron excitability12,13,14,15). Some researchers have suggested that the difference in imagined muscle contraction strength is not involved in the change in spinal motor neuron excitability12,13,14), while others have suggested that higher imagined muscle contraction strength results in greater facilitation of spinal motor neuron excitability15). In our previous study, the excitability of spinal motor neuron during MI under 50% maximal voluntary contraction (MVC) was similar to that under 10% and 30% MVC16).
Sympathetic nerve activity increases during actual movement, specifically during isometric muscle contraction17, 18). If MI shares neural mechanisms with motor execution, similar patterns in the changes of autonomic nervous system (ANS) activity during MI would be expected. So, ANS activity could be elicited during MI, as with motor execution. Previous research has demonstrated that the heart rate increases during MI19,20,21,22). The ANS regulates heart rate by increasing heart rate during sympathetic activity and decreasing it through parasympathetic activity. Therefore, MI may increase the heart rate through increased cardiac sympathetic activity. However, it is unclear whether the level of ANS activity during MI is affected by different imagined muscle contraction strengths. The aim of this research was to investigate the changes in spinal motor neuron excitability and ANS activity during MI of isometric thenar muscle activity at 10% and 50% MVC.
SUBJECTS AND METHODS
The subjects were 9 healthy young adults (males, 7; females, 2; mean age, 25.3 ± 5.3 years). All subjects provided their informed consent prior to the study’s commencement. This study was approved by the Research Ethics Committee of the Graduate School of Aomori University of Health and Welfare (approval number: 1408) and the Graduate School of Kansai University of Health Sciences (approval number: 14-18) and was conducted in accordance with the principles of the Declaration of Helsinki.
Subjects were positioned supine and instructed to fix one eye on the pinch meter display (Unipulse, Digital indicator F304A) throughout the test. To maintain the skin impedance below 5 kΩ, an abrasive gel was applied. The room temperature was maintained at 25 °C. The F-waves were recorded by electromyography [VIASYS; Viking Quest electromyograph (Natus Medical Inc.)]. After stimulating the left median nerve at the wrist, we recorded the F-wave of the left thenar muscle with a pair of round disk electrodes attached to the skin with a collodion adhesive. The electrodes were placed over the muscle belly and on the metacarpophalangeal joint of the thumb. The cathode was placed over the left median nerve, 3 cm proximal to the palmar crease, and the anode was placed 2 cm proximal to the crease. The maximal stimulus was determined by delivering 0.2-ms square-wave pulses of increasing intensity to elicit the maximal compound muscle action potentials. Supramaximal shocks (up to 120% of the maximum stimulus) were delivered at 0.5 Hz for the acquisition of F-waves. The bandwidth filter ranged from 2 Hz to 3 kHz.
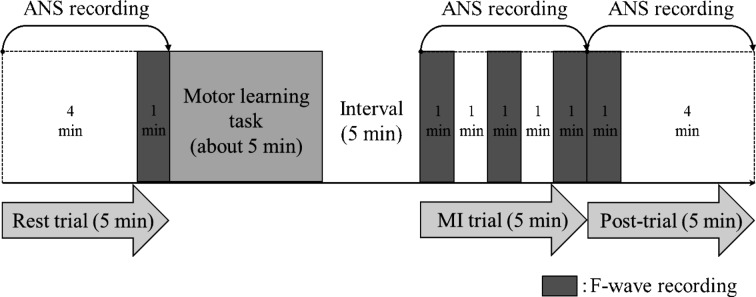
In the resting trial (rest), the F-wave was recorded while the muscle was relaxed. Next, the subjects held the sensor of the pinch meter while exerting maximum effort for 10 s to determine their 100% MVC. Subsequently, the subjects learned the motor task of isometric thenar muscle activity under 10% MVC. They practiced the activity using visual feedback while watching the digital display of the pinch meter until they were able to correctly perform the task, which took approximately 5 min. They were then instructed to imagine the 10% MVC motor task by holding the sensor between the thumb and index finger. The interval between the actual motor task and the MI trial was 5 min. The subjects used kinesthetic imagery for the MI task, which requires a subject to feel the movement and to perceive muscle contractions23). The F-waves were recorded during MI (10%MI) and immediately after the 10%MI trial (post-trial). This experimental condition, MI using 10% MVC, was labeled the 10%MI condition. This procedure was repeated using 50% MVC, and MI using 50% MVC was labeled the 50%MI condition. Both conditions were randomly performed on different days.
An F-wave is a compound action potential obtained as a result of re-excitation (“backfiring”) of an antidromic impulse following distal electrical stimulation of motor nerve fibers at the anterior horn cell24,25,26). F-waves were analyzed for their persistence, F/M amplitude ratio, and latency using 30 stimuli. In our study, persistence was defined as the number of measurable F-wave responses divided by 30 supramaximal stimuli. The F/M amplitude ratio was defined as the mean amplitude of all responses divided by the amplitude of the M-wave. Latency was defined as the mean latency from the time of stimulation to the onset of a measurable F-wave. Persistence reflects the number of backfiring anterior horn cells. The F/M amplitude ratio reflects the number of backfiring anterior horn cells and the excitability of individual anterior horn cells25, 26). Therefore, persistence and the F/M amplitude ratio are considered to be indices of spinal motor neuron excitability.
F-waves were recorded 4 min after initiation of the rest trial. In the MI trials, F-waves were recorded three times, immediately, 2 min, and 4 min after the initiation of MI, and the mean was used as the F-wave value in each MI trial. F-waves were also recorded immediately after the MI trial. The F-wave recording duration was 1 min (Fig. 1).
Fig. 1.
Experimental protocol
ANS activity was recorded using a heart rhythm scanner [Biocom Technologies; Heart Rhythm Scanner PE (Ark Trading Pacific Inc.)]. The pulse wave from the photoplethysmography sensor attached to the earlobe was recorded. The low frequency/high frequency (LF/HF) ratio was obtained by analyzing the pulse wave recorded by the Heart Rhythm Scanner PE, and it is considered to be an index of the sympathetic nerve activity. The European Society of Cardiology and the North American Society of Pacing and Electrophysiology recommend 5-min recordings for heart rate variability analysis27). The pulse wave recording was performed for 5 min at rest, during MI, and post-trial (Fig. 1).
The normality of F-wave data was confirmed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. The persistence, F/M amplitude ratio, latency, and LF/HF ratio during the three trials (rest, MI, and post-trial) under the two MI conditions (10% and 50% MVC) were compared using the Friedman Test and Scheffe’s post hoc test. The relative values obtained during the two MI conditions by dividing the values of persistence, F/M amplitude ratio, latency, and the LF/HF ratio at rest with those obtained during MI and at post-trial were also evaluated. The relative values of the two MI conditions were compared using the Wilcoxon signed rank test. Values of p < 0.05 were considered significant. We used IBM SPSS statistics ver.19 for statistical analysis.
RESULTS
The persistence and F/M amplitude ratio were significantly increased during 50%MI (82.3 ± 59.9% and 169.9 ± 280.6%, respectively) compared to rest (both p < 0.01; Table 1). The LF/HF ratio during 50%MI was also significantly increased (67.5 ± 87.2%) compared to rest (p < 0.05; Table 1). No significant differences were observed in persistence, F/M amplitude, and LF/HF ratio at post-trial compared to rest (Table 1).
Table 1. Change in F-wave and autonomic nervous system activity under the 50%MI condition.
| Rest | 50%MI | Post-trial | |
|---|---|---|---|
| Persistence (%) | 50.7 ± 26.1 | 92.4 ± 10.5** | 63.9 ± 26.4†† |
| F/M amplitude ratio | 1.14 ± 0.58 | 3.08 ± 2.23** | 1.44 ± 1.42†† |
| Latency (ms) | 25.4 ± 0.92 | 25.0 ± 1.31 | 25.6 ± 1.44 |
| LF/HF ratio | 1.74 ± 1.16 | 2.92 ± 2.17* | 2.07 ± 1.42 |
Mean ± SD
*p < 0.05, significant difference between rest and the 50%MI trial.
**p < 0.01, significant difference between rest and the 50%MI trial.
††p < 0.01, significant difference between the 50%MI and post-trial.
50%MI: Motor imagery of isometric thenar muscle activity at 50% MVC
Persistence during 10%MI tended to be increased (31.5 ± 56.5%) compared to rest (p = 0.062; Table 2). The F/M amplitude ratio and LF/HF ratio were increased (49.2 ± 91.7% and 121.6 ± 391.2%, respectively) compared to rest. However, no significant differences were observed between the F/M amplitude and LF/HF ratio during MI and rest (Table 2). No significant differences were observed in the persistence, F/M amplitude, and LF/HF ratio at post-trial compared to rest (Table 2).
Table 2. Changes in F-wave and autonomic nervous system activity under the 10%MI condition.
| Rest | 10%MI | Post-trial | |
|---|---|---|---|
| Persistence (%) | 65.2 ± 22.2 | 85.5 ± 9.64 | 60.8 ± 20.3 |
| F/M amplitude ratio | 1.07 ± 0.41 | 1.60 ± 0.78 | 1.21 ± 0.67 |
| Latency (ms) | 25.4 ± 1.96 | 25.1 ± 1.97 | 25.7 ± 2.08 |
| LF/HF ratio | 1.23 ± 0.75 | 2.73 ± 3.68 | 1.54 ± 0.52 |
Mean ± SD
10%MI: Motor imagery of isometric thenar muscle activity at 10% MVC
In both the 10%MI and 50%MI conditions, there were no significant differences in latency among any of the trials (Tables 1, 2).
Relative values of persistence during 50%MI tended to be higher than during 10%MI (p = 0.066; Table 3). The relative value of the F/M amplitude ratio during 50%MI was significantly higher than that during 10%MI (p < 0.05; Table 3). There were no significant differences in the latency or LF/HF ratio between the two MI conditions (Table 3).
Table 3. F-wave and autonomic nervous system activity between the 10%MI and 50%MI conditions.
| 50%MI | 10%MI | Significance | |
|---|---|---|---|
| Relative value of persistence (MI/rest) | 2.42 ± 1.39 | 1.69 ± 1.43 | |
| Relative value of persistence (post-trial/rest) | 1.36 ± 0.44 | 1.00 ± 0.36 | |
| Relative value of F/M amplitude ratio (MI/rest) | 3.45 ± 2.23 | 1.71 ± 0.73 | * |
| Relative value of F/M amplitude ratio (post-trial/rest) | 1.22 ± 0.73 | 1.22 ± 0.72 | |
| Relative value of latency (MI/rest) | 0.99 ± 0.04 | 0.99 ± 0.03 | |
| Relative value of latency (post-trial/rest) | 1.01 ± 0.04 | 1.01 ± 0.02 | |
| Relative value of LF/HF ratio (MI/rest) | 2.64 ± 3.35 | 1.75 ± 1.14 | |
| Relative value of LF/HF ratio (post-trial/rest) | 1.41 ± 0.72 | 1.61 ± 0.88 |
Mean ± SD. MI: motor imagery. *p < 0.05; significant difference between the 10%MI and 50%MI conditions
DISCUSSION
The excitability of spinal motor neuron during MI under the two MI conditions was higher than that at rest. This may attributable to the influence of descending pathways corresponding to the thenar muscle. Spinal motor neuron excitability is affected by cortical and subcortical activity during MI via the corticospinal and extrapyramidal tracts. Previous research has demonstrated the activation of the cerebral cortex (M1, S1, SMA, PM, Cb, and BG) during MI6,7,8,9). The SMA, PM, Cb, and BG have roles in planning and preparing movement and have connections to M1. The bulbar reticular formation (BRF), red nucleus (RN), Cb, and the caudate nucleus have connections to anterior horn cells. The BRF has connections to the M1, SMA, pM, and Cb, and the RN has connections to the Cb. Activation of the cerebral cortex during MI under the two MI conditions presumably increased the excitability of spinal motor neurons via the corticospinal and extrapyramidal tracts.
In addition, subjects performed MI while holding the sensor of a pinch meter. Therefore, the influence of tactile and proprioceptive inputs should be considered. Mizuguchi et al.28) reported that corticomotor excitability during MI was modulated by a combination of tactile and proprioceptive inputs while touching an object. Somatosensory inputs from the periphery are projected to the S1, which projects to M1. Therefore, somatosensory inputs from the periphery may influence corticospinal excitability during MI. In addition, Suzuki et al.11) compared the excitability of spinal motor neurons during MI with and without a pinch meter sensor. The subjects were instructed to imagine isometric thenar muscle activity under 50% MVC while holding a pinch meter sensor between the thumb and index finger (MI under the “with sensor” condition) on one day and not holding the sensor (MI under the “without sensor” condition) on another. F-waves during MI under both with and without sensor conditions were significantly greater than at rest. Furthermore, F-waves during MI were significantly higher under the “with sensor” condition than under the “without sensor” condition. Suzuki et al.11) suggested that it is important to use MI similar to actual movements in a clinical setting. Therefore, it is believed that tactile and proprioceptive inputs while holding the pinch meter sensor increase the excitability of spinal motor neurons as part of a synergistic effect.
In the present study, the excitability of spinal motor neurons during 50%MI was significantly higher than that during 10%MI. Suzuki et al.29) reported that the spinal motor neuron excitability increased linearly with muscle contraction strength. Similar to actual movement, it is thought that imagined muscle contraction strength may influence spinal motor neuron excitability. Mizuguchi et al.30) reported that corticospinal excitability during elbow flexion MI under 60% MVC was significantly increased compared with that under 10% and 30% MVC. In a study using movement-related cortical potentials (MRCPs), which are thought to reflect the cortical processes involved in movement planning and preparation31), SMA and pM showed greater activation in motor planning of larger force generation32). It is thought that MI under higher imagined muscle contraction strength resulted in greater facilitation of corticospinal excitability including M1. However, in our previous study, no significant difference was found in the spinal motor neuron excitability between MI of 10% and 50% MVC16). The difference between our present and previous studies is the practice time of motor task. Subjects who participated in the previous study performed a motor task for only 1 min; therefore, it is possible that they did not completely learn the motor task in 1 min. Subjects learned the motor task using visual feedback while watching the digital display of the pinch meter. Somatosensory and visual feedback are necessary for motor learning. When the visual and kinesthetic inputs are given simultaneously, humans become dependent on visual input. Our research used kinesthetic imagery for the MI task. Therefore, in our previous study, it is possible that subjects could not perform MI using the correctly imagined muscle contraction strength. Park and Li33) reported that MEP amplitude was higher during finger flexion or extension MI than during rest at 10%, 20%, 30%, 40%, 50%, and 60% of MVC, with no differences among the MI conditions. They suggested that differences in imagined muscle contraction strength cannot influence the magnitude of the change in corticospinal excitability. Mizuguchi et al.30) reported that the MEP amplitude during MI at 60% MVC was significantly increased compared to 10% and 30% MVC. Contrary to Park and Li, Mizuguchi et al. suggested that corticomotor excitability increased concurrently with changes in the magnitude of imagined contraction strength. Park and Li recorded MEPs during MI immediately after (8 s) actual muscle contraction. The MEP amplitude increases after actual muscle contraction, and continues to increase for several tens of seconds34). Therefore, Mizuguchi et al. suggested that the after effect of actual muscle contraction may have influenced the results of Park and Li. Also, MI ability is one factor that has an effect on the change in corticomotor excitability during MI. Previous research has demonstrated a significant correlation between the MEP amplitude during MI and MI ability35). Therefore, the corticospinal and spinal motor neuron excitability during MI might be facilitated under higher imagined muscle contraction. Thus, it is necessary to consider the after effects of actual muscle contraction and MI ability when interpreting the results.
In the present study, ANS activity under MI during both MI conditions was increased compared to rest. The LF/HF ratio during 50%MI was significantly greater than that at rest, but the difference was not significant in the 10%MI condition. In previous studies, sympathetic nerve activity could be elicited during MI19,20,21,22). Therefore, MI may increase sympathetic nerve activity due to the influence of the central command. The central command is defined as a feed-forward mechanism by which activation of cardiovascular and respiratory centers is accomplished by descending signals from the CNS36). M1, SMA, pM, Cb, and BG are activated during MI6,7,8,9) as are the anterior cingulate6, 37), dorsolateral prefrontal, and insula cortices38). The dorsolateral prefrontal cortex (DLPFC) has a role in motor cognition and has connections with the SMA, pM, and insula cortex. The anterior cingulate and insula cortices have roles in cardiovascular regulation. TMS to the M1 increases skin sympathetic nerve activity39), and transcranial direct stimulation (tDCS) to the M1 increases the LF/HF ratio40). tDCS is a non-invasive neuromodulatory technique that has been used to influence corticospinal excitability. The activation of the SMA, pM, DLPFC, and insula cortex during MI might influence M1 activity, and it is though that the M1 activity during MI stimulates the cardiac sympathetic nerve fibers via the corticospinal tract. Also, the rostral ventromedial medulla is part of the reticulospinal tract42) and is involved in regulation of sympathetic nerve activity and motor execution41). It is considered that activation of the cerebral cortex during MI increases cardiac sympathetic nerve activity via the corticospinal and reticulospinal tracts.
The change in sympathetic nerve activity during MI at 50% MVC tended to be higher than at 10% MVC, but it was not significant (Table 3). This result is similar to the changes in the spinal motor neuron excitability during MI between the 10%MI and 50%MI conditions. Based on the results of Mizuguchi et al.30), if central command during MI influences the changes in cardiac sympathetic nerve activity via the corticospinal tract, then differences in the imagined muscle contraction strength may affect cardiac sympathetic nerve activity. However, the difference in the change of sympathetic nerve activity between the 10%MI and 50%MI conditions was not significant with a lot of inter-individual variation. The corticospinal excitability during MI was affected by MI ability35), possibly because sympathetic nerve activity during MI was modulated by central command via the corticospinal tract and affected by MI ability. A major limitation of the present study is that we did not evaluate MI ability.
Finally, another possible factor that might have affected the changes in the spinal motor neuron excitability and cardiac sympathetic nerve activity is saccadic eye movement. Saccadic eye movement is an important selective process in visual perception, and is the shifts in the direction of gaze that rapidly and accurately aim the fovea at targets of interest43, 44). In the present study, subjects were instructed to fix one eye on the pinch meter display throughout the test. The frontal eye fields, DLPFC, parietal cortex, anterior cingulate cortex, and BG are all involved in saccadic eye movement45). These cerebral regions are also activated during MI. It may be that saccadic eye movement affects the spinal motor neuron excitability and cardiac sympathetic nerve activity.
In conclusion, MI at both 10% and 50% MVC can increase spinal motor neuron excitability and cardiac sympathetic nerve activity. In addition, MI at 50% MVC may be more effective than MI at 10% MVC.
REFERENCES
- 1.Guillot A, Di Rienzo F, Macintyre T, et al. : Imagining is not doing but involves specific motor commands: a review of experimental data related to motor inhibition. Front Hum Neurosci, 2012, 6: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yue G, Cole KJ: Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol, 1992, 67: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 3.Ranganathan VK, Siemionow V, Liu JZ, et al. : From mental power to muscle power—gaining strength by using the mind. Neuropsychologia, 2004, 42: 944–956. [DOI] [PubMed] [Google Scholar]
- 4.Sidaway B, Trzaska AR: Can mental practice increase ankle dorsiflexor torque? Phys Ther, 2005, 85: 1053–1060. [PubMed] [Google Scholar]
- 5.Guillot A, Tolleron C, Collet C: Does motor imagery enhance stretching and flexibility? J Sports Sci, 2010, 28: 291–298. [DOI] [PubMed] [Google Scholar]
- 6.Stephan KM, Fink GR, Passingham RE, et al. : Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol, 1995, 73: 373–386. [DOI] [PubMed] [Google Scholar]
- 7.Luft AR, Skalej M, Stefanou A, et al. : Comparing motion- and imagery-related activation in the human cerebellum: a functional MRI study. Hum Brain Mapp, 1998, 6: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotze M, Montoya P, Erb M, et al. : Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci, 1999, 11: 491–501. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda T, Watanabe S, Kuruma H, et al. : Neural correlates of chopsticks exercise for the non-dominant hand: comparison among the movement, images and imitations—A functional MRI study—. Rigakuryoho Kagaku, 2011, 26: 117–122(in Japanese). [Google Scholar]
- 10.Kasai T, Kawai S, Kawanishi M, et al. : Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res, 1997, 744: 147–150. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Bunno Y, Onigata C, et al. : Excitability of spinal neural function during several motor imagery tasks involving isometric opponens pollicis activity. NeuroRehabilitation, 2013, 33: 171–176. [DOI] [PubMed] [Google Scholar]
- 12.Hale BS, Raglin JS, Koceja DM: Effect of mental imagery of a motor task on the Hoffmann reflex. Behav Brain Res, 2003, 142: 81–87. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet M, Decety J, Jeannerod M, et al. : Mental simulation of an action modulates the excitability of spinal reflex pathways in man. Brain Res Cogn Brain Res, 1997, 5: 221–228. [DOI] [PubMed] [Google Scholar]
- 14.Aoyama T, Kaneko F: The effect of motor imagery on gain modulation of the spinal reflex. Brain Res, 2011, 1372: 41–48. [DOI] [PubMed] [Google Scholar]
- 15.Cowley PM, Clark BC, Ploutz-Snyder LL: Kinesthetic motor imagery and spinal excitability: the effect of contraction intensity and spatial localization. Clin Neurophysiol, 2008, 119: 1849–1856. [DOI] [PubMed] [Google Scholar]
- 16.Bunno Y, Yurugi Y, Onigata C, et al. : Influence of motor imagery of isometric opponens pollicis activity on the excitability of spinal motor neurons: a comparison using different muscle contraction strengths. J Phys Ther Sci, 2014, 26: 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, Mano T, Abe H, et al. : Responses in muscle sympathetic nerve activity to sustained hand-grips of different tensions in humans. Eur J Appl Physiol Occup Physiol, 1986, 55: 493–498. [DOI] [PubMed] [Google Scholar]
- 18.Seals DR: Influence of force on muscle and skin sympathetic nerve activity during sustained isometric contractions in humans. J Physiol, 1993, 462: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyer L, Weiss T, Hansen E, et al. : Dynamics of central nervous activation during motor imagination. Int J Psychophysiol, 1990, 9: 75–80. [DOI] [PubMed] [Google Scholar]
- 20.Decety J, Jeannerod M, Germain M, et al. : Vegetative response during imagined movement is proportional to mental effort. Behav Brain Res, 1991, 42: 1–5. [DOI] [PubMed] [Google Scholar]
- 21.Oishi K, Kimura M, Yasukawa M, et al. : Amplitude reduction of H-reflex during mental movement simulation in elite athletes. Behav Brain Res, 1994, 62: 55–61. [DOI] [PubMed] [Google Scholar]
- 22.Bolliet O, Collet C, Dittmar A: Autonomic nervous system activity during actual and mentally simulated preparation for movement. Appl Psychophysiol Biofeedback, 2005, 30: 11–20. [DOI] [PubMed] [Google Scholar]
- 23.Voisin JI, Mercier C, Jackson PL, et al. : Is somatosensory excitability more affected by the perspective or modality content of motor imagery? Neurosci Lett, 2011, 493: 33–37. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Saitoh E: Recommendations for the practice of the evoked EMG: H-reflex and F-wave—guidelines of the International Federation of Clinical Neurophysiology—. Rigakuryoho Kagaku, 2000, 15: 187–192(in Japanese). [Google Scholar]
- 25.Mesrati F, Vecchierini MF: F-waves: neurophysiology and clinical value. Neurophysiol Clin, 2004, 34: 217–243. [DOI] [PubMed] [Google Scholar]
- 26.Fisher MA: F-waves—physiology and clinical uses. ScientificWorldJournal, 2007, 7: 144–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik M, Bigger JT, Camm AJ, et al. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology: Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J, 1996, 17: 354–381. [PubMed] [Google Scholar]
- 28.Mizuguchi N, Sakamoto M, Muraoka T, et al. : The modulation of corticospinal excitability during motor imagery of actions with objects. PLoS ONE, 2011, 6: e26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki T, Fujiwara T, Takeda I: Excitability of the spinal motor neuron pool and F-waves during isometric ipsilateral and contralateral contraction. Physiother Theory Pract, 1993, 9: 19–24. [Google Scholar]
- 30.Mizuguchi N, Umehara I, Nakata H, et al. : Modulation of corticospinal excitability dependent upon imagined force level. Exp Brain Res, 2013, 230: 243–249. [DOI] [PubMed] [Google Scholar]
- 31.Wright DJ, Holmes PS, Smith D: Using the movement-related cortical potential to study motor skill learning. J Mot Behav, 2011, 43: 193–201. [DOI] [PubMed] [Google Scholar]
- 32.Oda S, Shibata M, Moritani T: Force-dependent changes in movement-related cortical potentials. J Electromyogr Kinesiol, 1996, 6: 247–252. [DOI] [PubMed] [Google Scholar]
- 33.Park WH, Li S: No graded responses of finger muscles to TMS during motor imagery of isometric finger forces. Neurosci Lett, 2011, 494: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balbi P, Perretti A, Sannino M, et al. : Postexercise facilitation of motor evoked potentials following transcranial magnetic stimulation: a study in normal subjects. Muscle Nerve, 2002, 25: 448–452. [DOI] [PubMed] [Google Scholar]
- 35.Williams J, Pearce AJ, Loporto M, et al. : The relationship between corticospinal excitability during motor imagery and motor imagery ability. Behav Brain Res, 2012, 226: 369–375. [DOI] [PubMed] [Google Scholar]
- 36.Hajduczok G, Hade JS, Mark AL, et al. : Central command increases sympathetic nerve activity during spontaneous locomotion in cats. Circ Res, 1991, 69: 66–75. [DOI] [PubMed] [Google Scholar]
- 37.Tyszka JM, Grafton ST, Chew W, et al. : Parceling of mesial frontal motor areas during ideation and movement using functional magnetic resonance imaging at 1.5 tesla. Ann Neurol, 1994, 35: 746–749. [DOI] [PubMed] [Google Scholar]
- 38.Mizuguchi N, Nakata H, Hayashi T, et al. : Brain activity during motor imagery of an action with an object: a functional magnetic resonance imaging study. Neurosci Res, 2013, 76: 150–155. [DOI] [PubMed] [Google Scholar]
- 39.Silber DH, Sinoway LI, Leuenberger UA, et al. : Magnetic stimulation of the human motor cortex evokes skin sympathetic nerve activity. J Appl Physiol 1985, 2000, 88: 126–134. [DOI] [PubMed] [Google Scholar]
- 40.Clancy JA, Johnson R, Raw R, et al. : Anodal transcranial direct current stimulation (tDCS) over the motor cortex increases sympathetic nerve activity. Brain Stimulat, 2014, 7: 97–104. [DOI] [PubMed] [Google Scholar]
- 41.Kerman IA, Enquist LW, Watson SJ, et al. : Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci, 2003, 23: 4657–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen GV, Cechetto DF: Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol, 1994, 350: 357–366. [DOI] [PubMed] [Google Scholar]
- 43.Hopp JJ, Fuchs AF: The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol, 2004, 72: 27–53. [DOI] [PubMed] [Google Scholar]
- 44.Liversedge SP, Findlay JM: Saccadic eye movements and cognition. Trends Cogn Sci, 2000, 4: 6–14. [DOI] [PubMed] [Google Scholar]
- 45.Gaymard B, Ploner CJ, Rivaud S, et al. : Cortical control of saccades. Exp Brain Res, 1998, 123: 159–163. [DOI] [PubMed] [Google Scholar]