Abstract
[Purpose] To assess the effects of different numbers of platelet-rich plasma (PRP) applications on pain and physical function in grade 3 knee osteoarthritis (OA). [Subjects and Methods] A total of 102 patients with grade 3 knee OA were randomly divided into three groups: Group 1 received a single injection of PRP, Group 2 received two injections of PRP two weeks apart, Group 3 received three injections of PRP at 2-weeks intervals. All patients were evaluated with a visual analog scale (VAS), the Western Ontario and McMaster Universities Arthritis Index (WOMAC), and the Timed-Up and Go test (TUG) before the treatment and at 1, 3 and 6 months after the treatment. [Results] Ninety-eight patients (15 males, 83 females) completed the study. The mean ages of the patients were 53.5±6.6, 54.9±5.3, and 55.1±5.6 years in Group 1, Group 2, and Group 3, respectively. Statistically significant improvements were noted in all of the evaluated measures in all of the groups. The mean differences of Group 1-Group 2 and Group 1-Group 3 WOMAC total, WOMAC pain, WOMAC stiffness, and WOMAC function scores were statistically significant. [Conclusion] PRP is an effective treatment for functional status and pain in moderate knee osteoarthritis and a minimum of two injections is appropriate.
Key words: Platelet-rich plasma effectiveness, Moderate, Knee osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is the most common chronic joint disorder, and it causes detrimental effects on the quality of life and functional status. These are characterized by progressively occurring cartilage destruction, osteophyte formation, and subchondral sclerosis1, 2). The histopathological findings of OA show that homeostasis between the destruction and repair mechanisms of the joint cartilage is disturbed by the increased expression of proinflammatory cytokines (IL-1, TNF alpha), matrix metalloproteinases, aggrecanases, nitric oxide, and prostaglandins. This causes degradation of the joints along with the insufficiency in the synthesis of growth factors (GFs), collagens, proteoglycans, and anti-inflammatory cytokines (IL-4, IL-10)3, 4).
Conservative treatments have been reported to increase the quality of life of patients particularly in the early phases, when the pathophysiology of the disease doesn’t change5). The effects of these treatments are short term and their local and systemic side effects cause frequent problems. Therefore, recent studies have focused on stimulating cartilage healing processes through administration of growth factors (GF), cytokine inhibitors, matrix metalloproteinase inhibitors, or IL-1 receptor antagonists5, 6).
Platelet-rich plasma (PRP) is an autologous concentration of a high number of platelets in a small volume of plasma, and it is prepared by centrifugation of blood. Platelets contain significant amounts of cytokines and growth factors which are capable of stimulating cellular growth, vascularization, proliferation, tissue regeneration, and collagen synthesis. Delivery of high concentrations of cytokines and GFs to damaged tissues by PRP is considered to have a beneficial effect on tendon and cartilage tissue regeneration7, 8). In some in vitro and in vivo studies, anti-inflammatory and reparative effects of PRP on cartilage, tendon, and ligament recovery have been shown9,10,11); however, there is no consensus on eligible patient selection, the number and frequency of injections, the preparation technique, or the appropriate platelet concentration5).
In knee OA, PRP injections aim to promote cartilage repair and relieve osteoarthritic symptoms, potentially delaying the need for joint replacement surgery12). Some studies have reported a reduction in PRP efficacy in moderate and advanced (Kellgren Lawrence grade 3–4) knee osteoarthritis, as this group of patients have higher pain and functional impairment, which require more medical attention13, 14). In some studies, it was suggested that in terms of PRP activity, OA and chondropenia level is more critical than platelet number and function4). Grade 4 OA generally requires surgical treatments such as tibial osteotomy and total knee replacement. In the present study the effects of PRP administration to control the disease activity of grade 3 knee OA either with one injection, two injections two weeks apart, or three injections separated by 2-week intervals on the patients’ pain, quality of life and physical activity levels were investigated.
SUBJECTS AND METHODS
Patients in the age range of 40–75 years who visited our physical medicine and rehabilitation outpatient clinic between May 2014–October 2014 because of single knee pain for a minimum 6 months were recruited for this study. OA was diagnosed according to the American College of Rheumatology (ACR) criteria15). Radiological assessment was conducted by standing anteroposterior and lateral knee radiography according to Kellgren- Lawrence grading system16). A total of 102 patients identified with grade 3 knee osteoarthritis (with multiple osteophytes, definite joint space narrowing, sclerosis and bony deformity) were studied. The exclusion criteria were bilateral symptomatic knee OA; age older than 75 years; receiving physical therapy, intra-articular steroid, hyaluronic acid or PRP injections in the last 6 months; recent history of severe trauma of the affected knee; active infection, inflammation or tumor existence around the knee; history of diabetes mellitus, severe cardiovascular diseases, coagulopathies, malignant, immunosuppressive, collagen vascular or autoimmune disorders; Hb values of < 11 g/dl or platelet values of < 150,000 per micro- liter; receiving treatment with anticoagulant or antiplatelet medications or systemic corticosteroids 10 days before injection, or use of NSAIDs 5 days before injection; genu varum or valgus greater than 5 degrees; pregnancy, or breastfeeding.
After receiving the approval of our Hospital’s Ethics Committee, the aims and methods of PRP therapy as well as the benefits and the possible adverse effects of study participation were presented to the patients in a written form. Only the participants who signed a written consent form were included in the study. The study participants attended a screening visit that included recording of medical history, physical examination, laboratory testing (complete blood count, erythrocyte sedimentation rate, C-reactive protein, coagulation profile, routine biochemistry), and a survey of medication use. Subjects’ age, gender, height and weight were recorded and their body mass index (BMI) was calculated.
The participants were randomized by block randomization into three groups: 34 participants in Group 1 received a single injection, 34 participants in Group 2 received two injections two weeks apart, and 34 participants in Group 3 received three injections of PRP separated by 2-weeks interval. One patient in Group 1, 2 patients in Group 2, and 1 patient in Group 3 did not complete the follow-up period due to personal reasons.
To prepare 4–5 cc PRP with platelet concentration of 4–6 times the average normal value, a 30–40 cc venous blood sample from antecubital vein was collected in a sterile sodium citrated tube using an 18G needle to avoid traumatizing platelets. Approximately 1 mL of whole blood was separated for a complete blood count. Then, the blood with anticoagulant was centrifuged twice: first at 1,800 rpm for 15 minutes to separate erythrocytes; then at 3,500 rpm for 5 minutes to concentrate platelets. The final product was 4–5 cc of PRP-containing leukocytes. Approximately 0.5 cc PRP was collected for platelet counting. Finally, 0.0425 mL of 10% calcium chloride per 1 mL of PRP was added to the final product to activate the platelets.
PRP in a sterile condition was injected by a physician using a classic lateral approach with a 22 G needle with the subjects in a supine position with the knee in full extension. Since some studies have indicated that a local anesthetic may have toxic effects on chondrocytes and affect platelet activation by modifying the ambient pH, a local anesthetic agent was not used before the injection17). The second and third injections were administered under the same conditions as the first injection. After the injections patients were told to actively flex and extend their knees a few times to allow the PRP to spread throughout the joint before gelling. Patients in each of the three groups were discharged to home after 15–20 minutes of rest with instructions to have rest, to limit weight bearing and to use cold packs 3–4 times a day for 10 minutes for 72 hours. During the follow-up period, the patients were asked to take acetaminophen only when necessary, or acetaminophen with codeine for persistent pain. The patients were instructed to not take them in the 48 hours before an assessment. Patients were prohibited from using other analgesics, NSAIDs, steroids or medications which might have influenced platelet count or function. Exercise or physical treatment was not allowed during the study period to eliminate synergistic effects.
Patients were evaluated before the treatment and at the 1 month, 3 months and 6 months after the treatment with a visual analog scale (VAS) for pain, the Western Ontario and McMaster Universities Arthritis Index (WOMAC), and the Timed- Up and Go test (TUG). Post-injection measurements were recorded by a different physician to ensure a blinded status.
VAS assessment was done with numbers from “0” to “10”, equidistantly marked on a 10 cm line. The patients were explained that “0” meant they were experiencing no pain, “5” moderate pain and “10” unbearable pain, and they were asked to mark the appropriate score on the line describing their own pain during rest and physical activity18). The WOMAC osteoarthritis index is a disease-specific questionnaire for the disease, which assesses pain, stiffness and physical functions of OA patients. It consists of 24 questions in total: 5 on pain, 2 on stiffness and 17 on physical functions. Individual subgroups scores or the total score can be calculated. A Likert scale (1: none, 2: low, 3: medium, 4: high, 5: very high) is used to assess all parameters on the WOMAC OA index. High WOMAC scores are indicative of intense pain and stiffness and impairment of the physical function. In our study, the patients’ WOMAC sub-scores (pain, stiffness, function) and total WOMAC score were calculated. The Turkish validity and reliability of the Turkish version of the WOMAC index was examined by Tuzun et al19). The Timed Up and Go test (TUG) was measured from when the patients rose from sitting with their feet on the floor and their arms resting on the armrest of a chair. The patients were asked to stand up without using their arms, walk for three meters, turn around, walk back and sit down. Measurement was ended when a subject’s buttocks regained contact with the chair. Three measurements were taken and the best value was recorded and categorized as: < 10 seconds = freely mobile, 10–19 seconds = mostly independent, 20–29 seconds = variable mobility, >30 =impaired mobility20).
The mean and standard deviation were calculated for continuous variables. The means of age and BMI of the groups were analyzed by one way ANOVA followed by the Bonferroni post-hoc test.
Repeated-measures ANOVA is used to compare the means of three or more matched groups. The term repeated-measures strictly applies to treatments repeatedly administered to each subject, and the term randomized block is used for randomly assigned treatments within a group (block) of matched subjects. A repeated-measures experimental design can be very powerful, as it controls factors that cause variability between subjects. If the matching is effective, the repeated-measures test will yield a smaller p value than ordinary ANOVA. The repeated-measures test is more powerful because it separates between-subject variability from within-subject variability21).
Data were analyzed using repeated ANOVA and multiple comparisons (the Bonferroni test) test. The SPSS statistical program was used to perform statistical analyses and values of p<0.05 were considered significant.
RESULTS
One hundred and two patients were enrolled in the study. Patients were randomly and equally divided into three treatment groups, and 98 (15 male and 83 female) patients completed the follow-up period (Group 1: n= 33, Group 2: n=32, Group 3: n=33) (Fig. 1).
Fig. 1.
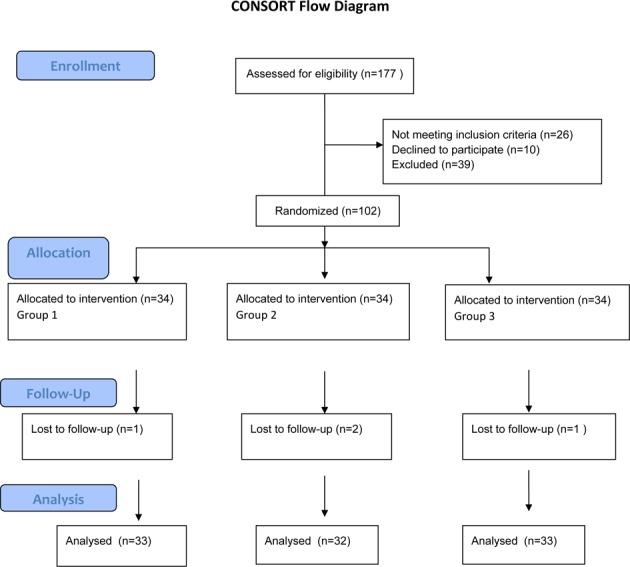
CONSORT flow diagram
The groups were homogenous in terms of age, gender and BMI; the results are presented in Table 1.
Table 1. The mean age and BMI of the patients.
| Variable | Treatment groups | ||
|---|---|---|---|
| Group-I Mean±SD | Group-II Mean±SD | Group-III Mean±SD | |
| Age (years) | 53.6±6.7 | 54.9±5.4 | 55.2±5.7 |
| BMI (k/m2) | 24.9±2.3 | 25.1±1.6 | 25.5±1.9 |
BMI: body mass index
VAS scores, TUG scores and WOMAC total and sub-scores were significantly better than preinjection scores in all of the three treatment groups during the follow-up period (p<0.001). Table 2 presents the mean and standard deviation values and comparisons of the treated groups during the follow-up period, and includes the results of repeated ANOVA and multiple comparisons.
Table 2. The mean and standard error of meam (SEM) values and the test values according to the repeated values for different four times.
| Treated Groups | Follow-ups | Outcome measures | |||||
|---|---|---|---|---|---|---|---|
| VAS Mean±SEM | W.Total Mean± SEM | W.Pain Mean± SEM | W.Stiffness Mean± SEM | W.Function Mean± SEM | TUG Mean± SEM | ||
| 1 | Pretreatment | 7.7±0.1 | 91.4±2.0 | 17.9±0.5 | 6.5±0.1 | 67.0±1.4 | 13.0±0.2 |
| 1 month | 5.6±0.2 | 81.7±2.1 | 14.9±0.5 | 5.0±0.2 | 61.8±1.6 | 12.8±0.2 | |
| 3 months | 6.5±0.2 | 89.3±5.6 | 20.7±5.0 | 5.4±0.2 | 63.1±1.4 | 13.0±0.2 | |
| 6 months | 7.2±0.2 | 87.6±1.9 | 16.9±0.4 | 6.1±0.2 | 64.6±1.4 | 13.1±0.2 | |
| 2 | Pretreatment | 7.7±1.2 | 81.6±3.0 | 17.2±0.5 | 6.6±0.2 | 58.0±2.0 | 12.7±0.2 |
| 1 month | 3.3±0.2 | 65.9±2.4 | 12.6±0.4 | 3.9±0.2 | 49.4±2.0 | 12.1±0.2 | |
| 3 months | 4.8±1.2 | 69.6±2.3 | 13.7±0.4 | 4.5±0.2 | 51.3±2.0 | 12.4±0.2 | |
| 6 months | 6.4±0.2 | 74.5±2.4 | 15.2±0.5 | 5.5±0.2 | 53.7±2.0 | 12.5±0.2 | |
| 3 | Pretreatment | 8.4±1.2 | 89.9±1.7 | 18.9±0.3 | 7.1±0.2 | 63.8±1.3 | 12.6±0.1 |
| 1 month | 2.4±0.1 | 67.4±1.6 | 11.9±0.3 | 3.4±0.2 | 52.0±1.3 | 11.6±0.1 | |
| 3 months | 3.0±1.2 | 69.8±1.8 | 12.5±0.3 | 3.8±0.2 | 53.5±1.4 | 11.7±0.1 | |
| 6 months | 4.5±1.2 | 75.1±1.7 | 14.1±0.2 | 4.9±0.2 | 56.0±1.4 | 11.7±0.3 | |
VAS: Visual analog Scale, W: WOMAC, TUG: Timed Up and Go Test, SD: standard deviation
The mean differences, the SEM of mean differences, p value and 95% confidence intervals of two groups were compared and the mean differences of Group 1-Group 2, Group 1-Group 3, and Group 2-Group 3 in VAS and TUG scores were found to be significant (p<0.001). Also the mean differences of Group 1-Group 2 and Group 1-Group 3 in WOMAC total, WOMAC pain, WOMAC stiffness and WOMAC function scores were also found to be statistically significant (p<0.001, Table 2). No significant complications were observed other than transient increases in local pain or swelling during the treatment and follow-up periods.
DISCUSSION
One of the major results of this study was the effectiveness of PRP treatment for pain and physical function in grade 3 knee OA. However the effectiveness of a single injection was found to be significantly lower than that of two or three injections. In this study, during the follow up period, significant improvements were observed in the VAS, WOMAC and TUG values of all of the three groups compared to their pre-injection values, and they showed a tendency of gradual decrease over time.
According to the study of Kon et al. which examined PRP effectiveness on the knee joint, better results were achieved in patients with a low degree of cartilage degeneration9). Chang’s meta-analysis showed that PRP effectiveness was higher at the degenerative chondropathy stage, and the effect decreased at two or lower doses when degeneration was worse5). In a study administering a single injection of PRP, a longer sustained period of pain relief was observed in milder cases of OA22), while Sampson et al. noted in a study of 14 patients’ cartilage thicknesses measured by ultrasonography, that responses to PRP decreased as the level of OA and chondropenia increased4). As joint degeneration increases, factors such as decrease of viable cells, muscle function deficiency, joint instability due to increased ligament laxity, decrease in anabolic response to growth factors, loss of chondrocyte and thinning of cartilage plate may diminish the effectiveness of PRP12).
Despite poorer results, patients with advanced OA still benefit from PRP. In a comparitive study of PRP and hyaluronic acid (HA) in grade 1–3 knee OA, the PRP group showed significantly better results after 6 months and the worst results were observed in HA-treated subjects with grade 3 knee OA23). Kon et al. speculate that, additional biological mechanisms, not currently known, are responsible for the improvement of OA symptoms after PRP treatment6). In the advanced stages of OA, PRP might not have a direct effect on the chondrocyte anabolic process, but an anti-inflammatory effect through the regulation of joint homeostasis and the cytokine level6, 24). However, contrary to this opinion, Calis et al. showed that PRP administered three times at weekly intervals to patients with grade 3 and 4 knee OA reported improvements in their quality of life, and reduced levels of pain, and had increased cartilage thickness as measured by ultrasonography at the 6-month follow up25).
There is not enough data regarding the effectiveness of PRP in the regeneration of substantial and irreversible bone and cartilage damage26). Accordingly, objective studies conducted using magnetic resonance imaging or arthroscopic methods will be valuable in this regard. To our knowledge, there are no studies in the literature which have compared different doses of PRP administered to patients with grade 3 knee osteoarthritis. In the present study, 3 PRP injections separated by 2-week intervals were found to be more effective for the improvement of pain and mobility than 2 injections in Grade 3 OA patients; however, no significant differences were observed in the WOMAC values. A significant effect was observed in the early period after a single injection of PRP, but the effect decreased in a short time. Based on the present results, we recommend 2 or 3 injections of PRP for patients with moderate knee OA, and physicians’ decisions should be based on various factors such as the level of pain, level of activity, cost-effectiveness, and BMI. We further speculate that repeating the application after 6 months may further relieve symptoms for a longer period and delay OA progression.
In the studies conducted so far, the lack of standardization of PRP dosing regimens makes it difficult to compare outcomes of studies for the evaluation of clinical effectiveness27). The amount and effectiveness of platelet concentration and the GFs related to platelets in PRP content will vary according to PRP preparation techniques. For example, using an activator, the existence of leukocytes in PRP content, application frequency, and platelet number range are currently debated issues. In addition, the follow-up period in many studies was short term, and there were no control groups5, 27). PRP preparation technique in this study was standardized by our transfusion medicine department and no commercial filters were used. The platelet numbers injected in this study were between 1.1 billion–1.4 billion, 4–6 fold higher than the baseline value, a number similar to that used in many studies, and it was also within the recommended range28).
This study had certain strengths and limitations. The strength of this study was the prospective randomized design. The absence of a control group and the relatively small patient numbers were the limitations of the study.
Considering the evidence, this minimally invasive injection procedure appears to be safe and effective, and since PRP injections biologically change the articular cartilage, they may be a worthwhile treatment option even in moderate knee osteoarthritis. Further studies are required with larger sample sizes with longer follow-ups and objective outcome measures.
In conclusion, PRP is an effective and reliable treatment for functional status and pain for Grade 3 OA, and a minimum of two injections appears to be appropriate.
REFERENCES
- 1.Nam CW, Kim K, Lee HY: The influence of exercise on an unstable surface on the physical function and muscle strength of patients with osteoarthritis of the knee. J Phys Ther Sci, 2014, 26: 1609–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koca I, Boyacı A, Tutoglu A, et al. : The relation between quadriceps thickness, radiological staging and clinical parameters in knee osteoarthritis. J Phys Ther Sci, 2014, 26: 931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangone G, Orioli A, Pinna A, et al. : Infiltrative treatment with Platelet Rich Plasma (PRP) in knee osteoarthritis. Clin Cases Min Bone Metab, 2014, 11: 67–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim G, Kim E: Anti-inflammation effects of low intensity laser therapy on monosodium iodoacetate-induced osteoarthritis in rats. J Phys Ther Sci, 2013, 25: 173–175. [Google Scholar]
- 5.Chang KV, Hung CY, Aliwarga F, et al. : Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil, 2014, 95: 562–575. [DOI] [PubMed] [Google Scholar]
- 6.Kon E, Buda R, Filardo G, et al. : Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc, 2010, 18: 472–479. [DOI] [PubMed] [Google Scholar]
- 7.Dragoo JL, Wasterlain AS, Braun HJ, et al. : Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med, 2014, 42: 610–618. [DOI] [PubMed] [Google Scholar]
- 8.Smyth NA, Murawski CD, Fortier LA, et al. : Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy, 2013, 29: 1399–1409. [DOI] [PubMed] [Google Scholar]
- 9.Steinert AF, Middleton KK, Araujo PH, et al. : Platelet-rich plasma in orthopedic surgery and sports medicine: pearls, pitfalls and new trends in research. Oper Tech Orthop, 2012, 22: 91–103. [Google Scholar]
- 10.Rayegani SM, Raeissadat SA, Taheri MS, et al. : Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev Pavia, 2014, 6: 5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayabalan P, Hagerty S, Cortazzo MH: The use of platelet-rich plasma for the treatment of osteoarthritis. Phys Sportsmed, 2014, 42: 53–62. [DOI] [PubMed] [Google Scholar]
- 12.Interventional procedure overview of platelet-rich plasma injections for osteoarthritis of the knee. NICE Interventional Procedure Guidance. http://www.guidance.nice.org.uk. (Accessed May, 2014).
- 13.Riddle DL, Stratford PW: Unilateral vs bilateral symptomatic knee osteoarthritis: associations between pain intensity and function. Rheumatology (Oxford), 2013, 52: 2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kon E, Filardo G, Di Matteo B, et al. : PRP for the treatment of cartilage pathology. Open Orthop J, 2013, 7: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misso ML, Pitt VJ, Jones KM, et al. : Quality and consistency of clinical practice guidelines for diagnosis and management of osteoarthritis of the hip and knee: a descriptive overview of published guidelines. Med J Aust, 2008, 189: 394–399. [DOI] [PubMed] [Google Scholar]
- 16.Kijowski R, Blankenbaker D, Stanton P, et al. : Arthroscopic validation of radiographic grading scales of osteoarthritis of the tibiofemoral joint. AJR Am J Roentgenol, 2006, 187: 794–799. [DOI] [PubMed] [Google Scholar]
- 17.Mishra A, Woodall J, Jr, Vieira A: Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med, 2009, 28: 113–125. [DOI] [PubMed] [Google Scholar]
- 18.Dixon JS, Bird HA: Reproducibility along a 10 cm vertical visual analogue scale. Ann Rheum Dis, 1981, 40: 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tüzün EH, Eker L, Aytar A, et al. : Acceptability, reliability, validity and responsiveness of the Turkish version of WOMAC osteoarthritis index. Osteoarthritis Cartilage, 2005, 13: 28–33. [DOI] [PubMed] [Google Scholar]
- 20.Yim-Chiplis PK, Talbot LA: Defining and measuring balance in adults. Biol Res Nurs, 2000, 1: 321–331. [DOI] [PubMed] [Google Scholar]
- 21.Celik MY: Research Methods and Biostatistics, Dicle University Press, 2014, pp 18–21. [Google Scholar]
- 22.Jang SJ, Kim JD, Cha SS: Platelet-rich plasma (PRP) injections as an effective treatment for early osteoarthritis. Eur J Orthop Surg Traumatol, 2013, 23: 573–580. [DOI] [PubMed] [Google Scholar]
- 23.Cerza F, Carnì S, Carcangiu A, et al. : Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med, 2012, 40: 2822–2827. [DOI] [PubMed] [Google Scholar]
- 24.Marmotti A, Rossi R, Castoldi F, et al. : PRP and articular cartilage: a clinical update. Biomed Res Int, 2015, 2015: 542502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Çalış HT, Sutbeyaz ST, Guler E, et al. : Efficacy of intra-articular autologous platelet rich plasma application in knee osteoarthritis. Arch Rheumatol, 2015, 30: 198–205. [Google Scholar]
- 26.Ayhan E, Kesmezacar H, Akgun I: Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthod, 2014, 5: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo TN, Pouliot MA, Kim HJ, et al. : Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med, 2011, 39: 266–271. [DOI] [PubMed] [Google Scholar]
- 28.Everts PA, Brown Mahoney C, Hoffmann JJ, et al. : Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors, 2006, 24: 165–171. [DOI] [PubMed] [Google Scholar]


