Abstract
Previous studies have demonstrated augmented levels of diacylglycerols (DAG) in the frontal cortex and plasma of Alzheimer’s disease (AD) patients. We extended these findings from non-targeted liopidomics studies to design a lipidomics platform to interrogate DAGs and monoacylglycerols (MAG) in the frontal cortex and plasma of MCI subjects. Control subjects included both aged normal controls and controls with normal cognition, but AD pathology at autopsy, individuals termed non-demented AD neuropathology (NDAN). DAGs with saturated, unsaturated, and polyunsaturated fatty acid substituents were found to be elevated in MCI frontal cortex and plasma. Tandem mass spectrometry of the DAGs did not reveal any differences in the distributions of the fatty acid substitutions between MCI and control subjects. While triacylglycerols were not altered in MCI subjects there were increases in monoacylglycerol levels both in the frontal cortex and plasma. In toto, increased levels of DAGs and MAGs appear to occur early in AD pathophysiology and require both further validation in a larger patient cohort and elucidation of the lipidomics alteration(s) that lead to the accumulation of DAGs in MCI subjects.
Keywords: diacylglycerols, monoacylglycerols, Alzheimer’s disease, Mild Cognitive Impairment
INTRODUCTION
Mild cognitive impairment is the prodromal phase prior to conversion to Alzheimer’s disease in a significant proportion of dementia patients [1]. Increasing our understanding of the molecular changes that occur in MCI patients will be extremely useful in defining the biochemical basis both for the development of MCI and for the conversion from MCI to dementia. In this regard, non-targeted lipidomics studies have revealed elevated levels of diacylglycerols (DAG) in the plasma of Alzheimer’s disease (AD) patients [2–3]; the frontal cortex of AD subjects [4–5], and the temporal cortex of mixed dementia subjects with AD and subcortical ischemic vascular dementia [6], but not in subjects only with subcortical ischemic vascular dementia [6]. These data indicate that altered DAG levels may be specific to the AD process. In addition, in our previous work we detected elevated DAGs in the plasma [2] and frontal cortex [5] of MCI subjects. Therefore, it appears that augmented levels of brain and circulating DAGs occur early in the AD disease process.
These may represent new seminal observations in research efforts to define early biochemical alterations in the etiology of sporadic AD. DAG pools in all tissues are tightly regulated since these glycerolipids serve multiple critical roles. These roles include: i) precursors for structural glycerophospholipids [7], ii) precursors for triacylglycerols (TAG) and monoacylglycerols (MAG), including the endocannabinoid 2-arachidonyl-glycerol [8–10], iii) mediators of signal transduction via activation of protein kinase C (PKC), protein kinase D (PKD), Ca(2+)/calmodulin-dependent protein kinase (PKCaMII), RasGRP1/Ras/Erk MAPK, chimaerins, and munc13 proteins that regulate neurotransmitter release [11–15], iv) regulation of immunological synapse function [16] and respiratory burst in immune cells like microglia [17]; v) required in the Golgi for transport carrier biogenesis [18]; vi) nuclear signal transduction [19], and vii) structural roles in the endoplasmic reticulum and nuclear envelope [20–21].
In light of the diverse structural and signal transduction roles of DAG pools, we undertook a targeted lipidomics analysis of DAGs, and MAGs in the frontal cortex and plasma of MCI subjects. These analyses were conducted with high- resolution (0.2 to 3 ppm mass error) mass spectrometric [22].
Materials and methods
Study participants
The plasma samples used in this study were from subjects described in a previous publication [2]. These plasma samples underwent 2 freeze-thaw cycles. Similarly, the frontal cortex samples were from donors described in a previous publication [5]. The patient demographics are briefly outlined in Table 1. Written informed consent was obtained from all participants of the Oregon Health and Science University aging study. Tissue and postmortem CSF were provided to the Oregon Brain Bank by volunteer subjects who gave informed consent. The study was approved by the Oregon Health and Science University Institutional Review Board.
Table 1.
Patient information for the clinical groups.
| Plasma Samples: | ||||
|---|---|---|---|---|
| MMSE Stratification | MMSE | Age | Age Range | N |
| 25–30 | 29.0 ± 1.5 | 68.4 ± 10.8 | 58–89 | 25 |
| 19–24 | 21.1 ± 2.0 | 69.8 ± 7.7 | 57–79 | 16 |
| 10–18 | 15.4 ± 2.4 | 74.8 ± 3.5 | 57–82 | 8 |
| 4–9 | 7.2 ± 2.2 | 68.4 ± 6.6 | 57–76 | 5 |
| Brain Samples: | ||
|---|---|---|
| Group | Age | N |
| Young Controls | 67.3 ± 11.8 | 20 |
| Old Controls | 91.5 ± 4.2 | 8 |
| MCI | 90.5 ± 7.9 | 19 |
Lipid Extraction and Analysis
Lipids were extracted from 100 μL of EDTA plasma or brain tissue (10 to 20 mg) with methy-tert-butyl ether and methanol containing [2H8]arachidonic acid [13C18]stearic acid, [2H5]MAG 18:1, [2D5]DAG 36:0, [13C3]DAG 36:2, glyburide, and bromocriptine as internal standards [2, 5, 23–25]. Extracts were dried by centrifugal vacuum evaporation and dissolved in isopropanol : methanol : chloroform (4:2:1) containing 15 mM ammonium acetate. Constant infusion lipidomics (5 μL per min) were performed utilizing high-resolution (140,000 at 200 amu) data acquisition, with sub-millimass accuracy on an orbitrap mass spectrometer (Thermo Q Exactive) [26]. Washes (500 μL) with methanol followed by hexane/ethyl acetate (3:2), between samples, were used to minimize ghost effects.
In positive ion electrospray ionization (ESI), the cations of monoacylglycerols (MAG) and the ammonium adducts of diacylglycerols (DAG) were quantitated. The cations of bromocriptine and glyburide were used to monitor for potential mass axis drift. For the majority of DAGs and MAGs, relative concentrations were obtained by determining the ratio of the endogenous lipid peak area to the peak area of a stable isotope internal standard. For the plasma DAGs 34:2, 36:2, and 38:4, absolute plasma concentrations were measured using analytical standards of each DAG for the 8 point standard curves.
Fatty Acid Analysis of DAGs and MAGs
MS2 of DAGs was conducted to determine if there were alterations in the fatty acid substitutions of DAGs in MCI subjects. Precursor ions were selected with unit mass resolution while the product ions were monitored with high-resolution analysis. In positive ion ESI the major ions monitored resulted from the loss of NH3 and the individual fatty acids, with the loss of the sn-2 fatty acid predominating. Using these data the dominant fatty acid combination for each DAG is presented as 1.0 and the minor fatty acid combinations presented as a fraction of 1.0 The dominant ion for MS2 of MAG was the fatty acid substitution with loss of OH.
Plasma Glucose
50 μL of plasma was extracted with cold acetonitrile:methanol (8:1) containing [13C6]glucose as the internal standard. The samples were centrifuged at 30,000 ×g and 4°C for 20 min. To 100 μL of the supernatant were added 100 μL of Girard’s Reagent T (20 mg/ml methanol) and 20 μL of glacial acetic acid. The samples were heated at 70°C, with shaking, for 30 min. Next the samples were cooled and dried by vacuum centrifugation prior to dissolution in acetonitrile:methanol (4:1) for direct flow analyses in positive ESI [294.1659 (endogenous)/300.1861(internal standard)]. Washes (500 μL) with methanol followed by hexane/ethyl acetate (3:2), between samples, were used to minimize ghost effects. Standard curves utilized 8-point calibration.
Statistical Analysis
R values (ratio of endogenous lipid peak area to the peak area of an appropriate internal standard) were calculated. For plasma glucose and several DAGs absolute levels were calculated utilizing 8 point standard curves with analytical standards and [13C6]glucose, [2H5]DAG 36:0, or [13C3]DAG 36:2 as the internal standard. In the case of brain samples R values were corrected for the wet weight of the tissue analyzed. Data are presented as mean ± SEM. Data were analyzed with the Kruskal-Wallis test, followed by the Dunn’s t test to compare groups to the controls.
Results
DAGs
DAG levels were significantly elevated both in the frontal cortex (Fig. 1) and the plasma (Fig. 2) of MCI subjects, suggesting that this an early biochemical change in the lipidome that may play a role in the pathogenesis of AD. Increased DAGs included DAGs with saturated, unsaturated, and polyunsaturated fatty acid substitutions. MS/MS analysis of the fatty acid substituents of these augmented DAG pools revealed that the fatty acid profiles of monitored DAGs were not altered in the cortex (Table 2) or plasma (Table 3) of MCI or dementia patients.
Figure 1.
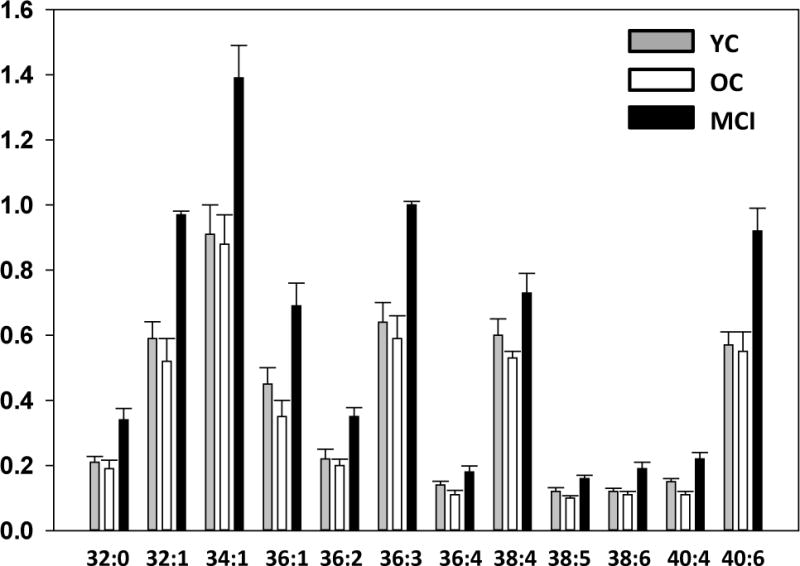
Levels of diacylglycerols (DAG) in the frontal cortex gray matter of subjects previously diagnosed as mild cognitive impairment (MCI; > 85 yr.; N=19). The control groups included young controls (YC; < 85 yr.; N=20) with no neurological or cognitive impairment and old controls (OC; >85 yr; N=8.) with no cognitive impairment but significant AD neuropathology at autopsy. Y axis is the ratio of the peak area of the endogenous lipid to the peak area of a stable isotope internal standard, corrected for tissue wet weight. The data are presented as mean ± SEM. All DAG levels in the MCI group were significantly (p < 0.05) increased above YC values while OC were not different from YC.
Figure 2.
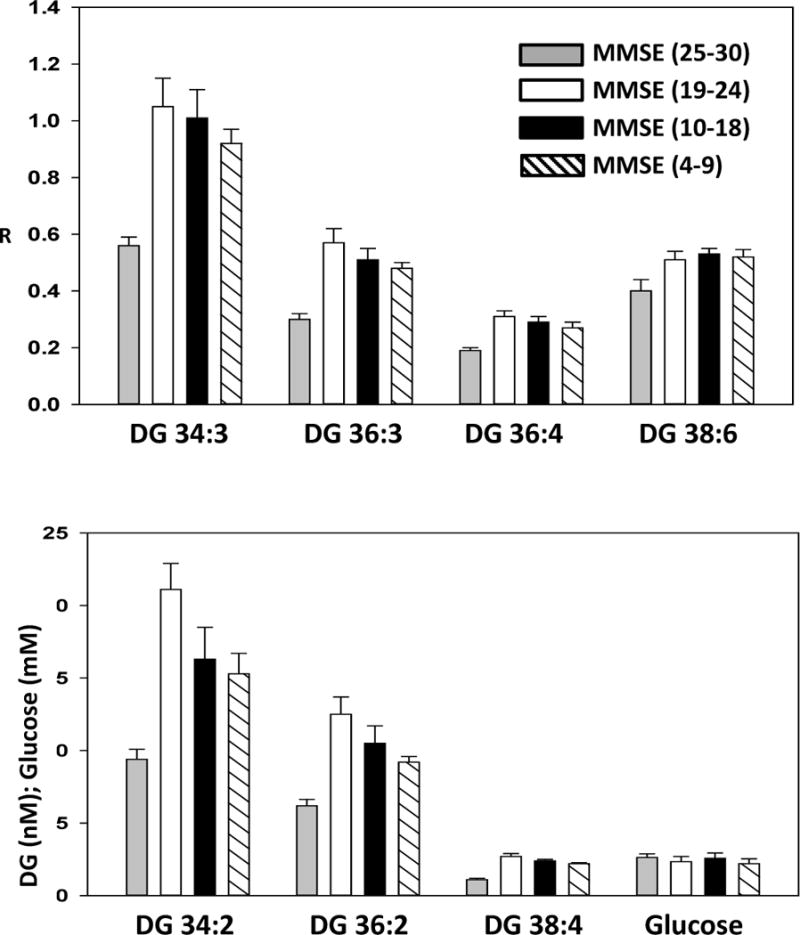
Levels of diacylglycerols (DAG) in the plasma of control subjects (MMSE 25–30), MCI subjects (MMSE 19–24), moderate dementia patients (MMSE 10–18), and severe dementia patients (MMSE 4–9). Y axis is the ratio of the peak area of the endogenous lipid to the peak area of a stable isotope internal standard, in the upper graph but is presented as nM in the lower graph. The data are presented as mean ± SEM. All DAG levels in the MCI, moderate dementia, and severe dementia groups were significantly (p < 0.05) increased above young controls (YC) values.
Table 2.
Fatty acid substituents of DAGs in brain samples, as determined by MS2. In positive ion ESI the major ions monitored resulted from the loss of NH3 and the individual fatty acids, with the loss of the sn-2 fatty acid predominating. Using these data the dominant fatty acid combination for each DAG is presented as 1.0 and the minor fatty acid combinations presented as a fraction of 1.0.
| DAG | Fatty Acids | YC (N = 19) | OC (N=8) | MCI (N=17) |
|---|---|---|---|---|
| 32:0 | 16:0/16:0 | 1.0 | 1.0 | 1.0 |
| 32:1 | 16:0/16:1 | 1.0 | 1.0 | 1.0 |
| 18:0/14:1 | 0.16 ± 0.010 | 0.19 ± 0.031 | 0.15 ± 0.005 | |
| 34:1 | 16:0/18:1 | 1.0 | 1.0 | 1.0 |
| 16:1/18:0 | 0.021 ± 0.0011 | 0.024 ± 0.0031 | 0.020 ± 0.0013 | |
| 36:1 | 18:0/18:1 | 1.0 | 1.0 | 1.0 |
| 16:0/20:1 | 0.047 ± 0.0021 | 0.043 ± 0.0051 | 0.047± 0.0012 | |
| 36:2 | 18:1/18:1 | 1.0 | 1.0 | 1.0 |
| 18:0/18:2 | 0.085 ± 0.0052 | 0.88 ± 0.14 | 0.83 ± 0.0054 | |
| 36:3 | 18:1/18:2 | 1.0 | 1.0 | 1.0 |
| 18:0/18:3 | 0.077 ± 0.0063 | 0.078 ± 0.0094 | 0.079 ± 0.0055 | |
| 36:4 | 16:0/20:4 | 1.0 | 1.0 | 1.0 |
| 18:2/18:2 | 0.048 ± 0.007 | 0.048 ± 0.012 | 0.061 ± 0.010 | |
| 38:4 | 18:1/20:3 | 1.0 | 1.0 | 1.0 |
| 18:0/20:4 | 0.27 ± 0.016 | 0.30 ± 0.019 | 0.31 ± 0.022 | |
| 16:1/22:3 | 0.15 ± 0.0042 | 0.14 ± 0.0037 | 0.16 ± 0.0052 | |
| 38:5 | 18:1/20:4 | 1.0 | 1.0 | 1.0 |
| 18:2/20:3 | 0.018 ± 0.0022 | 0.022 ± 0.003 | 0.02 ± 0.002 | |
| 38:6 | 16:0/22:6 | 1.0 | 1.0 | 1.0 |
| 18:2/20:4 | 0.095 ± 0.005 | 0.10 ± 0.016 | 0.097 ± 0.0041 | |
| 40:6 | 16:0/24:6 | 1.0 | 1.0 | 1.0 |
Table 3.
Fatty acid substituents of DAGs in plasma samples, as determined by MS2. In positive ion ESI the major ions monitored resulted from the loss of NH3 and the individual fatty acids, with the loss of the sn-2 fatty acid predominating. Using these data the dominant fatty acid combination for each DAG is presented as 1.0 and the minor fatty acid combinations presented as a fraction of 1.0.
| DAG | Fatty Acids | MMSE = 25–30 | MMSE = 19–24 | MMSE = 10–18 | MMSE = 4–9 |
|---|---|---|---|---|---|
| N = 17 | N = 14 | N = 8 | N = 5 | ||
| 34:2 | 16:0/18:2 | 1.0 | 1.0 | 1.0 | 1.0 |
| 18:1/16:1 | 0.14 ± 0.0089 | 0.14 ± 0.017 | 0.19 ± 0.032 | 0.11 ± 0.0071 | |
| 34:3 | 16:1/18:2 | 1.0 | 1.0 | 1.0 | 1.0 |
| 16:0/18:3 | 0.62 ± 0.055 | 0.61 ± 0.024 | 0.60 ± 0.055 | 0.57 ± 0.037 | |
| 36:2 | 18:1/18:1 | 1.0 | 1.0 | 1.0 | 1.0 |
| 18:0/18:2 | 0.47 ± 0.031 | 0.60 ± 0.043* | 0.39 ± 0.054 | 0.46 ± 0.036 | |
| 36:3 | 18:1/18:2 | 1.0 | 1.0 | 1.0 | 1.0 |
| 16:0/20:3 | 0.20 ± 0.016 | 0.25 ± 0.014 | 0.21 ± 0.018 | 0.18 ± 0.0088 | |
| 36:4 | 18:2/18:2 | 1.0 | 1.0 | 1.0 | 1.0 |
| 18:1/18:3 | 0.26 ± 0.012 | 0.25 ± 0.010 | 0.30 ± 0.022 | 0.24 ± 0.009 | |
| 16:1/20:3 | 0.021 ± 0.0035 | 0.024 ± 0.0023 | 0.028 ± 0.004 | 0.0014 ± 0.0027 | |
| 38:4 | 18:0/20:4 | 1.0 | 1.0 | 1.0 | 1.0 |
| 18:1/20:3 | 0.13 ± 0.008 | 0.13 ± 0.007 | 0.16 ± 0.019 | 0.15 ± 0.016 | |
| 38:6 | 16:0/22:6 | 1.0 | 1.0 | 1.0 | 1.0 |
| 18:2/20:4 | 0.30 ± 0.036 | 0.43 ± 0.035 | 0.44 ± 0.042 | 0. 38± 0.037 |
Plasma Glucose
Plasma glucose levels were not significantly different between groups (Fig 2). While there were several subjects with high plasma glucose levels in each cohort, there was no correlation between plasma glucose and plasma DAG levels.
MAGs
MAG levels also were elevated in the plasma of MCI patients, except for MAG 16:0 (Fig. 3; upper panel) and in the frontal cortex of MCI subjects (Fig. 3; lower panel).
Figure 3.
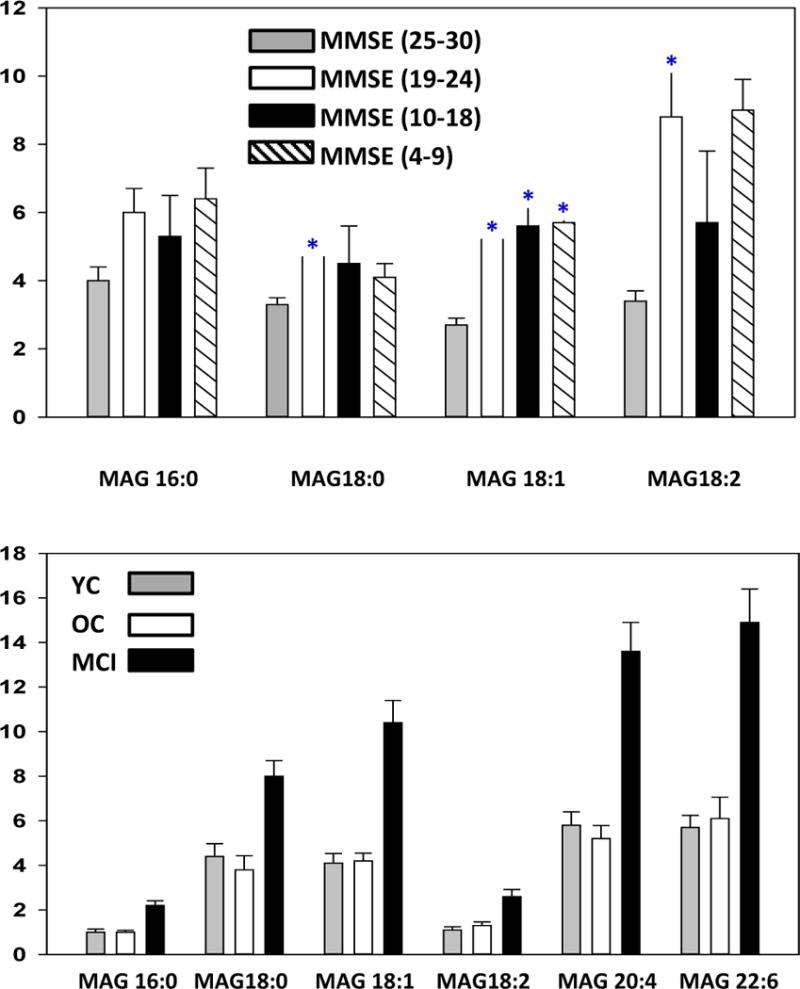
Levels of monoacylglycerols (MAG) in plasma (Upper panel) and frontal cortex gray matter (Lower panel). Tissue samples were from subjects previously diagnosed as mild cognitive impairment (MCI; > 85 yr.; N=19). The tissue controls included young controls (YC; < 85 yr.; N=20) with no neurological or cognitive impairment and old controls (OC; >85 yr; N=8.) with no cognitive impairment but significant AD pathology at autopsy. The plasma samples were obtained from control subjects (MMSE 25–30), MCI subjects (MMSE 19–24), moderate dementia patients (MMSE 10–18), and severe dementia patients (MMSE 4–9). Y axis is the ratio of the peak area of the endogenous lipid to the peak area of a stable isotope internal standard and were corrected for wet weight in the case of tissues. The data are presented as mean ± SEM. *, p < 0.05 vs. controls.
DISCUSSION
Our data confirm and extend the previous demonstrations of augmented DAG levels in AD frontal cortex [4–5] and mixed dementia temporal cortex [6], and AD plasma [2–3], to include MCI patients. Since MCI generally represents the prodromal phase in the progression to dementia, it is important to define if DAGs participate in the development of MCI and/or in the conversion of MCI to frank dementia.
DAGs function in a number of critical roles as structural lipids, as precursors for glycerophospholipid synthesis, and as mediators of signal transduction The levels of DAGs are strictly regulated to maintain their functions in these diverse roles [8]. This regulation appears to involve 4 major metabolic pathways for DAGs (Fig. 4A): i) conversion to glycerophospholipids; ii) phosphorylation via DAG kinase (DGK) to generate phosphatidic acids; iii) hydrolysis by diacylglycerol lipase (DGL), lipoprotein lipase (LPL), and hormone-sensitive lipase (HSL) to generate MAGs; and iv) acylation by DAG acyltransferase (DGAT) to synthesize TAGs. With regard to these potential regulatory pathways for DAGs, miR-34a, a negative regulator of DAG kinase [14], is up-regulated in the hippocampus and frontal cortex in AD [27], suggesting that conversion of DAGs to PA by DAG kinase (Fig. 4A) may be decreased in AD brain. Previous reports of lipase activities in AD are less consistent with our observations of DAG and MAG pools in AD and MCI, therefore requiring further investigation. These include increased DAG lipase in hippocampal microglia [28] and hippocampal membranes [29] in AD while MAG lipase levels appear to be decreased in AD hippocampal pyramidal neurons [28] but augmented in hippocampal membrane preparations [29].
Figure 4.
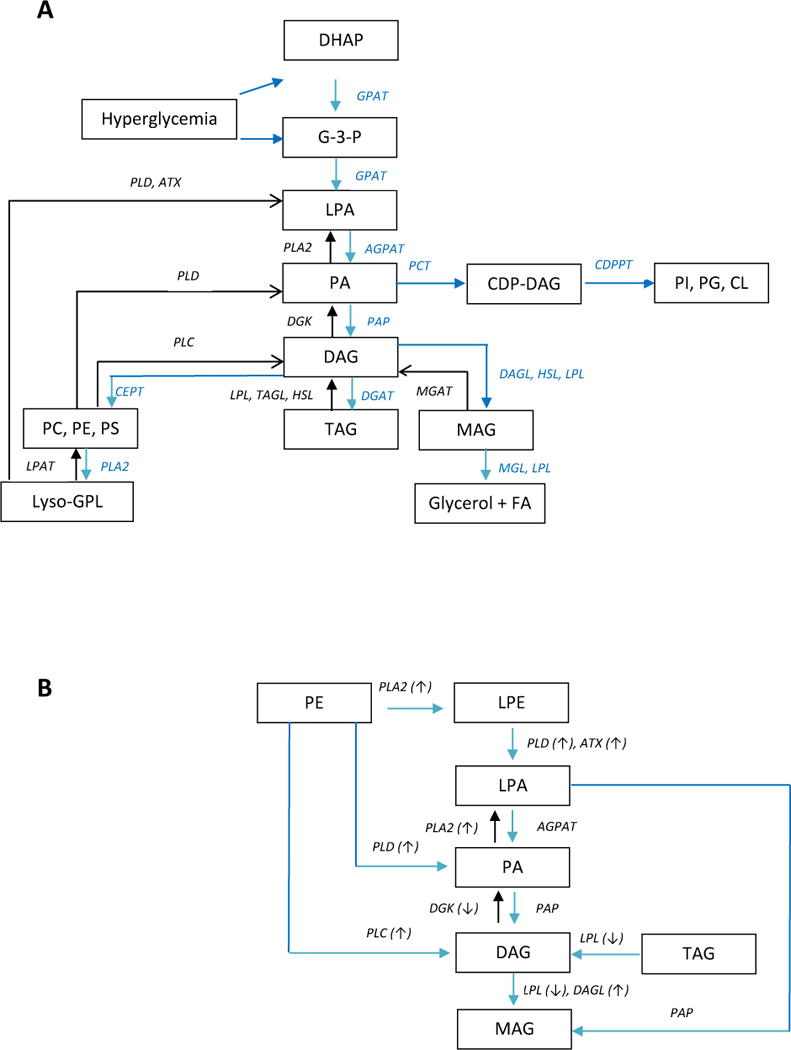
Presentation of DAG synthesis and metabolism. Figure A presents a broad overview of the interrelationships of DAGs with a number of other lipid pools while Figure B focusses on pathways involving glycerophospholipid metabolism and the associated enzymes reported to be altered in AD (see Discussion). AGPAT, acylglycerol-3-phosphate acyltransferase; ATX, autotaxin (lysophospholipase D); CDPPT, CDP-diacylglycerol-inositol/glycerol phosphatidyltransferase; CEPT, choline and ethanolamine phosphotransferases; CL, cardiolipin; DAG, diacylglycerol; DAGL, diacylglycerol lipase; DGAT, diacylglycerol acyltransferase; DHAP, dihydroxyacetone phosphate; DGK, diacylglycerol kinase; FA, fatty acid; G-3-P, glycerol-3-phosphate; GPAT, glycerol-3-phosphate acyltransferase; GPL, glycerophospholipid; HSL, hormone-sensitive lipase; LPA, lysophosphatidic acid; LPAT, lysophospholipid acyltransferase; LPL, lipoprotein lipase; MAG, monoacylglycerol; MGL, monoacylglycerol lipase; PA, phosphatidic acid; PAP, phosphatidic acid phosphatase; PC, phosphatidylcholines; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PLA2, phospholipase A2; PLC, phospholipase C; PLD, phospholipase D; PCT, phosphatidic acid cytidyltransferase; PS, phosphatidylserine; TAG, triacylglycerol; TAGL triacylglycerol lipase. Enzymes that have been reported to be elevated in AD are highlighted with an up arrow while enzymes that are decreased are highlighted with a down arrow.
With regard to metabolic sources of DAGs, glycerophospholipid degradation (Fig 4A and B) represents a major pathway with a large precursor pool. Since phosphatidylglycerol and phosphatidylinositol levels appear to be unaltered in AD frontal cortex [4–5] and MCI cortex [5] alterations in the CDP-glycerol pathway do not appear to be involved. In contrast, the selective deceases in phosphatidylethanolamines (PE) in AD frontal cortex [4–5], in the absence of alterations in phosphatidylserines, phosphatidylinositols, or phosphatidylcholines, suggest that PE degradation may contribute to elevated DAG levels. Augmentation of DAG levels, via PE degradation, are most likely to proceed via a combination of direct conversion to DAG by phospholipase C (PLC); metabolism to phosphatidic acid by phospholipase D (PLD) and subsequent conversion to DAG by phosphatidic acid phosphatase (PAP); and deacylation of PE to lysophosphatidylethanolamine (LPE) by phospholipase A2 (PLA2) with sequential metabolism of LPE to DAG (Fig. 4B). In this regard, a number of these enzymes involved in DAG synthesis (Fig. 4B) have been found to be altered in AD. Specific enzyme and gene changes include: 1) Increased levels of several PLC isoforms in AD brain [30–31]; 2) PLD3 gene variants have been reported as a risk factor for AD [32–35] and PLD accumulation occurs in neuritic plaques in AD [36]. Increased gene expression of autotaxin (lysophosphatidate phosphatase) has also been reported for AD frontal cortex [37]; and 3) PLA2 gene variants which have been reported as a risk factor for AD [38–39]. The conjecture that these metabolic pathways may be the main routes to augmented DAG levels in MCI and AD cannot rule out potential contributions by other glycerophospholipids since the size of the pools of these lipids far exceeds that of the DAGs. Alterations in the steady-state levels of glycerophospholipid pools may be minimally impacted but still substantially augment DAG levels. In this regard, while decreased PE levels were observed in AD frontal cortex [4–5], there were no such decrements in PE levels in MCI frontal cortex [5]. Similarly, phosphatidic acid and TAG levels are unaltered in AD [4–5] and MCI cortex [5]. As discussed above these data are not sufficient to exclude their potential contribution to elevated DAG levels in MCI and AD. However, the low levels of TAGs in the CNS, the observations that TAG levels are unaltered in AD and MCI frontal cortex [4–5], and the report of low lipoprotein lipase (LPL, Fig. 4A) in AD brain and CSF [40] strongly suggest that TAG pools are not a major metabolic source for DAGs in MCI and AD brain. Decrements in LPL levels may be the result of neuroinflammation in AD [41] which potently down-regulates LPL mRNA [42].
Other metabolic sources of DAG include de novo synthesis from the glycolytic pathway and from sphingolipid metabolism. Metabolic syndrome, which may represent a risk factor for AD in some patients [43], can lead to elevated DAG levels via augmented glycolysis [44]. However, hyperglycemia was not a factor in our MCI and AD cohorts, indicating that this is a minor source for increased circulating levels of DAGs. In the case of sphingolipid metabolism, DAGs are produced from phosphatidylcholines in the synthesis of sphingomyelins via the transfer of the phosphocholine headgroup to a ceramide, catalyzed by sphingomyelin synthase [8]. However, there are no significant alterations in the levels of sphingomyelins or phosphatidylcholines in the frontal cortex of AD [4–5] or MCI [5] subjects.
Another aspect of our study was the inclusion of brain samples from control subjects termed non-demented AD neuropathology (NDAN). These individuals possessed normal cognition, but at autopsy were found to have significant AD neuropathology [45–46]. Despite the presence of AD neuropathology, this group did not demonstrate elevations in DAGs or MAGs, suggesting that these biochemical changes may be specific to the processes leading to the deterioration of cognitive function.
In toto, these data suggest that alterations in a number of enzymes regulating lipid metabolism may be involved in the observed changes in DAG levels in MCI and AD. However, the majority of these observations have been reported for tissue samples obtained from AD patients and not from MCI patients. This complicates interpreting the role(s) of these enzymes in the early phases of the AD disease process. Therefore, mechanism(s) for augmented DAG levels in the frontal cortex in MCI remain to be more accurately defined (Fig. 4A and B). The data to-date suggests that further investigation of the following pathways is a rational next step: 1) glycerophospholipid → DAG; 2) glycerophospholipid → PA → DAG; 3) glycerophospholipid → lysoglycerophospholipid → lysophosphatidic acid→ phosphatidic acid →DAG; and 4) lysophosphatidic acid → MAG (Fig. 4). As a part of these investigations, we also need to assess lipin family proteins which are key regulators in lipid metabolism both as phosphatidic acid phosphatases and as transcriptional co-activators regulating the expression of genes involved in lipid metabolism [47–48]. In this regard, lipin 1γ is highly expressed in human brain [48].
Acknowledgments
We thank the participants of the aging study and their families for their valuable contribution to aging and Alzheimer’s research. This research was funded by Lincoln Memorial University, DeBusk College of Osteopathic medicine and by NIA-AG08017
Footnotes
Competing Interests
The authors declare no competing interests.
Author contributions
All authors contributed to the design of the experiment, analysis of the data, and writing the manuscript. RLW performed the neuropathology and PLW conducted the lipidomics studies.
References
- 1.Hye A, Riddoch-Contreras J, Baird AL, Ashton NJ, Bazenet C, Leung R, Westman E, Simmons A, Dobson R, Sattlecker M, Lupton M, Lunnon K, Keohane A, Ward M, Pike I, Zucht HD, Pepin D, Zheng W, Tunnicliffe A, Richardson J, Gauthier S, Soininen H, Kłoszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement. 2014;10:799–807. doi: 10.1016/j.jalz.2014.05.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood PL, Phillipps A, Woltjer RL, Kaye JA, Quinn JF. Increased lysophosphatidylethanolamine and diacylglycerol levels in Alzheimer’s disease plasma. JSM Alzheimer’s Disease and Related Dementia. 2014;1:1001. [Google Scholar]
- 3.González-Domínguez R, García-Barrera T, Gómez-Ariza JL. Application of a novel metabolomic approach based on atmospheric pressure photoionization mass spectrometry using flow injection analysis for the study of Alzheimer’s disease. Talanta. 2015;131:480–9. doi: 10.1016/j.talanta.2014.07.075. [DOI] [PubMed] [Google Scholar]
- 4.Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G, Di Paolo G. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012;287:2678–88. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL. Non-targeted lipidomics of CSF and frontal cortex gray and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatrica. 2015 doi: 10.1017/neu.2015.18. [DOI] [PubMed] [Google Scholar]
- 6.Lam SM, Wang Y, Duan X, Wenk MR, Kalaria RN, Chen CP, Lai MK, Shui G. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol Aging. 2014;35:2369–81. doi: 10.1016/j.neurobiolaging.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood PL. Lipidomics of Alzheimer’s disease: current status. Alzheimers Res Ther. 2012;4:5. doi: 10.1186/alzrt103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrasco S, Mérida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2007;32:27–36. doi: 10.1016/j.tibs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab. 2009;297:E10–8. doi: 10.1152/ajpendo.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savinainen JR, Saario SM, Laitinen JT. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf) 2012;204:267–76. doi: 10.1111/j.1748-1716.2011.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brose N, Betz A, Wegmeyer H. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr Opin Neurobiol. 2004;14:328–40. doi: 10.1016/j.conb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Madani S, Hichami A, Cherkaoui-Malki M, Khan NA. Diacylglycerols containing Omega 3 and Omega 6 fatty acids bind to RasGRP and modulate MAP kinase activation. J Biol Chem. 2004;279:1176–83. doi: 10.1074/jbc.M306252200. [DOI] [PubMed] [Google Scholar]
- 13.Heimfarth L, Loureiro SO, Reis KP, de Lima BO, Zamboni F, Gandolfi T, Narvaes R, da Rocha JB, Pessoa-Pureur R. Cross-talk among intracellular signaling pathways mediates the diphenyl ditelluride actions on the hippocampal cytoskeleton of young rats. Chem Res Toxicol. 2011;24:1754–64. doi: 10.1021/tx200307u. [DOI] [PubMed] [Google Scholar]
- 14.Shin J, Xie D, Zhong XP. MicroRNA-34a enhances T cell activation by targeting diacylglycerol kinase ζ. PLoS One. 2013;8:e77983. doi: 10.1371/journal.pone.0077983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genc O, Kochubey O, Toonen RF, Verhage M, Schneggenburger R. Munc 18-1 is a dynamically regulated PKC target during short-term enhancement of transmitter release. Elife. 2014;3:e01715. doi: 10.7554/eLife.01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauveau A, Le Floc’h A, Bantilan NS, Koretzky GA, Huse M. Diacylglycerol kinase α establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci Signal. 2014;7:ra82. doi: 10.1126/scisignal.2005287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlam D, Bohdanowicz M, Chatgilialoglu A, Steinberg BE, Ueyama T, Du G, Grinstein S, Fairn GD. Diacylglycerol kinases terminate diacylglycerol signaling during the respiratory burst leading to heterogeneous phagosomal NADPH oxidase activation. J Biol Chem. 2013;288:23090–104. doi: 10.1074/jbc.M113.457606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarri E, Sicart A, Lázaro-Diéguez F, Egea G. Phospholipid synthesis participates in the regulation of diacylglycerol required for membrane trafficking at the Golgi complex. J Biol Chem. 2011;286:28632–43. doi: 10.1074/jbc.M111.267534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García del Caño G, Montaña M, Aretxabala X, González-Burguera I, López de Jesús M, Barrondo S, Sallés J. Nuclear phospholipase C-β1 and diacylglycerol LIPASE-α in brain cortical neurons. Adv Biol Regul. 2014;54:12–23. doi: 10.1016/j.jbior.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Domart MC, Hobday TM, Peddie CJ, Chung GH, Wang A, Yeh K, Jethwa N, Zhang Q, Wakelam MJ, Woscholski R, Byrne RD, Collinson LM, Poccia DL, Larijani B. Acute manipulation of diacylglycerol reveals roles in nuclear envelope assembly & endoplasmic reticulum morphology. PLoS One. 2012;7:e51150. doi: 10.1371/journal.pone.0051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peddie CJ, Blight K, Wilson E, Melia C, Marrison J, Carzaniga R, Domart MC, O’Toole P, Larijani B, Collinson LM. Correlative and integrated light and electron microscopy of in-resin GFP fluorescence, used to localise diacylglycerol in mammalian cells. Ultramicroscopy. 2014;143:3–14. doi: 10.1016/j.ultramic.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood PL. Mass spectrometry strategies for targeted clinical metabolomics and lipidomics in psychiatry, neurology, and neuro-oncology. Neuropsychopharmacology. 2014;39:24–33. doi: 10.1038/npp.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood PL, Shirley NR. Lipidomics analysis of postmortem interval: Preliminary evaluation of human skeletal muscle. Metabolomics. 2013;3:3. [Google Scholar]
- 24.Wood PL, Bravermann NE. Lipidomics analysis of peroxisomal disorders: Discovery of deficits in phosphatidyglycerol levels in Rhizomelic Chondrodysplasia Type 1. J Data Mining in Genomics and Proteomics. 2014;S1:001. [Google Scholar]
- 25.Wood PL. Accumulation of N-acylphosphatidylserines and N-acylserines in the frontal cortex in schizophrenia. Neurotransmitter. 2014;1:e263. doi: 10.14800/nt.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuhmann K, Almeida R, Baumert M, Herzog R, Bornstein SR, Shevchenko A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. J Mass Spectrom. 2012;47:96–104. doi: 10.1002/jms.2031. [DOI] [PubMed] [Google Scholar]
- 27.Goodall EF, Heath PR, Bandmann O, Kirby J, Shaw PJ. Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front Cell Neurosci. 2013;7:178. doi: 10.3389/fncel.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulder J, Zilberter M, Pasquaré SJ, Alpár A, Schulte G, Ferreira SG, Köfalvi A, Martín-Moreno AM, Keimpema E, Tanila H, Watanabe M, Mackie K, Hortobágyi T, de Ceballos ML, Harkany T. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain. 2011;134:1041–60. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farooqui AA, Liss L, Horrocks LA. Stimulation of lipolytic enzymes in Alzheimer’s disease. Ann Neurol. 1988;23:306–8. doi: 10.1002/ana.410230317. [DOI] [PubMed] [Google Scholar]
- 30.Shimohama S, Sasaki Y, Fujimoto S, Kamiya S, Taniguchi T, Takenawa T, Kimura J. Phospholipase C isozymes in the human brain and their changes in Alzheimer’s disease. Neuroscience. 1998;82:999–1007. doi: 10.1016/s0306-4522(97)00342-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D, Dhillon H, Prasad MR, Markesbery WR. Regional levels of brain phospholipase Cgamma in Alzheimer’s disease. Brain Res. 1998;811:161–5. doi: 10.1016/s0006-8993(98)00935-4. [DOI] [PubMed] [Google Scholar]
- 32.Cruchaga C, Karch CM, Jin SC, Benitez BA, Cai Y, Guerreiro R, Harari O, Norton J, Budde J, Bertelsen S, Jeng AT, Cooper B, Skorupa T, Carrell D, Levitch D, Hsu S, Choi J, Ryten M, UK Brain Expression Consortium. Hardy J, Ryten M, Trabzuni D, Weale ME, Ramasamy A, Smith C, Sassi C, Bras J, Gibbs JR, Hernandez DG, Lupton MK, Powell J, Forabosco P, Ridge PG, Corcoran CD, Tschanz JT, Norton MC, Munger RG, Schmutz C, Leary M, Demirci FY, Bamne MN, Wang X, Lopez OL, Ganguli M, Medway C, Turton J, Lord J, Braae A, Barber I, Brown K, Alzheimer’s Research UK Consortium. Passmore P, Craig D, Johnston J, McGuinness B, Todd S, Heun R, Kölsch H, Kehoe PG, Hooper NM, Vardy ER, Mann DM, Pickering-Brown S, Brown K, Kalsheker N, Lowe J, Morgan K, David Smith A, Wilcock G, Warden D, Holmes C, Pastor P, Lorenzo-Betancor O, Brkanac Z, Scott E, Topol E, Morgan K, Rogaeva E, Singleton AB, Hardy J, Kamboh MI, St George-Hyslop P, Cairns N, Morris JC, Kauwe JS, Goate AM. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature. 2014;505:550–4. doi: 10.1038/nature12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenthal SL, Kamboh MI. Late-Onset Alzheimer’s Disease Genes and the Potentially Implicated Pathways. Curr Genet Med Rep. 2014;2:85–101. doi: 10.1007/s40142-014-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chouraki V, Seshadri S. Genetics of Alzheimer’s disease. Adv Genet. 2014;87:245–94. doi: 10.1016/B978-0-12-800149-3.00005-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Yu JT, Tan L. PLD3 in Alzheimer’s Disease. Mol Neurobiol. 2014;51:480–6. doi: 10.1007/s12035-014-8779-5. [DOI] [PubMed] [Google Scholar]
- 36.Satoh J, Kino Y, Yamamoto Y, Kawana N, Ishida T, Saito Y, Arima K. PLD3 is accumulated on neuritic plaques in Alzheimer’s disease brains. Alzheimers Res Ther. 2014;6:70. doi: 10.1186/s13195-014-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umemura K, Yamashita N, Yu X, Arima K, Asada T, Makifuchi T, Murayama S, Saito Y, Kanamaru K, Goto Y, Kohsaka S, Kanazawa I, Kimura H. Autotaxin expression is enhanced in frontal cortex of Alzheimer-type dementia patients. Neurosci Lett. 2006;400:97–100. doi: 10.1016/j.neulet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Cordeiro Q, Noguti R, Bottino CM, Vallada H. Study of association between genetic polymorphisms of phospholipase A2 enzymes and Alzheimer’s disease. Arq Neuropsiquiatr. 2010;68:189–93. doi: 10.1590/s0004-282x2010000200007. [DOI] [PubMed] [Google Scholar]
- 39.Martínez-García A, Sastre I, Recuero M, Aldudo J, Vilella E, Mateo I, Sánchez-Juan P, Vargas T, Carro E, Bermejo-Pareja F, Rodríguez-Rodríguez E, Combarros O, Rosich-Estrago M, Frank A, Valdivieso F, Bullido MJ. PLA2G3, a gene involved in oxidative stress induced death, is associated with Alzheimer’s disease. J Alzheimers Dis. 2010;22:1181–7. doi: 10.3233/JAD-2010-101348. [DOI] [PubMed] [Google Scholar]
- 40.Gong H, Dong W, Rostad SW, Marcovina SM, Albers JJ, Brunzell JD, Vuletic S. Lipoprotein lipase (LPL) is associated with neurite pathology and its levels are markedly reduced in the dentate gyrus of Alzheimer’s disease brains. J Histochem Cytochem. 2013;61:857–68. doi: 10.1369/0022155413505601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood PL. The cerebellum in AD: A Case for arrested neuroinflammation? In: Wood PL, editor. Neuroinflammation Mechanisms and Management. 2nd. Humana Press; Totowa, NJ: 2003. pp. 295–300. [Google Scholar]
- 42.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl) 2002;80:753–69. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 43.Siervo M, Harrison SL, Jagger C, Robinson L, Stephan BC. Metabolic syndrome and longitudinal changes in cognitive function: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41:151–61. doi: 10.3233/JAD-132279. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava SP, Shi S, Koya D, Kanasaki K. Lipid mediators in diabetic nephropathy. Fibrogenesis Tissue Repair. 2014;7:12. doi: 10.1186/1755-1536-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kramer PL, Xu H, Woltjer RL, Westaway SK, Clark D, Erten-Lyons D, Kaye JA, Welsh-Bohmer KA, Troncoso JC, Markesbery WR, Petersen RC, Turner RS, Kukull WA, Bennett DA, Galasko D, Morris JC, Ott J. Alzheimer disease pathology in cognitively healthy elderly: a genome-wide study. Neurobiol Aging. 2011;32:2113–22. doi: 10.1016/j.neurobiolaging.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood PL, Wood JA. Critical assessment of the status of Alzheimer’s disease Biomarkers. J Parkinson’s disease & Alzheimer’s disease. 2014;1:4. [Google Scholar]
- 47.Brindley DN, Pilquil C, Sariahmetoglu M, Reue K. Phosphatidate degradation: phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim Biophys Acta. 2009;1791:956–61. doi: 10.1016/j.bbalip.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Rui BB, Tang LY, Hu CM. Lipin family proteins - key regulators in lipid metabolism. Ann Nutr Metab. 2015;66:10–8. doi: 10.1159/000368661. [DOI] [PubMed] [Google Scholar]


