Abstract
The Src Family kinases (SFKs) are nonreceptor protein tyrosine kinases that are implicated in many normal and pathological processes in the nervous system. The SFKs Fyn, Src, Yes, Lyn and Lck are expressed in the brain. This review will focus on Fyn, as Fyn mutant mice have striking phenotypes in the brain and Fyn has been shown to be involved in ischemic brain injury in adult rodents, and with our work, in neonatal animals. An understanding of Fyn’s role in neurodevelopment and disease will allow researchers to target pathological pathways while preserving protective ones.
Keywords: Fyn tyrosine kinase, brain, development, ischemia
Fyn Structure and Regulation
Fyn is a 59kDa protein that is expressed in neurons and glia in the nervous system (1). Alternative splicing produces three Fyn isoforms. FynB, which uses exon 7A, is enriched in the brain (2). In the rodent embryo, Fyn is present in axonal tracts and growth cones, the telencephalon, hippocampal formation, cerebral cortex, and thalamic and hypothalamic nuclei (1, 3). There are elevated Fyn protein levels in white matter beginning at postnatal day 10 that coincides with myelination (3). In the mature brain, Fyn has decreased expression in axonal tracts and is predominantly found in the cerebellum, telencephalon and brain stem (1, 3). Fyn expression and kinase activity increase with development (3, 4).
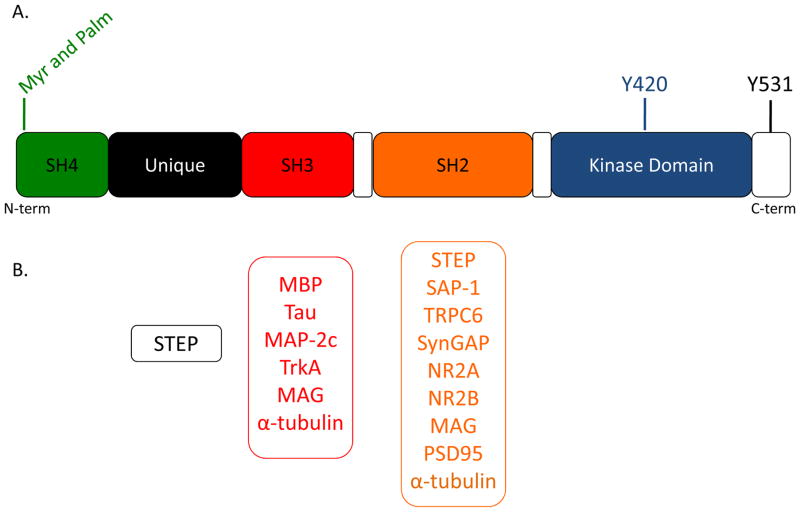
Fyn shares a similar structure to other SFKs, an N-terminal SH4 domain, unique region, SH3 and SH2 domains, linker regions and a C-terminal kinase domain (Figure 1A). Myristoylation at glycine 2 and palmitoylation at cysteine 3 and 6 allow Fyn to target to the plasma membrane and lipid rafts (5–7). The SH3 domain is a protein-protein interaction module that recognizes proline-rich regions (8) and the SH2 domain recognizes phosphorylated tyrosine (pY) (9). Fyn interacts with a wide range of proteins through these domains (Figure 1B), and thereby regulates various intracellular signaling pathways (10–17).
Figure 1.
Structure of FynB A) Domain structure of FynB including N-terminal myristoylation (Myr) and palmitylation (Palm) sites as well as regulatory tyrosine residues. B) Fyn binding partners in the unique region (black), SH3 domain (red) and SH2 domain (orange). These are the partners that have been published with demonstrated interactions with specific SH2 or SH3 domain. MAG: myelin-associated glycoprotein; SAP-1: stomach cancer-associated protein-tyrosine phosphatase-1; TRPC-6: transient receptor potential channel member 6
SFKs exist in active and inactive conformations that are partially driven by phosphorylation of two critical tyrosine residues (Y531 and Y420, numbering according to the amino acid sequence of human Fyn, Figure 2). Y531 is located in the extreme C-terminus. When this residue is phosphorylated, it forms an intramolecular interaction with the SH2 domain. This conformation makes the kinase active site and SH3 domain inaccessible (18, 19). Y420 is located within the activation loop of the kinase domain. When this site is phosphorylated, it activates SFKs and makes the SH3 domain available for protein-protein interactions (20).
Figure 2.
Inactive and active conformations of Fyn Phosphorylation of Y531 in the C-terminus leads to intramolecular interactions that prevent kinase activity and protein-protein interactions, while phosphorylation of Y420 leads to an open structure that is catalytically active and accessible to binding partners.
The dynamic control of phosphorylation of Y420 and Y531 provides an important level of regulation of Fyn activity and its ability to interact with other proteins. C-terminal Src kinase (Csk) and striatal enriched phosphatase (STEP) are Fyn negative regulators in the mammalian brain. Csk phosphorylates Fyn on Y531, while STEP dephosphorylates Y420 [10]. On the other hand, protein tyrosine phosphatase α (PTPα) activates Fyn by dephosphorylating Y531 (21–23). Once activated, Fyn can phosphorylate substrates in the brain with diverse cellular functions (Table 1). A broad range of substrates and binding partners situate Fyn upstream of many cellular processes in the brain.
Table 1.
Direct Fyn substrates in the brain
| Substrate | Site(s) | Outcome of phosphorylation | Refs |
|---|---|---|---|
| PTPRT (Protein Tyrosine Phosphatase Receptor T) | Y912 | Decreases phosphatase activity Promotes homophilic interactions Inhibits synapse formation |
(89) |
| TrkA receptor | ND | Promotes transactivation of TrkA by G-Coupled Protein Receptors (GPCRs) | (13) |
| Nav1.2 | ND | Decreases sodium currents | (90) |
| γ2 subunit GABAA receptor |
Y365, Y367 | Prevents clathrin-mediated endocytosis Enhances synaptic inhibition |
(48) (47) |
| NR2A subunit NMDA receptor |
ND | ND | (91) |
| NR2B subunit NMDA receptor |
Y932, Y1039, Y1070,Y1109, Y1252,Y1336, Y1472 | Y1472: prevents clathrin-mediated endocytosis Y1336: promotes calpain cleavage of NR2B; increased interaction with PI3-K |
(91) (53) (58) (56) (57) |
| TCGAP | Y406 | Negatively regulates activity | (92) |
| p250GAP | ND | Increases association with Fyn | (93) |
| p190RhoGAP | ND | ND | (94) |
| Cdk5 | Y15 | Increases kinase activity Promotes sema3a induced growth cone collapse |
(95) |
| Tau | Y18 | Prevents inhibition of anterograde fast axonal transport | (96) (97) |
| MAP-2c | Y67 | Increased interaction with Grb2 | (98) (99) |
| Psd93 | Y348 | ND | (78) |
| Psd95 | Y523 | Increases NMDA receptor currents | (73) |
| rSLM-1 | ND | Prevents splice site selection | (100) |
| α-synuclein | Y125 | ND | (101) |
| c-Cbl | ND | ND | (102) |
| N-WASP | Y253 | Arp2/3 complex mediated actin polymerization Neurite extension |
(103) |
| Dab1 | Y185,Y198 | Permits phosphorylation of other Y sites of Dab1 Increases interaction with Fyn Akt activation Degradation of Dab1 |
(32) (31) (104) |
Functional role of Fyn in the immature and mature nervous system
Several conclusions about Fyn function in the developing and adult nervous system can be drawn from transgenic mice where Fyn has been deleted, mutated or overexpressed. The Soriano lab generated Fyn null mice that lack expression of all Fyn isoforms (24). Yagi et al produced Fyn mutant mice in which the SH2, SH3 and kinase domain are replaced with lacZ, producing a Fyn-β-galatosidase fusion protein that is catalytically inactive (25). A Fyn kinase dead mutant exists which has a point mutant in the ATP binding pocket (K296R) (26). Finally, there are two mouse models that overexpress Fyn in excitatory neurons. Native or constitutively active (Y531F) Fyn is driven by the calcium/calmodulin-dependent protein kinase IIα (CaMKIIα) promoter where Fyn is overexpressed postnatally in the forebrain (27, 28). Studies using these transgenic mice have implicated Fyn in migration, myelination, synaptic plasticity and the regulation of excitatory and inhibitory receptors.
Neuronal Migration
Development of the neocortex involves the coordinated migration of neurons from the ventricular zone radially toward the pial surface. Fyn is expressed in the leading processes of migratory cortical neurons during corticogenesis (29). On a molecular level, Fyn has been implicated in the Reelin pathway. Reelin is an extracellular molecule that activates signaling cascades eventually leading to “inside-out” layering of neurons in the cerebral cortex, where early-generated neurons are located at a deeper position and later-generated neurons are present superficially (30).
Reelin and the intracellular adaptor protein Dab1 lead to activation of Fyn. Fyn phosphorylates Dab1 that initiates signal transduction cascades critical for neuronal migration (31, 32). Fyn null mice have abnormal stratification of layer II-III neurons with sparing of neurons in the deeper layers (29). Fyn knockout (KO) embryos have an intermediate migration defect, however Fyn Src double KO mice have a reeler phenotype suggesting that both kinases function downstream of Reelin and are necessary for cortical layer formation (33).
In addition, Fyn kinase is reported to be a component of the neural cell adhesion molecule (NCAM) and netrin signaling pathways regulating neurite outgrowth and attraction (34, 35)
Oligodendrocyte Maturation
One function of oligodendrocytes (OL) is myelination of neurons in the central nervous system (36). Fyn null mice have significantly less myelin and OLs. Fyn kinase dead mutant mice are also hypomyelinated, suggesting that Fyn kinase activity is required for myelination (26). In vitro, fewer OLs develop in the absence of Fyn and fewer cells are morphologically mature. Fyn KO OLs are insensitive to IGF-1 induced maturation (37). Many of these phenotypes are recapitulated in Fyn KO mice backcrossed to the C57BL/6 background as early as postnatal day 6. These mice show severe hydrocephalus with defects in oligodendrocyte development (38).
Fyn KO mice have decreased mylein basic protein (MBP) throughout development (36). While this may be due to decreased number of OLs, Fyn also regulates MBP at the mRNA level. Activated Fyn phosphorylates RNA binding protein hnRNP A2, which stimulates transport and translation of MBP mRNA (39). These studies suggest that Fyn does not participate in oligodendrocyte migration, but functions in oligodendrocyte maturation and may affect myelin production at transcriptional and translational levels (36).
Synaptic Plasticity
Synaptic plasticity refers to the ability of neuronal connections, synapses, to change over time. One experimental model of synaptic plasticity is long-term potentiation (LTP), in which repetitive stimulation of excitatory synapses leads to a long-lasting increase in synaptic strength (40). Fyn KO mice have impaired LTP in the hippocampus in response to weak intensity tetanus (41). Interestingly, LTP is normal due to Src compensation until 14 weeks of age in Fyn KO mice when compensatory Src expression is reduced and the LTP deficit begins to be observed (27). Mice overexpressing constitutively active Fyn have a lower threshold for LTP in response to a weak stimulus. These studies suggest that Fyn is not required for the initiation of LTP, but rather plays a modulatory role influencing the threshold of LTP induction (42).
In addition to the LTP deficit, Fyn KO mice have impaired spatial memory in the Morris water maze. Anatomically, Fyn deletion results in an abnormal localization and an increased number of granule cells in the dentate gyrus and target cells in CA3 (41). Fyn KO mice also have decreased spine density in the hippocampus at 3 months and 1 year of age (43). These anatomical changes may contribute to aberrant hippocampal function in adult Fyn KO mice.
GABAergic synaptic transmission
Inhibitory neurotransmission is mediated by γ-aminobutric acid (GABA) through activation of GABA receptors. GABA type A receptors (GABAAR) are heteropentameric GABA-gated chloride channels that are derived from 19 genes (α1–6, β1–3, γ1–3, δ, ε, θ, π, ρ1–3) (44). Several lines of evidence suggest that Fyn regulates GABAAR expression and function.
Fyn KO slices from the olfactory bulb were insensitive to the GABAAR antagonists, bicuculline and picrotoxin (45). Functional deficits in GABAA –gated chloride flux are also evident in the cerebellum of Fyn KO mice (46). GABAAR agonists have hypnotic effects, however Fyn KO mice are less sensitive to the hypnotic effects of β2/β3 agonists, but not to an α1 selective agonist (46).
The γ2 subunit of GABAAR is a Fyn substrate (Table 1). Fyn phosphorylates Y365 and Y367 that are within a consensus tyrosine-based endocytosis motif (YGYECL). Phosphorylation at Y365/7 prevents endocytosis of the GABAAR, leading to increased surface expression of GABAARs and synaptic inhibition (47). Homozygotes for the mutation of Y365/7 to phenylalanine (Y365/7F) is embryonic lethal. Heterozygous Y365/7F mice have an increased size of inhibitory synapses and increased miniature inhibitory postsynaptic currents (mIPSCs) in the CA3 region of the hippocampus. Heterozygous mice also have impaired spatial object recognition (48). A recent study also implicated Fyn kinase activity in GABAergic synapse formation downstream of NCAM in postnatal mouse cortex (49).
These studies suggest that Fyn deletion leads to abnormal GABAergic synaptic transmission with behavioral, functional and developmental consequences in different brain regions. Fyn may regulate GABAARs specifically via the β2, β3 and γ2 subunits in these different locations.
NMDA Receptor Surface Expression and Cleavage
The N-methyl-D-aspartate receptor (NMDAR) is typically a di-heteromeric glutamate receptor composed of an obligatory NR1 subunit and modulatory subunits NR2A-D. NMDARs participate in fast excitatory synaptic transmission (50) and form large multi-protein complexes at synaptic membranes (51). The NR2A and NR2B subunits have multiple tyrosine residues on their C-terminal tails that are phosphorylated by SFKs Src and Fyn (50). Exogenous Fyn upregulates NMDAR currents possibly by tyrosine phosphorylation (52). Fyn-mediated NMDAR tyrosine phosphorylation is also involved in regulation of the susceptibility of kindling and seizure (28).
Fyn deletion results in decreased tyrosine phosphorylation (pY) of NR2B and Fyn overexpression (Fyn OE) leads to elevated pY NR2B. Interestingly, Fyn KO mice have normal levels of pY NR2A, while Fyn OE mice have increased pY NR2A (28). These results suggest that in vivo Fyn may preferentially phosphorylate the NR2B subunit of the NMDAR.
In vitro, Fyn phosphorylates seven tyrosine residues on NR2B (53)(Table 1). In the developing cortex, we found that pY1070NR2B, pY1252NR2B, pY1336NR2B and pY1472NR2B expression was highest in synaptic membranes, while pY1336NR2B was also present in extrasynaptic membranes (54). One study reported that pY1252 NR2B is higher in synaptic lipid rafts compared to the post-synaptic density (PSD) (55). While the physiological function of pY1252 and pY1070NR2B is unknown, pY1336 promotes calpain cleavage of NR2B in response to glutamate exposure in vitro (56) and is associated with increased interaction of NR2B with phosphatidylinositol 3-kinase (PI-3K) (57).
Among Fyn-mediated NR2B tyrosine phosphorylation sites, Y1472 has been studied the most extensively. Phosphorylation of Y1472 maintains surface expression and synaptic localization of the NR2B-containing NMDARs as it is within the tyrosine endocytosis motif YEKL (58–60). Although pY1472 leads to increased surface expression and less endocytosis of NR2B, it does not affect excitatory synaptic transmission (59, 60). Interestingly, mice in which Y1472 has been replaced with phenylalanine (Y1472F) have impaired fear-related learning and decreased LTP in the amygdala, but normal LTP and spatial memory in the hippocampus [60]. pY1472 affects NR2B tyrosine phosphorylation, as Y1472F mice have 80% less tyrosine phosphorylation in the amygdala [60] and 70% less in the cortex compared to WT mice [61]. Y1472F mice also have changes in the NR2B complex, with less α-actinin and CaMKII associated with NR2B (60). pY1472 leads to activation of the CaMKII pathway in the amygdala and spinal cord (60, 62). Taken together, these results suggest that pY1472 affects NR2B tyrosine phosphorylation, surface expression, complex formation and downstream signaling cascades.
In summary, Fyn is involved in many processes critical for the development of the brain. Fyn regulates neuronal migration during corticogenesis, oligodendrocyte maturation, myelin production, long-term potentiation, and excitatory and inhibitory neuronal receptors.
Fyn in adult ischemic brain injury
Stroke is a leading cause of death and disability worldwide (63). Preclinical studies in rodents using SFK inhibitors suggest that targeting this kinase family may be protective in ischemic brain injury in humans (64). PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) and PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) are ATP-analogues which compete with ATP for the ATP binding pocket of SFKs, thereby decreasing the ability of SFKs to phosphorylate substrates. Both compounds have some selectivity for Fyn among SFKs (65). Experiments using the adult rodent middle cerebral artery occlusion (MCAO) model have shown that PP2 reduces infarct volume and blood brain barrier leakage (66). PP2 protects hippocampal CA1 pyramidal neurons from transient ischemia (67). PP1 decreases infarct volume, edema, neurological deficits and increases survival when given after an ischemic insult (68).
While these inhibitors demonstrate that SFKs contribute to ischemic brain injury, few studies have examined the relative contribution of specific SFKs. Using a permanent MCAO model in adult rodents, Paul et al found that Src KO mice have decreased infarct volume compared to control mice. However, brain injury in Fyn KO mice (which are on C57BL/6, 129s hybrid background) did not differ from control C57BL/6 or 129s mice (68). This study would suggest that Src may be more important to the pathogenesis of stroke in adults. However it is unknown how brain injury in Fyn KO mice compares to control mice on a hybrid background. Recently, Du et al found in an in vitro model of ischemia, oxygen glucose deprivation, that Src or Fyn knockdown leads to decreased apoptotic cell death. Fyn knockdown with siRNA had a greater neuroprotective effect (69). This study would suggest that Fyn and Src both contribute to injury, and that Fyn may be more important for apoptotic cell death, which is more prevalent in the neonatal brain.
How does Fyn contribute to ischemic brain injury in adult rodents? Consistent with its role as an adaptor protein, Fyn is part of multiple protein complexes that assemble in response to ischemia. It may also phosphorylate proteins implicated in cell death pathways.
Fyn exists in at least three ischemia-induced complexes with the NMDAR, PSD95, L-type voltage gated calcium channel (LVGCC), and SynGAP (Figure 3A). Fyn interacts with NMDAR subunits NR2A and NR2B in response to ischemia (17). While it is unknown whether Fyn directly phosphorylates the NMDAR in this setting, its interaction with the NMDAR coincides with elevated tyrosine phosphorylation (70). Tyrosine phosphorylation of NR2A and the NR2A-Fyn association are enhanced by PSD95 after transient brain ischemia (71). Studies have shown that PSD95 and the NMDAR couple to the neurotoxic nitric oxide signaling pathway and disrupting this interaction is protective (72). It is possible that Fyn may promote this interaction, since the NMDAR and PSD95 are Fyn substrates (Table 1).
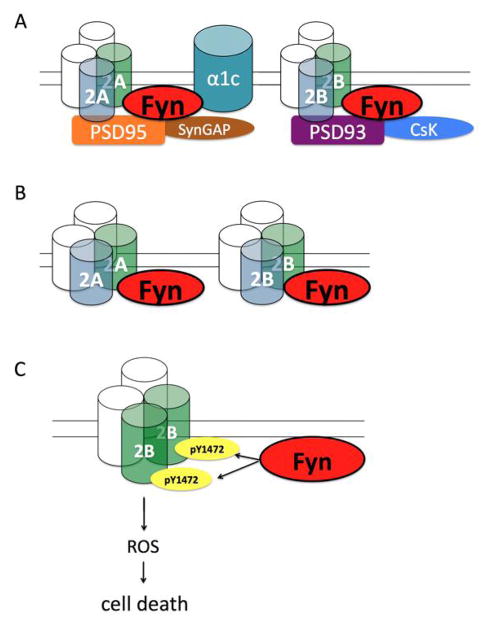
Figure 3.
Fyn complexes during ischemia in adult and neonatal rodents A) In response to ischemia in adult rodents, Fyn interacts with two receptors that flux calcium, the NMDA receptor and α1c subunit of L-type voltage gated calcium channel. PSD95 facilitates the interaction between Fyn and NR2A. Fyn also associates with SynGAP. Additionally, PSD93 interacts with NR2A/NR2B as well as with Fyn. Tyrosine-phosphorylated PSD93 binds to Csk, a negative regulator of SFKs. PSD93 enhances Fyn/NR2B association and NR2B tyrosine phosphorylation in adult brain ischemia. B) Fyn forms a complex with NMDA receptor subunits NR2A and NR2B during neonatal hypoxic-ischemic brain injury. These proteins are tyrosine phosphorylated by Fyn, which strengthens complex formation and likely contributes to pathogenic signaling in the setting of ischemia. C) Phosphorylation of Y1472 NR2B by Fyn mediates cell death by reactive oxygen species (ROS) generation in a calcium independent manner.
Fyn phosphorylates PSD95 at Y523, which leads to upregulation of glutamate receptor channel activity in cultured hippocampal neurons (73). The Fyn-NR2A-PSD95 complex is positively regulated by protein tyrosine kinases and negatively regulated by protein tyrosine phosphatases (71). Two neuroprotective agents, Chinese traditional medicine Sy-21 and lithium, are associated with decreased pY NR2A and decreased formation of the Fyn-PSD95-NR2A complex (74, 75). These results suggest that tyrosine phosphorylation of this complex by Fyn may contribute directly to ischemic brain injury by increasing calcium influx through the NMDAR via PSD95 while also increasing nitric oxide signaling by promoting the NMDAR-PSD95 interaction.
To add another layer of complexity, the Fyn-NR2A-PSD95 complex is enhanced by NMDAR and L-type voltage gated calcium channel (L-VGCC) activity [76]. Fyn interacts directly with the α1c subunit of L-VGCC during the peak of its tyrosine phosphorylation (77). Fyn also interacts with PSD95 associated GTPase, SynGAP during ischemia. Pei et al found that SynGAP tyrosine phosphorylation increases after ischemia, as does its association with Fyn. It is unknown whether Fyn regulates SynGAP phosphorylation or how SynGAP participates in ischemia (16).
Similar to PSD95, PSD93, another member of the membrane-associated guanylate kinase family of proteins, is also found to be a Fyn substrate (78) and interacts with NR2A/NR2B as well as with Fyn (79) in mouse cortex. Interestingly, tyrosine-phosphorylated PSD93 binds to Csk, a negative regulator of SFKs activity, and thereby forms a feedback loop to modulate SFKs-mediated phosphorylation of PSD proteins (78). In adult brain ischemia, PSD93 enhances Fyn/NR2B association and Fyn-mediated tyrosine phosphorylation of NR2B, including NR2B phosphorylation at Y1472, and contributes to brain injury (80). We did not see protection from PSD93 deficiency in neonatal brain hypoxia-ischemia (HI), possibly due to compensation of PSD95 (81). While PSD95 and PSD93 have unique properties, they may coordinate with Fyn in regulating NMDAR function and downstream signaling in normal neuronal plasticity and in response to pathological conditions.
Taken together, these studies suggest that, as an intrinsic PSD tyrosine kinase and one of the core glutamate receptor kinase, Fyn is involved in phosphorylation and assembly/remodeling of the PSD complexes in response to ischemia in the adult brain. Fyn may phosphorylate several proteins such as PSD95, PSD93, SynGAP and membrane channels leading to increased calcium flux and cell death signaling pathways (Figure 3A). While these studies implicate Fyn in the pathogenesis of ischemic brain injury in adults, much less is known about the contribution of Fyn to ischemic brain injury in neonates.
Fyn in neonatal hypoxic-ischemic brain injury
Neonatal encephalopathy affects 1–6/1000 live births (82). Encephalopathy is derived from the Greek words enkephalos (brain) and pathos (disease) and refers to a disorder of the brain resulting in global dysfunction. Neonatal hypoxic-ischemic encephalopathy (HIE) is caused by hypoxia-ischemia during the prenatal, perinatal or postnatal periods (82). Neonatal HIE is modeled in postnatal day 7 (P7) and 10 rodents by exposing them to unilateral common carotid artery ligation followed by systemic hypoxia. This leads to injury in the cortex, hippocampus and striatum ipsilateral to the ligation (83–85).
Age-dependent differences in subunit expression and phosphorylation of NMDAR are associated with selective vulnerability of neonatal brain to HIE. While NR2B expression declines with age, tyrosine phosphorylation of NR2B, as well as pY1472NR2B, increase with age (53). By contrast, upregulation of NR2A protein level is paralleled by a decrease of its tyrosine phosphorylation with development (86). HI induced a rapid reduction of NR2A, leaving predominately NR2B tyrosine phosphorylation at P7 in rat brain. At P21, NR2B was reduced after HI and both NR2A and NR2B tyrosine phosphorylation was increased (86). Although it is uncertain whether SFKs are responsible for these phosphorylation events, we found that in the mouse, NR2B and Fyn are expressed at much higher levels at P7 in both synaptic and extrasynaptic membranes than in the adult brain, suggesting the importance of Fyn in regulating NR2B in the developing brain (54). SFKs are activated in response to HI in neonatal mice. SFK activity correlates with elevated NR2A and NR2B tyrosine phosphorylation. Fyn has increased association with NR2A and NR2B in response to injury. Significantly, SFK inhibitor PP2 was protective (87). To address the specific contribution of Fyn, we subjected mice overexpressing Fyn in neurons to neonatal HI. Fyn OE mice have increased brain injury and mortality following injury. This is associated with increased tyrosine phosphorylation of NR2A and NR2B as well as increased calpain activity. NR2B phosphorylation at Y1252 and Y1472 is higher in Fyn OE mice in synaptic fractions (88). These studies are consistent with the adult ischemia literature, namely that Fyn forms a complex with the NMDAR during HI and PP2 is protective (Figure 3B).
Next, we explored the functional consequence of Fyn-mediated NMDAR tyrosine phosphorylation in neonatal HI brain injury. We found that mice with a knock-in mutation of Y1472 to phenylalanine (YF-KI) had less brain injury than WT mice subjected to neonatal HI. This correlated with decreased activity of proteases implicated in apoptotic and necrotic cell death. Intriguingly, YF-KI mice had reduced superoxide production and decreased expression of NADPH oxidase subunits gp91phox and p47phox in vivo. In vitro, YF-KI neurons had decreased superoxide generation in response to NMDA without affecting calcium accumulation. Additionally, YF-KI neurons were protected from NMDA and glutamate-induced cell death (61). Taken together, these studies demonstrate that Fyn-mediated phosphorylation of NR2B contributes to cell death, in part, by regulating superoxide-related oxidative signaling during HI and excitotoxicity (Figure 3C).
Given the crucial function of Fyn in neurodevelopment, future investigations of the mechanisms and functional consequences of Fyn-mediated cell death in neonatal HI will accelerate our progress towards developing therapies that selectively target critical signaling nodes mediating brain injury without disrupting normal brain development.
Acknowledgments
This work was supported by grants F31NS073145 to Dr. Knox; R21NS059613 and RO1NS084057 to Dr. Jiang. We thank Dr. Donna Ferriero for her careful review of the manuscript.
References
- 1.Bare DJ, Lauder JM, Wilkie MB, Maness PF. p59fyn in rat brain is localized in developing axonal tracts and subpopulations of adult neurons and glia. Oncogene. 1993;8:1429–1436. [PubMed] [Google Scholar]
- 2.Schenone S, Brullo C, Musumeci F, Biava M, Falchi F, Botta M. Fyn kinase in brain diseases and cancer: the search for inhibitors. Curr Med Chem. 2011;18:2921–2942. doi: 10.2174/092986711796150531. [DOI] [PubMed] [Google Scholar]
- 3.Umemori H, Wanaka A, Kato H, Takeuchi M, Tohyama M, Yamamoto T. Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system. Brain Res Mol Brain Res. 1992;16:303–310. doi: 10.1016/0169-328x(92)90239-8. [DOI] [PubMed] [Google Scholar]
- 4.Inomata M, Takayama Y, Kiyama H, Nada S, Okada M, Nakagawa H. Regulation of Src family kinases in the developing rat brain: correlation with their regulator kinase, Csk. J Biochem. 1994;116:386–392. doi: 10.1093/oxfordjournals.jbchem.a124536. [DOI] [PubMed] [Google Scholar]
- 5.Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;269:16701–16705. [PubMed] [Google Scholar]
- 6.Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 9.Moran MF, Koch CA, Anderson D, Ellis C, England L, Martin GS, Pawson T. Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc Natl Acad Sci USA. 1990;87:8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- 11.Smith GS, De Avila M, Paez PM, Spreuer V, Wills MK, Jones N, Boggs JM, Harauz G. Proline substitutions and threonine pseudophosphorylation of the SH3 ligand of 18. 5-kDa myelin basic protein decrease its affinity for the Fyn-SH3 domain and alter process development and protein localization in oligodendrocytes. J Neurosci Res. 2012;90:28–47. doi: 10.1002/jnr.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usardi A, Pooler AM, Seereeram A, Reynolds CH, Derkinderen P, Anderton B, Hanger DP, Noble W, Williamson R. Tyrosine phosphorylation of tau regulates its interactions with Fyn SH2 domains, but not SH3 domains, altering the cellular localization of tau. FEBS Journal. 2011;278:2927–2937. doi: 10.1111/j.1742-4658.2011.08218.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal R, Chao MV. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol Cell Neurosci. 2006;33:36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- 15.Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279:18887–18894. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- 16.Pei L, Teves RL, Wallace MC, Gurd JW. Transient cerebral ischemia increases tyrosine phosphorylation of the synaptic RAS-GTPase activating protein, SynGAP. J Cereb Blood Flow Metab. 2001;21:955–963. doi: 10.1097/00004647-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Takagi N, Cheung HH, Bissoon N, Teves L, Wallace MC, Gurd JW. The effect of transient global ischemia on the interaction of Src and Fyn with the N-methyl-D-aspartate receptor and postsynaptic densities: possible involvement of Src homology 2 domains. J Cereb Blood Flow Metab. 1999;19:880–888. doi: 10.1097/00004647-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Cooper JA, Gould KL, Cartwright CA, Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 20.Gonfloni S, Weijland A, Kretzschmar J, Superti-Furga G. Crosstalk between the catalytic and regulatory domains allows bidirectional regulation of Src. Nat Struct Mol Biol. 2000;7:281–286. doi: 10.1038/74041. [DOI] [PubMed] [Google Scholar]
- 21.Bhandari V, Lim KL, Pallen CJ. Physical and functional interactions between receptor-like protein-tyrosine phosphatase alpha and p59fyn. J Biol Chem. 1998;273:8691–8698. doi: 10.1074/jbc.273.15.8691. [DOI] [PubMed] [Google Scholar]
- 22.Ponniah S, Wang DZ, Lim KL, Pallen CJ. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang PS, Wang J, Xiao ZC, Pallen CJ. Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J Biol Chem. 2009;284:33692–33702. doi: 10.1074/jbc.M109.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 25.Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y. A role for Fyn tyrosine kinase in the suckling behaviour of neonatal mice. Nature. 1993;366:742–745. doi: 10.1038/366742a0. [DOI] [PubMed] [Google Scholar]
- 26.Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci USA. 1997;94:4761–4765. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima N, Ishibashi H, Obata K, Kandel ER. Higher seizure susceptibility and enhanced tyrosine phosphorylation of N-methyl-D-aspartate receptor subunit 2B in fyn transgenic mice. Learn Mem. 1998;5:429–445. [PMC free article] [PubMed] [Google Scholar]
- 29.Yuasa S, Hattori K, Yagi T. Defective neocortical development in Fyn-tyrosine-kinase-deficient mice. Neuroreport. 2004;15:819–822. doi: 10.1097/00001756-200404090-00016. [DOI] [PubMed] [Google Scholar]
- 30.Honda T, Kobayashi K, Mikoshiba K, Nakajima K. Regulation of cortical neuron migration by the Reelin signaling pathway. Neurochem Res. 2011;36:1270–1279. doi: 10.1007/s11064-011-0407-4. [DOI] [PubMed] [Google Scholar]
- 31.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 32.Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- 33.Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beggs HE, Soriano P, Maness PF. NCAM-dependent neurite outgrowth is inhibited in neurons from Fyn-minus mice. J Cell Biol. 1994;127(3):825–833. doi: 10.1083/jcb.127.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Beggs H, Jürgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7(11):1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krämer-Albers EM, White R. From axon-glial signalling to myelination: the integrating role of oligodendroglial Fyn kinase. Cell Mol Life Sci. 2011;68:2003–2012. doi: 10.1007/s00018-010-0616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperber BR, McMorris FA. Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. J Neurosci Res. 2001;63:303–312. doi: 10.1002/1097-4547(20010215)63:4<303::AID-JNR1024>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Goto J, Tezuka T, Nakazawa T, Sagara H, Yamamoto T. Loss of Fyn tyrosine kinase on the C57BL/6 genetic background causes hydrocephalus with defects in oligodendrocyte development. Mol Cell Neurosci. 2008;38:203–212. doi: 10.1016/j.mcn.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 39.White R, Gonsior C, Krämer-Albers EM, Stöhr N, Hüttelmaier S, Trotter J. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J Cell Biol. 2008;181:579–586. doi: 10.1083/jcb.200706164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 41.Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 42.Lu YF, Kojima N, Tomizawa K, Moriwaki A, Matsushita M, Obata K, Matsui H. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11:75–82. doi: 10.1046/j.1460-9568.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 43.Babus LW, Little EM, Keenoy KE, Minami SS, Chen E, Song JM, Caviness J, Koo SY, Pak DTS, Rebeck GW, Turner RS, Hoe HS. Decreased dendritic spine density and abnormal spine morphology in Fyn knockout mice. Brain Res. 2011;1415:96–102. doi: 10.1016/j.brainres.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitazawa H, Yagi T, Miyakawa T, Niki H, Kawai N. Abnormal synaptic transmission in the olfactory bulb of Fyn-kinase-deficient mice. J Neurophysiol. 1998;79:137–142. doi: 10.1152/jn.1998.79.1.137. [DOI] [PubMed] [Google Scholar]
- 46.Boehm SL, Peden L, Harris RA, Blednov YA. Deletion of the fyn-kinase gene alters sensitivity to GABAergic drugs: dependence on beta2/beta3 GABAA receptor subunits. J Pharmacol Exp Ther. 2004;309:1154–1159. doi: 10.1124/jpet.103.064444. [DOI] [PubMed] [Google Scholar]
- 47.Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. Mol Cell Neurosci. 2010;44:129–134. doi: 10.1016/j.mcn.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tretter V, Revilla-Sanchez R, Houston C, Terunuma M, Havekes R, Florian C, Jurd R, Vithlani M, Michels G, Couve A, Sieghart W, Brandon N, Abel T, Smart TG, Moss SJ. Deficits in spatial memory correlate with modified {gamma}-aminobutyric acid type A receptor tyrosine phosphorylation in the hippocampus. Proc Natl Acad Sci U S A. 2009;106:20039–20044. doi: 10.1073/pnas.0908840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chattopadhyaya B, Baho E, Huang ZJ, Schachner M, Di Cristo G. Neural cell adhesion molecule-mediated Fyn activation promotes GABAergic synapse maturation in postnatal mouse cortex. J Neurosci. 2013;33:5957–5968. doi: 10.1523/JNEUROSCI.1306-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salter M, Kalia L. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 51.Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 52.Köhr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol (Lond) 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 54.Jiang X, Knox R, Pathipati P, Ferriero D. Developmental localization of NMDA receptors, Src and MAP kinases in mouse brain. Neurosci Lett. 2011;503:215–219. doi: 10.1016/j.neulet.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delint-Ramirez I, Fernández E, Bayés A, Kicsi E, Komiyama NH, Grant SGN. In vivo composition of NMDA receptor signaling complexes differs between membrane subdomains and is modulated by PSD-95 and PSD-93. J Neurosci. 2010;30:8162–8170. doi: 10.1523/JNEUROSCI.1792-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu H, Hsu F, Gleichman A, Baconguis I, Coulter D, Lynch D. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hisatsune C, Umemori H, Mishina M, Yamamoto T. Phosphorylation-dependent interaction of the N-methyl-D-aspartate receptor epsilon 2 subunit with phosphatidylinositol 3-kinase. Genes Cells. 1999;4:657–666. doi: 10.1046/j.1365-2443.1999.00287.x. [DOI] [PubMed] [Google Scholar]
- 58.Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- 59.Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knox R, Brennan-Minnella AM, Lu F, Yang D, Nakazawa T, Yamamoto T, Swanson RA, Ferriero DM, Jiang X. NR2B phosphorylation at Tyrosine 1472 contributes to brain injury in a rodent model of neonatal hypoxia-ischemia. Stroke. 2014;45:3040–3047. doi: 10.1161/STROKEAHA.114.006170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumura S, Kunori S, Mabuchi T, Katano T, Nakazawa T, Abe T, Watanabe M, Yamamoto T, Okuda-Ashitaka E, Ito S. Impairment of CaMKII activation and attenuation of neuropathic pain in mice lacking NR2B phosphorylated at Tyr1472. Eur J Neurosci. 2010;32:798–810. doi: 10.1111/j.1460-9568.2010.07348.x. [DOI] [PubMed] [Google Scholar]
- 63.Davis SM, Donnan GA. Clinical practice. Secondary prevention after ischemic stroke or transient ischemic attack. N Engl J Med. 2012;366:1914–1922. doi: 10.1056/NEJMcp1107281. [DOI] [PubMed] [Google Scholar]
- 64.Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8:892–909. doi: 10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 66.Takenaga Y, Takagi N, Murotomi K, Tanonaka K, Takeo S. Inhibition of Src activity decreases tyrosine phosphorylation of occludin in brain capillaries and attenuates increase in permeability of the blood-brain barrier after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1099–1108. doi: 10.1038/jcbfm.2009.30. [DOI] [PubMed] [Google Scholar]
- 67.Hou XY, Liu Y, Zhang GY. PP2, a potent inhibitor of Src family kinases, protects against hippocampal CA1 pyramidal cell death after transient global brain ischemia. Neurosci Lett. 2007;420:235–239. doi: 10.1016/j.neulet.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 68.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 69.Du CP, Tan R, Hou XY. Fyn kinases play a critical role in neuronal apoptosis induced by oxygen and glucose deprivation or amyloid-β peptide treatment. CNS Neurosci Ther. 2012;18:754–761. doi: 10.1111/j.1755-5949.2012.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheung HH, Takagi N, Teves L, Logan R, Wallace MC, Gurd JW. Altered association of protein tyrosine kinases with postsynaptic densities after transient cerebral ischemia in the rat brain. J Cereb Blood Flow Metab. 2000;20:505–512. doi: 10.1097/00004647-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Chen M, Hou X, Zhang G. Tyrosine kinase and tyrosine phosphatase participate in regulation of interactions of NMDA receptor subunit 2A with Src and Fyn mediated by PSD-95 after transient brain ischemia. Neurosci Lett. 2003;339:29–32. doi: 10.1016/s0304-3940(02)01439-8. [DOI] [PubMed] [Google Scholar]
- 72.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 73.Du CP, Gao J, Tai JM, Liu Y, Qi J, Wang W, Hou XY. Increased tyrosine phosphorylation of PSD-95 by Src family kinases after brain ischaemia. Biochem J. 2009;417:277–285. doi: 10.1042/BJ20080004. [DOI] [PubMed] [Google Scholar]
- 74.Chen M, Wang Y, Liu Y, Hou XY, Zhang QG, Meng FJ, Zhang GY. Possible mechanisms underlying the protective effects of SY-21, an extract of a traditional Chinese herb, on transient brain ischemia/reperfusion-induced neuronal death in rat hippocampus. Brain Res. 2003;989:180–186. doi: 10.1016/s0006-8993(03)03331-6. [DOI] [PubMed] [Google Scholar]
- 75.Ma J, Zhang GY. Lithium reduced N-methyl-d-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia. Neurosci Lett. 2003;348:185–189. doi: 10.1016/s0304-3940(03)00784-5. [DOI] [PubMed] [Google Scholar]
- 76.Hou XY, Zhang GY, Yan JZ, Chen M, Liu Y. Activation of NMDA receptors and L-type voltage-gated calcium channels mediates enhanced formation of Fyn-PSD95-NR2A complex after transient brain ischemia. Brain Res. 2002;955(1–2):123–132. doi: 10.1016/s0006-8993(02)03376-0. [DOI] [PubMed] [Google Scholar]
- 77.Hou XY, Zhang GY, Yan JZ, Liu Y. Increased tyrosine phosphorylation of α1C subunits of L-type voltage-gated calcium channels and interactions among Src/Fyn, PSD-95 and α1C in rat hippocampus after transient brain ischemia. Brain Res. 2003;979:43–50. doi: 10.1016/s0006-8993(03)02845-2. [DOI] [PubMed] [Google Scholar]
- 78.Nada S, Shima T, Yanai H, Husi H, Grant SG, Okada M, Akiyama T. Identification of PSD-93 as a substrate for the Src family tyrosine kinase Fyn. J Biol Chem. 2003;278:47610–47621. doi: 10.1074/jbc.M303873200. [DOI] [PubMed] [Google Scholar]
- 79.Sato Y, Tao YX, Su Q, Johns RA. Post-synaptic density-93 mediates tyrosine-phosphorylation of the N-methyl-D-aspartate receptors. Neuroscience. 2008;153(3):700–708. doi: 10.1016/j.neuroscience.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang M, Li Q, Chen L, Li J, Zhang X, Chen X, Zhang Q, Shao Y, Xu Y. PSD-93 deletion inhibits Fyn-mediated phosphorylation of NR2B and protects against focal cerebral ischemia. Neurobiol Dis. 2014;68:104–111. doi: 10.1016/j.nbd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Jiang X, Mu D, Sheldon RA, Glidden DV, Ferriero DM. Neonatal hypoxia-ischemia differentially upregulates MAGUKs and associated proteins in PSD-93-deficient mouse brain. Stroke. 2003;34(12):2958–2963. doi: 10.1161/01.STR.0000102560.78524.9D. [DOI] [PubMed] [Google Scholar]
- 82.Ferriero D. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 83.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 84.Ditelberg JS, Sheldon RA, Epstein CJ, Ferriero DM. Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatr Res. 1996;39:204–208. doi: 10.1203/00006450-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 86.Gurd JW, Bissoon N, Beesley PW, Nakazawa T, Yamamoto T, Vannucci SJ. Differential effects of hypoxia-ischemia on subunit expression and tyrosine phosphorylation of the NMDA receptor in 7- and 21-day-old rats. J Neurochem. 2002;82(4):848–856. doi: 10.1046/j.1471-4159.2002.01026.x. [DOI] [PubMed] [Google Scholar]
- 87.Jiang X, Mu D, Biran V, Faustino J, Chang S, Rincón C, Sheldon R, Ferriero D. Activated Src kinases interact with the N-methyl-D-aspartate receptor after neonatal brain ischemia. Ann Neurol. 2008;63:632–641. doi: 10.1002/ana.21365. [DOI] [PubMed] [Google Scholar]
- 88.Knox R, Zhao C, Miguel-Perez D, Wang S, Yuan J, Ferriero D, Jiang X. Enhanced NMDA receptor tyrosine phosphorylation and increased brain injury following neonatal hypoxia-ischemia in mice with neuronal Fyn overexpression. Neurobiol Dis. 2013;51:113–119. doi: 10.1016/j.nbd.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lim SH, Kwon SK, Lee MK, Moon J, Jeong DG, Park E, Kim SJ, Park BC, Lee SC, Ryu SE, Yu DY, Chung BH, Kim E, Myung PK, Lee JR. Synapse formation regulated by protein tyrosine phosphatase receptor T through interaction with cell adhesion molecules and Fyn. EMBO J. 2009;28:3564–3578. doi: 10.1038/emboj.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahn M, Beacham D, Westenbroek RE, Scheuer T, Catterall WA. Regulation of Na(v)1. 2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J Neurosci. 2007;27:11533–11542. doi: 10.1523/JNEUROSCI.5005-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki T, Okumura-Noji K. NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- 92.Liu H, Nakazawa T, Tezuka T, Yamamoto T. Physical and functional interaction of Fyn tyrosine kinase with a brain-enriched Rho GTPase-activating protein TCGAP. J Biol Chem. 2006;281:23611–23619. doi: 10.1074/jbc.M511205200. [DOI] [PubMed] [Google Scholar]
- 93.Taniguchi S, Liu H, Nakazawa T, Yokoyama K, Tezuka T, Yamamoto T. p250GAP, a neural RhoGAP protein, is associated with and phosphorylated by Fyn. Biochem Biophys Res Commun. 2003;306:151–155. doi: 10.1016/s0006-291x(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 94.Brouns MR, Matheson SF, Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat Cell Biol. 2001;3:361–367. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- 95.Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, Ohno S, Nakamura F, Goshima Y. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002;35:907–920. doi: 10.1016/s0896-6273(02)00857-7. [DOI] [PubMed] [Google Scholar]
- 96.Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, Leitman E, Binder LI, Brady ST. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging. 2012;33:826.e15–30. doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zamora-Leon SP, Lee G, Davies P, Shafit-Zagardo B. Binding of Fyn to MAP-2c through an SH3 binding domain. Regulation of the interaction by ERK2. J Biol Chem. 2001;276:39950–39958. doi: 10.1074/jbc.M107807200. [DOI] [PubMed] [Google Scholar]
- 99.Zamora-Leon SP, Bresnick A, Backer JM, Shafit-Zagardo B. Fyn phosphorylates human MAP-2c on tyrosine 67. J Biol Chem. 2005;280:1962–1970. doi: 10.1074/jbc.M411380200. [DOI] [PubMed] [Google Scholar]
- 100.Stoss O, Novoyatleva T, Gencheva M, Olbrich M, Benderska N, Stamm S. p59(fyn)-mediated phosphorylation regulates the activity of the tissue-specific splicing factor rSLM-1. Mol Cell Neurosci. 2004;27:8–21. doi: 10.1016/j.mcn.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Nakamura T, Yamashita H, Takahashi T, Nakamura S. Activated Fyn phosphorylates α-Synuclein at tyrosine residue 125. Biochem Biophys Res Commun. 2001;280:1085–1092. doi: 10.1006/bbrc.2000.4253. [DOI] [PubMed] [Google Scholar]
- 102.Nishio H, Otsuka M, Kinoshita S, Tokuoka T, Nakajima M, Noda Y, Fukuyama Y, Suzuki K. Phosphorylation of c-Cbl protooncogene product following ethanol administration in rat cerebellum: possible involvement of Fyn kinase. Brain Res. 2002;950:203–209. doi: 10.1016/s0006-8993(02)03038-x. [DOI] [PubMed] [Google Scholar]
- 103.Suetsugu S, Hattori M, Miki H, Tezuka T, Yamamoto T, Mikoshiba K, Takenawa T. Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev Cell. 2002;3:645–658. doi: 10.1016/s1534-5807(02)00324-6. [DOI] [PubMed] [Google Scholar]
- 104.Feng L, Cooper JA. Dual functions of Dab1 during brain development. Mol Cell Biol. 2009;29:324–332. doi: 10.1128/MCB.00663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]