Abstract
Recent clinical trials in patients with heart failure with preserved ejection fraction (HFpEF) have provided important insights into participant selection strategies. Historically, HFpEF trials have included patients with relatively preserved left ventricular ejection fraction ranging from 40% to 55% and a clinical history of heart failure. Contemporary HFpEF trials have also incorporated inclusion criteria such as hospitalization for HFpEF, altered functional capacity, cardiac structural and functional abnormalities, and abnormalities in neurohormonal status (e.g., elevated natriuretic peptide levels). Careful analyses of the impact of these patient selection criteria on outcomes in prior trials provide valuable lessons for future trial design. We review recent and ongoing HFpEF clinical trials from a patient selection perspective and appraise trial patient selection methodologies in relation to outcomes. This review reflects discussions between clinicians, scientists, trialists, regulators, and regulatory representatives at the 10th Global CardioVascular Clinical Trialists Forum in Paris, France on December 6, 2013.
Keywords: clinical protocols, methodology, natriuretic peptides, patient selection
Heart failure with preserved ejection fraction (HFpEF) currently represents almost half of all heart failure (HF) patients and, with the growing elderly population, is projected to become the predominant form of HF in the future. HFpEF represents a large unmet need in cardiovascular medicine (1,2). Over 5 million Americans and 23 million people worldwide have HF, of which patients with HFpEF constitute more than 50% and this percentage will continue to rise with our aging population (1,3–5). In general, outcomes in HFpEF are similarly poor as those in patients with heart failure with reduced ejection fraction (HFrEF) with respect to hospitalization and mortality risk. Despite the therapeutic advances for patients with HFrEF through landmark clinical trials on neurohormonal modulation and device therapy, clinical trials in patients with HFpEF have been challenging and results have been neutral. Important lessons can be learned from these prior trials. In this paper, we summarize recent and ongoing HFpEF clinical trials and appraise trial methodologies from the perspective of patient selection in order to critically inform the design of future randomized clinical trials for clinicians, researchers, and patients.
Guideline Definitions for HFpEF
Recommendations for the diagnosis of patients with HFpEF are similar in scope and depth across the most recent U.S. and European guidelines (6–9). The most recent American College of Cardiology (ACC)/American Heart Association (AHA) guidelines defined HFpEF as patients with ejection fraction (EF) ≥50% with symptoms suggestive of HF and exclusion of other potential noncardiac etiologies of HF. The guidelines also include subpopulations of borderline HFpEF with EF 41% to 49% and HFpEF with improved EF >40% for patients previously with reduced EF (6). The 2012 European Society of Cardiology (ESC) guidelines defined 4 requirements to diagnose HFpEF, including: 1) symptoms typical of HF; 2) signs typical of HF; 3) normal or only mildly reduced left ventricular EF without left ventricular dilation; and 4) relevant structural heart disease (left ventricular hypertrophy/left atrial enlargement) and/or diastolic dysfunction (Table 1)(8,9). The underlying pathophysiologic mechanisms behind HFpEF involve, in part, a diffuse inflammatory state that develops from the constellation of such frequently co-existing comorbidities as chronic obstructive lung disease, anemia, diabetes mellitus, renal dysfunction and obesity in patients with HFpEF (10,11).. The proinflammatory state limits the available nitric oxide in the coronary microvasculature and shifts cardiac remodeling towards hypertrophy and interstitial fibrosis, which increases left ventricular diastolic stiffness and the conditions for HFpEF(12).
Table 1.
Summary of HFpEF Diagnosis Guidelines
| Guidelines | Diagnosis |
|---|---|
| ESC (9) | The following 4 criteria are required: 1) symptoms typical of HF; 2) signs typical of HF; 3) normal or only mildly reduced LVEF and LV not dilated; and 4) relevant structural heart disease (LV hypertrophy/LA enlargement) and/or diastolic dysfunction |
| ACC/AHA–HFpEF (6) |
Diastolic HF. Multiple criteria have been used; exclude other potential noncardiac causes of symptoms suggestive of HF. |
| ACC/AHA–EF 41%-49% |
Borderline or intermediate EF; these patients have similar characteristics, treatment patterns, and outcomes to those with HFpEF |
| ACC/AHA– improved EF |
Patients previously with HFrEF; improved or recovered EF clinically distinct from patients with preserved or reduced EF. |
| HFSA (7) | Patients with EF ≥50% with symptoms suggestive of HF. Use echocardiography, ECG, stress imaging, or cardiac catheterization to distinguish HF with preserved LVEF and other cardiac disorders. |
ACC = American College of Cardiology; AHA = American Heart Association; ECG = electrocardiogram; ESC = indicates European Society of Cardiology; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HFSA = Heart Failure Society of America LA = left atrial; LV = left ventricle; LVEF = left ventricular ejection fraction.
Definitions in Clinical Trials
The first large clinical trial that focused on patients with HFpEF, the CHARM (Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity) Preserved trial, required an EF >40%, New York Heart Association (NYHA) class II-IV symptoms for >4 weeks, and any prior hospital admission for a cardiac reason (13). This definition was analogous to HFrEF trials at the time, where EF cutpoints <35% and <45% were used in addition to HF symptoms or known history of HF (14,15). As the results from clinical trials and secondary analyses in these HFpEF populations without use of guideline criteria revealed low event rates and limited benefits from traditional HF therapies, clinical trialists subsequently adjusted entry criteria (16). The EF criterion was increased, echocardiographic parameters were incorporated, and eventually natriuretic peptide (NP) levels were included in a combined definition that also required HF symptoms (Table 2). Preserved EF ≥50%, symptoms and/or hospitalization for HF, echocardiographic findings, and elevated NP levels exemplified the prevailing thought that the underpinning of HFpEF pathophysiology was primarily a disease of elderly women with stiff left ventricles from long-standing hypertension and concomitant diabetes mellitus. However, clinical trials, cohort studies, and registry analyses have demonstrated that the HFpEF population is heterogeneous, particularly with respect to comorbidities (17). Future clinical trials in HFpEF may benefit from further refinement of these key patient selection criteria in order to optimize the potential for success.
Table 2.
Inclusion Criteria in Selected Previous and Ongoing Clinical Trials and Registries of Patients With HFpEF
| Trial | Clinical Dx HF | Prior HF Admission |
EF % |
Atrial Fibrillation/ Atrial Flutter |
BNP * pg/ml |
NT- proBNP *pg/ml |
Event Rate† (1-yr) |
Primary Endpoint | Clinical Findings |
|---|---|---|---|---|---|---|---|---|---|
| CHARM- PRESERVED (11) |
NYHA II-IV >4 weeks, hospitalized for cardiac reason, history of hospital admission for cardiac reason |
68.8 | >40 | 29.3% | -- | -- | 9.1% | CV death or HF hospitalization |
No reduction in CV death; fewer patients in treatment group had HF hospitalization |
| PEP-CHF (18) | Age ≥70, on diuretics, Echo with DD, 3/9 clinical, 2/4 echo criteria, CV hospitalization within 6 months |
100 | >40 | 22% | -- | 453 | 11.96% | Composite of all- cause mortality and unplanned HF hospitalization |
No reduction in all-cause mortality or HF hospitalization |
| DIG-PEF (87) | NSR, HF symptoms |
-- | >45 | 0 | -- | -- | 7.8% | Combined HF hospitalization or HF mortality |
Digoxin had no effect on mortality or all-cause CV hospitalization |
| SENIORS (28) | Age ≥70, HF history + ≥1 HF hospitalization or EF ≤35% within past 6 months |
-- | >35 | 37.1% | -- | -- | 19.2% | All-cause mortality or CV hospitalizations |
No difference of nebivolol effect on elderly patients with HFpEF vs. HFrEF |
| I-PRESERVE (19) |
NYHA II-IV | 44% | ≥45 | 17% | -- | 320 | 10.54% | All-cause mortality or CV hospitalization |
No improvement from irbesartan in primary endpoints |
| ELANDD (88) | NYHA II-III, Echo DD |
-- | >45 | -- | -- | 147 | -- | Change in 6MWD after 6 months |
No change in 6MWD or peak VO2 |
| J-DHF (89) | Modified Framingham criteria for HF within 12 months |
60% | >40 | 45.6% | 235 – mean |
-- | 8.5 | Composite of CV death and unplanned HF hospitalization |
No improvement from carvedilol |
| ALDO-DHF (52) | Ambulatory NYHA Class II-III HF, Echo DD |
4% | ≥50 | 9% | -- | 148 | -- | Coprimary: change in diastolic function (E/e’) and peak VO2 at 12 months |
Improved DD but no change in peak VO2 |
| Ex-DHF (90) | Symptomatic NYHA II-III outpatients, age ≥45 yrs, DD grade ≥1, NSR, 1 CV risk factor |
-- | ≥50 | -- | -- | -- | -- | Change in peak VO2 after 3 months |
Peak VO2 increased 3.3 ml/min/kg, E/e’ and LAVI decreased |
| PARAMOUNT (21) |
NYHA II-IV | 45% | ≥45 | 43% | -- | >400 | n/a | Change in NT- proBNP from BL to 12 weeks |
LCZ696 reduced NT- proBNP more than valsartan at 12 weeks |
| RELAX (51) | Stable outpatients with HF, elevated NT-proBNP or elevated filling pressures, reduced exercise capacity |
39% | ≥50 | 50 | -- | 648 | 12.6 | Change in peak VO2 after 24 weeks of therapy |
No improvement in peak VO2 |
| RELAX-AHF (91) |
Admitted for AHF (dyspnea at rest or minimum exertion, pulmonary congestion on chest radiograph & BNP ≥350 pg/ml or NT- proBNP ≥1,400 pg/ml and eGFR 30-75 ml/min per 1.73 m2 |
29.5% | ≥50 | 61.2% | -- | 3992 | 12.75%‡ | Change from BL in VAS AUC to day 5 and proportion of patients with improved dyspnea by Likert scale during first 24 h |
Improved dyspnea relief by the VAS- AUC and Likert scale |
| RAAM-PEF (92) | NYHA II-III, clinical HF and BNP ≥100 pg/ml within 2 months |
60.9% | ≥50 | 13% | 284 | -- | -- | Change in 6MWD | No change in 6MWD |
| SHIFT- PRESERVED (68) |
Signs or symptoms of HF, Echo with DD, Exercise capacity <80% age/sex predicted, E/e’ >13 after exercise |
-- | ≥50 | -- | 62 | -- | -- | Exercise capacity, E/e’ |
Improved peak VO2 and improved E/e’ after exercise |
| TOPCAT (48) | History of hospitalization within previous 12 months with HF component or elevated BNP within 60 days |
71.5% | ≥45 | >100 235 |
>360 1017 |
20.4% | Composite of CV mortality, aborted cardiac arrest, or hospitalization for HF |
No improvement in composite endpoint |
|
| Enrollment on the basis of HF hospitalization past 1 yr |
-- | -- | -- | -- | -- | 19.1% | -- | -- | |
| Enrollment on the basis of BNP/NT- proBNP criteria |
-- | -- | -- | -- | -- | 23.6% | -- | -- | |
| Enrollment in the Americas |
-- | -- | -- | -- | -- | 12.6% | -- | -- | |
| Enrollment in Eastern Europe |
-- | -- | -- | -- | -- | 2.3% | -- | -- | |
| PARAGON-HF (93) |
NYHA II-IV requiring treatment with diuretics for ≥30 days, LAE or LVH by echo, and HF hospitalization within 9 months or elevated NT- proBNP |
≥45 | Elevate d |
Composite endpoint of CV death and total HF hospitalizations (first and recurrent) |
-- | ||||
| SOCRATES- PRESERVED (94) |
Worsening HF requiring hospitalization or IV diuretic as outpatient |
-- | ≥45 | -- | -- | -- | -- | NT-proBNP from BL to 12 weeks, change of LAVI, safety |
-- |
| EDIFY(95) | Stable symptomatic NYHA II-II ≥3 months, HR >70, E/e’ >13 or e’ lateral <10 cm/s and e’ septal <8 cm/s or LAVI >34 ml/m2 |
-- | -- | -- | >80 | >220 | -- | Diastolic function (E/e’), NT-proBNP and 6MWD |
-- |
| Ontario, Canada (30) |
1st time admission for HF (only) on the basis of Framingham HF criteria |
-- | ≥50% | 31.8 | -- | -- | 31.1% | HF survival rate similar between HFrEF and HFpEF |
22.2% 1-yr mortality, 13.5% 1-yr Readmission for HF, 9.4% 30-day mortality or readmission for HF |
| Olmsted County (4) |
All consecutive patients hospitalized at Mayo Clinic Hospitals from 1987-2001 (ICD code 428 and DRG code 127) |
-- | ≥50 | 41.3 | -- | -- | 29% | Prevalence and survival of patients with HFpEF over a 15-yr period |
Increased prevalence of HFpEF with similar rate of death over a 15-yr period |
| ADHERE (96) | AHF as new-onset HF or decompensated chronic HF with symptoms requiring hospitalization; ICD-9 discharge diagnosis of HF |
63% | ≥40 | 21% (1st ECG) |
-- | -- | 2.8%§ | Prevalence and outcomes of patients with HFpEF |
In-hospital mortality was lower in patients with HFpEF vs. HFrEF. |
| OPTIMIZE-HF (23) |
Hospitalized new- onset or worsening HF as primary cause of admission or significant HF symptoms that developed during hospitalization with HF as primary discharge diagnosis |
-- | ≥40 | 33% | 602 | -- | 35.3%║ | Prevalence and outcomes of patients with HFpEF (in- hospital mortality, rehospitalization rate) |
HFpEF and HFrEF had similar lengths of hospital stay, in- hospital mortality was lower in HFpEF |
| ≥50 | 32% | 537 | -- | 36.8%║ | |||||
| MAGGIC (25) | Meta-analysis of observational studies and RCTS through 2006, with eligible studies including patients with HF and death from any cause where EF criterion was not used for study entry |
-- | ≥50 | 27% | -- | -- | 12.1%¶ | Survival of HFpEF vs HFrEF patients |
HFpEF patients have a lower risk of death than patients with HFrEF but absolute mortality in HFpEF is still high |
| GWTG-HF (5) | Hospitalization for acute, decompensated HF on the basis of clinical diagnosis. |
54% 56% |
≥50 40-50 |
34% 34% |
551 761 |
3401 5495 |
2.5%# 2.3%# |
Trend in therapies and outcomes |
Hospitalizatio n for HFpEF is increasing relative to HFrEF, with in-hospital mortality for HFpEF declining over study period |
AHF = acute heart failure; BL = baseline; BNP = B-type natriuretic peptide; CV = cardiovascular; DD = diastolic dysfunction; DRG = diagnosis related group; Dx = diagnosis; E/e’ = peak early transmitral ventricular filling velocity/early diastolic tissue Doppler velocity; EF = ejection fraction; eGFR = estimated glomerular filtration rate; ICD = International Classification of Diseases; IV = intravenous; LAE = left atrial enlargement; LAVI = left atrial volume index; LVH = left ventricular hypertrophy; NSR = normal sinus rhythm; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; VAS-AUC = visual analog scale-area under curve; VO2 = peak oxygen consumption; 6MWD = 6 min walk distance. Other abbreviations as in Table 1.
NP levels are median levels unless otherwise specified and if used as inclusion criteria are listed with > or < symbols
Data are from placebo groups in clinical trials unless otherwise noted
RELAX-AHF event rate is cardiovascular death or HF/renal failure hospitalization through Day 60
ADHERE event rate is in-hospital mortality
OPTIMIZE-HF event rate is post-discharge mortality/rehospitalization at 60-90 days
MAGGIC meta-analysis event rate was number of deaths/100 patient-years
GWTG-HF event rate is in-hospital mortality.
Ejection Fraction
EF was the first inclusion criterion used to differentiate patients with HFrEF from HFpEF. The first 3 large HFpEF trials studied renin-angiotensin aldosterone system (RAAS) inhibition with EF cutoffs of 40% to 45% (13,18,19). More recent trials have split between using an EF cutoff ≥45% and ≥50%. The PARAMOUNT (Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion) and TOPCAT (Treatment of Preserved Cardiac Function HF Aldosterone Antagonist trial) trials used an EF cutoff for participant inclusion of ≥45% (20,21). This cutoff choice between trials in HFrEF and HFpEF have left a largely unstudied intermediate EF group including 10% to 15% (5,22) of the overall HF population with an EF between 40% and 50%. The CHARM pooled analyses and the American Heart Association’s Get With the Guidelines-Heart Failure (GWTG-HF) initiative have a bell-shaped EF distribution with 17% (n = 1,295) and 14% (n = 15,184) of patients with an EF of 40% to 50%, respectively. However, the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry and Olmstead County study have bimodal EF distributions, with relatively few patients with an EF of 40% to 50%, suggesting that real-world populations may have fewer patients in this intermediate range than clinical trials (4,22,23). The clinical characteristics and clinical outcomes of patients with an EF between 40% and 50% appear to be intermediate compared with patients with HFrEF and HFpEF, and the etiology of the mild reduction in EF is unknown (partial EF recovery or primary HFpEF)(24).
It is unknown which EF cutoff provides the most reliable discriminator to enhance enrollment of the HFpEF phenotype and associated event rates. The MAGGIC (Meta-analysis Global Group in Chronic Heart Failure) meta-analysis demonstrated a clear increase in event rates when EF <40% was compared with >40% (25). The utility of this EF cutpoint was also demonstrated in the OPTIMIZE-HF registry, where multivariable analyses revealed in-hospital mortality risk for patients with EF between 40% and 50% was similar to those with EF >50%. Specifically, in-hospital mortality decreased by 17% for every 10% increase in EF up to 38%, with no further association with increased mortality above an EF of 38% (23). The CHARM pooled analyses demonstrated an increased risk for all-cause mortality and cardiovascular death with EF <45% (26). When event rates in the CHARM pooled analyses were evaluated for patients with HFpEF using an EF ≥40% and repeating the same analysis with an EF ≥50%, the event rates were unchanged (27). A secondary analysis from the SENIORS (Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure) trial revealed similar primary event rates for patients with HFpEF defined on the basis of an EF >35% or ≥40% (28). The placebo arms of HF clinical trials using lower EF cutoffs, such as EF >40%, reveal similar event rates to clinical trials with EF cutoffs ≥45% or ≥50% (Table 2). Although the event rates from the placebo arms across EF cutoffs from 40% to 50% are similar, using an EF cutoff of ≥40% or ≥45% risks including patients with an intermediate EF who may have different characteristics, such as more ischemic heart disease.
The historical precedence for using EF cutoffs for HFrEF <40% or ≤ 35% versus ≥40%, 45%, or 50% for HFpEF, combined with equal event rates across a range of EFs ≥40% to 50%, leaves us with 3 future clinical design options, including: 1) using an EF ≥40% to prevent an intermediate EF gap; 2) using a higher EF of ≥45% or ≥50% and defining the created intermediate EF group; or 3) lessening the impact of EF as an inclusion criterion. There is no clear absolute EF inclusion criterion; however, insightful use of EF as an inclusion criterion with an eye towards the preferred HFpEF phenotype will lead to successful trials. For example, if a clinical trial is studying a pharmaceutical therapy aimed at HFpEF patients with hypertension and associated structural remodeling, then use of a higher EF (e.g., 50%) inclusion criterion will enrich the trial with the preferred phenotype. However, if a promising new therapy appears to work across a more heterogeneous HFpEF population, then use of a lower EF (e.g., 40%) inclusion criterion will make the results of the trial generalizable. Ultimately, the appropriate use of EF as an inclusion criterion requires appropriate insight into the HFpEF phenotype that will benefit most from the therapy under investigation.
Prior Heart Failure Hospitalization
Prior hospitalization for HF is a powerful predictor of future outcomes. Investigators pooled the CHARM clinical trials and used a time-updated Cox proportional-hazards model to show that the mortality rate increased after HF hospitalization for upwards of 6 months from time of discharge to randomization (27). This observation was also independent of EF. Longer hospitalization and repeat hospitalization also increased the risk of mortality. The time period early after discharge for hospitalization for HF represents a particularly high-risk window for mortality. This high-risk period may also represent an opportunity to enrich event rates in clinical trials (29). A large population study in Ontario, Canada reported 1-year HF readmission rates of 16.1% and 13.5% (p = 0.09) for HFrEF and HFpEF, respectively, whereas the unadjusted combined 1-year endpoint of death and HF readmission was 36.1% and 31.1% for HFrEF and HFpEF, respectively (p = 0.01)(30). A recent analysis of the CHARM trials revealed that event rates for mortality and hospitalization were higher in patients with previous HF hospitalization compared with those without prior HF hospitalization. Specifically, the time interval between hospitalization and randomization was inversely related and the overall rates between HFrEF and HFpEF were similar (27). Patients hospitalized for HF within the previous 6 months in the placebo arm of the I-PRESERVE (Irbesartan in Heart Failure with Preserved Systolic Function) trial had 11.5 events per 100 patient-years compared with 7.0 events per 100 patient-years in those not recently hospitalized for HF (19).
Recent HFpEF trials have incorporated the inclusion criterion of prior hospitalization for HF on the basis of the high risk associated with recent HF hospitalization, as identified in the CHARM program (27), but this inclusion criterion has also complicated the interpretation of trial results. The recent TOPCAT trial demonstrated that there were regional differences in how patients entered the trial, specifically related to the HF hospitalization criterion. In addition to ≥1 sign and symptom of HF, an EF ≥45%, and controlled systolic blood pressure, patients were required to have a history of hospitalization for HF within the previous 12 months or an elevated NP level within 60 days before randomization (B-type natriuretic peptide [BNP] ≥100 pg/ml or N-terminal pro-B-type natriuretic peptide [NT-proBNP] ≥360 pg/ml). In the Americas, 39.6% of patients qualified for the trial on the basis of HF hospitalization within the preceding 12 months compared with 60.4% of patients from Eastern Europe. Unadjusted Cox models by geographic region and treatment group revealed that patients enrolled in the American control group had a primary outcome event rate of 31.8%, compared with 8.4% in patients enrolled in the Eastern European control group. Furthermore, post-hoc analyses of TOPCAT revealed a 4-fold higher event rate in patients enrolled in Russia and Georgia compared with the Americas, where the primary outcomes of cardiovascular death and hospitalization were significantly reduced by spironolactone (31). These data support observations seen in other international clinical trials whereby the patients enrolled in different regions/countries have distinct underlying characteristics, treatment protocols/standards, and event rates (20,32–34). Hospitalization for HF is an important inclusion criterion that can drive increased event rates in clinical trials, but the clinical definition of HF is subjective, with geographical differences in characteristics and standards potentially leading to different event rates.
The use of prior hospitalization as an inclusion criterion in HFpEF clinical trials can and should be a powerful driver of event rates. Using hospitalizations for HF as remote as 12 months has proven successful, but event rates occur early after discharge; thus, the use of hospitalizations for HF within 6 months will increase event rates. Geographical and international differences in the definition and treatment of patients with HFpEF necessitates further confirmation of clinical HFpEF that can include adjudication or combining HF hospitalization with a specific threshold NP level. Confirming HF hospitalizations or combining HF hospitalization and NP level entry criteria will decrease variability in HFpEF patients and enrich HFpEF event rates.
Clinical Diagnosis of Heart Failure
HF is a diagnosis on the basis of a clinical assessment and physical examination, along with supporting data from the chest radiograph and additional testing. Despite the added clinical and laboratory information, the diagnosis remains largely subjective, with clinical gestalt on the basis of history, physical examination, and routinely obtained laboratory and hemodynamic measurements. The traditional findings associated with HF, including dyspnea, paroxysmal nocturnal dyspnea, jugular venous distension, and pulmonary rales have projected sensitivities of 39%, 17%, and 29%, respectively, compared with left ventricular dysfunction, whereas their specificities are 80%, 98%, and 77%, respectively (35,36). The NYHA functional class is a subjective measurement used in HF trials that is strongly associated with worse outcomes in patients with HFrEF and HFpEF (37–39). Ultimately, hospitalizing a patient for HF is determined by the physician’s interpretation of the patient’s overall status. Dyspnea severity is the inherent symptom that influences decision-making. A recent analysis revealed that 50% of patients had dyspnea at rest and that these patients had increased rates of comorbidities, mortality, and HF readmission risk (40).
There are differences in presentations and management across geographical regions that can challenge the design and interpretation of clinical trial results (41). Dyspnea responds quickly to intravenous diuretics, with upwards of three-fourths of patients responding within 6 h sitting upright versus 47% supine (42). There are multiple dyspnea scales; those commonly used are the 5-point and 7-point Likert scales, and the 10-cm visual analog scale. A post-hoc analysis of URGENT (Ularitide Global Evaluation in Acute Decompensated Heart Failure) Dypsnea revealed that up to 40% of patients did not have improved dyspnea in the first 6 h; for those who did improve, patient characteristics differed across all 3 scales, with the c-index ranging from 0.71 to 0.83, suggesting that improvement in dyspnea may differ from scale to scale (43). The RELAX-AHF trial evaluating serelaxin used a visual analog scale area under the curve (VAS-AUC) endpoint to assess if serelaxin-treated patients would have improved dyspnea with baseline dyspnea, congestion on chest radiograph, elevated NP levels to Day 5 of the VAS-AUC, and the proportion of patients with moderate or marked dyspnea by the Likert scale during the first 24 h (44). Dyspnea relief, measured by VAS-AUC from baseline to Day 5, was improved in the overall population and in HFrEF and HFpEF compared with placebo; however, dyspnea relief at 24 h using the Likert scale was significantly improved in HFpEF patients compared with placebo, but not in HFrEF with treatment (interaction p = 0.03). The primary dyspnea endpoint of 448 mm improvement using the VAS-AUC score was significant (p = 0.007); however, the coprimary endpoint using the Likert scale endpoint was not significant through 5 days (p = 0.7). The dyspnea scoring tools vary from tool to tool and may not always correspond to hard outcomes. The clinical diagnosis of HF on the basis of physician assessment, combined with the use of auxiliary tools, such as NYHA classification and dyspnea scores, will enhance HFpEF clinical trials with patients experiencing HF symptoms. In patients without clear HF, adding together dyspnea severity, acute HF diagnostic prediction models, clinical assessment, and NP levels has excellent diagnostic accuracy (45).
Natriuretic Peptide Levels
NP levels, such as BNP and its cosecreted biologically inactive compound, NT-proBNP, are useful markers for diagnosing HF and provide prognostic information for patients presenting with dyspnea. In more recent HFpEF trials, NP levels have been used as key inclusion criteria to: 1) increase the specificity of the HFpEF diagnosis; and 2) select patients at higher risk. Post-hoc analyses from I-PRESERVE reveal that NT-proBNP is the most powerful independent factor for the primary outcome of all-cause mortality or cardiovascular hospitalization in patients with HFpEF (46,47). The PEP-CHF (Perindopril in Elderly People with Chronic Heart Failure) trial demonstrated an increased number of deaths and hospitalization for HF with higher quartiles of NT-proBNP levels (18). In the TOPCAT trial, patients enrolled on the basis of NP level had primary outcome event rates of 23.6% of patients enrolled on the basis of hospitalization in the past year, compared with 19.1% in the placebo group (Table 2)(48). Using NP-level thresholds for HFpEF clinical trial entry criteria have driven the HFpEF trials with higher event rates.
The COACH (Coordinating study evaluating Outcomes of Advising and Counselling in Heart Failure) substudy confirmed that NP levels are lower in patients with HFpEF compared with HFrEF, although the clinical outcomes were similar for a given BNP level (49). NP levels are also markers of the stage of disease and may potentially guide selection of patients in the “modifiable” zone, which identifies patients who may be neither “too well” nor “too sick” to benefit from an intervention. A post-hoc analysis of I-PRESERVE demonstrated that patients with NT-proBNP levels above the median of 339 pg/ml were independently associated with the primary endpoint of all-cause mortality and cardiovascular hospitalizations; whereas, patients with NT-proBNP levels below the median had beneficial effects from irbesartan, even after adjustment for 20 covariates (50). In the RELAX trial evaluating phosphodiesterase-5 inhibition in patients with HFpEF, the median NT-proBNP levels (648 pg/ml) were even higher than in I-PRESERVE with similar results: no improvement in the primary endpoints of peak VO2 or 6 min walk distance (50,51). The I-PRESERVE and the RELAX trials suggest that there is an upper boundary for a modifiable zone, above which a more advanced disease state exists where therapies may provide little, if any benefit. In the ALDO-DHF (Aldosterone receptor blockade in Diastolic Heart Failure) trial, the median NT-proBNP level of 148 pg/ml demonstrated improvement of 1 coprimary endpoint, E/e’, but no improvement in the other coprimary endpoint, change in peak VO2, suggesting the possibility of a lower boundary, where patients are too well to benefit from therapy, in addition to an upper boundary (52). The cutoff for NPs provides a distinct opportunity to increase the specificity of the diagnosis of HFpEF as well as event rates; however, choosing too high a level will potentially identify patients too advanced in their disease state to benefit from interventions such as RAAS therapy (49).
NP levels are highly affected by the confounding comorbidities of atrial fibrillation, renal insufficiency, and obesity. NP levels are lower in obese patients with HFpEF and independently associated with a favorable adiposity profile (53). Compared with patients with normal BMI, NP levels are significantly lower in obese and overweight patients after adjustment for important clinical characteristics, including atrial fibrillation (median values of 227 pg/ml and 608 pg/ml, respectively)(54,55). For example, the NT-proBNP level was revised in the RELAX trial evaluating sildenafil secondary to the “falsely” low NP levels found in obese patients with hemodynamically validated HFpEF. NP levels are known to be higher in patients with atrial fibrillation (56); however, patients with atrial fibrillation and obesity have an inverse relationship between BMI and circulating levels of NT-proBNP, suggesting that the underlying pathophysiology of obesity may reduce NP levels (57). HFpEF patients with renal impairment are known to have elevated NP levels, with NT-proBNP rising more than BNP; more than 79% of HFpEF patients with BNP levels >1,000 pg/ml have chronic renal insufficiency (58,59).
Choosing a threshold and ceiling level for trial entry on the basis of NP level requires considering several key factors for success: 1) the tradeoff between specificity and sensitivity for the diagnosis of HFpEF; 2) feasibility of patient recruitment; 3) clinical setting (acute decompensated HFpEF vs. chronic stable HFpEF); and 4) comorbidities. More recent clinical trials have raised the entry criteria level for NP levels for BNP from TOPCAT levels of 100 pg/ml and 360 pg/ml for BNP and NT-proBNP, respectively, to PARAMOUNT’s NT-proBNP threshold of 400 pg/ml. The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trials used a cutoff of 150 pg/ml and 600 pg/ml, respectively, for BNP and NT-proBNP for patients without a HF hospitalization in the previous 12 months and 100 pg/ml and 400 pg/ml, respectively, for patients with a HF hospitalization in the previous 12 months. NP levels are 1 of the key inclusion criteria that are most specific for patients with HFpEF with resultant increases in event rates. Careful adjustment upward or downward of NP threshold on the basis of comorbidities will enrich the preferred HFpEF phenotype and result in higher event rates.
Atrial fibrillation
Atrial fibrillation is 1 of the most common comorbidities in patients with HFpEF and coexists in 21% to 33% of patients in large registries and 9% to 61% of patients in HFpEF clinical trials (Table 2). HFpEF patients with atrial fibrillation are older, have higher NP levels, larger left atrial volume indexes, and are independently associated with death after adjustment for covariates compared with HFpEF patients in sinus rhythm (60). The PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized for Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) trial is 1 of many with a high enrollment of patients with atrial fibrillation (57%) raising the question of whether acute symptoms may be related to exacerbated atrial fibrillation rather than to acutely decompensated HFpEF (61). Most drugs targeting HFpEF may not improve patients with a primary problem of atrial fibrillation; thus, studying these patients may confound results and limit our ability to detect HFpEF-specific treatment effects. However, basing entry criteria on NT-proBNP or LA size would bias for the selection of patients with atrial fibrillation (who have higher levels of NP and larger LA, independent of HF). Furthermore, some have argued that atrial fibrillation should be considered part and parcel of the HFpEF disease syndrome because almost two-thirds of HFpEF patients have atrial fibrillation during the course of their disease: approximately 29% of patients with HFpEF have atrial fibrillation before diagnosis; 23% at the time of diagnosis; and 32% go on to develop atrial fibrillation within 3 to 4 years of follow-up (60). In PARAMOUNT, the mandate for a NT-proBNP cutoff for entry resulted in an over-representation of patients with atrial fibrillation, in whom higher levels of NT-proBNP are expected, related to atrial fibrillation and the resultant increased left atrial size and circulatory volume, leading to increased release of NPs. If, as expected, more patients have atrial fibrillation without true HFpEF, then there would be a significant impact on PARAMOUNT’s goal to detect LCZ696’s efficacy in patients with HFpEF, as LCZ696 is not known to affect the underlying pathophysiology of atrial fibrillation. The significant number of enrolled patients with atrial fibrillation led to a cap on the absolute percentage of patients with atrial fibrillation that could be recruited.
Strategies to address this dilemma include: 1) introduction of a cap on the proportion of patients with atrial fibrillation who can be recruited; and 2) using different NT-proBNP cutoffs for those with and without atrial fibrillation.
Hemodynamics
Hemodynamics, measured invasively and noninvasively, yield an objective assessment of pressures in the venous circulation. Central venous pressure increases when increased circulatory volume occurs from tricuspid regurgitation or right ventricle failure, and can be estimated fairly accurately through echocardiography. Hemodynamic measurements help discern which patients have the diagnosis of HF when the usual selection criteria are not conclusive. Pulmonary hypertension is frequently caused by left-sided HF and defined as pulmonary artery systolic pressure (PASP) >35 mm Hg, derived from the tricuspid velocity and highly prevalent, with estimates as high as 83% of patients with HFpEF (62), whereas pulmonary capillary wedge pressure (PCWP) is estimated from the ratio of early transmitral flow velocity to early mitral annular diastolic velocity. Normal filling pressures and other hemodynamic parameters such as PCWP, PASP and left ventricular end-diastolic pressure (LVEDP) at rest followed by normal filling pressures during exercise, exclude the diagnosis of HF. In contrast, if filling pressures increase in proportion to the increase in PCWP, then a diagnosis of HFpEF is suggested (63–65). Other related diagnoses, such as pulmonary arterial hypertension, can also be clearly identified during right heart catheterization.
However, outside of small studies evaluating hemodynamics in patients with equivocal diagnoses, invasive hemodynamic measurements are infrequently used and there is limited data from clinical trials confirming that these measurements enhance event rates. The RELAX trial evaluating phosphodiesterase-5 inhibition utilized an alternative entry criteria for elevated LVEDP or PCWP if other criteria were not met in a small number of unreported patients (51). Cardiopulmonary exercise testing (CPET) provides additive information to hemodynamics that may help exclude HF with normal tests and confirm or suggest a HF diagnosis with abnormal results (66). CPET measurements obtained during exercise include the gas exchange parameters, peak oxygen consumption (VO2), and the slope of the relationship between ventilation and carbon dioxide production (Ve/VCO2 slope), are independently associated with mortality, and are strong independent predictors of mortality (39). A study evaluating serelaxin demonstrated significant reductions in peak PCWP without changes in cardiac index, and a CPET with echocardiography study in HFpEF patients treated with ivabradine, revealing improved METS, peak VO2, and reduced E/e’ provided the impetus to pursue larger clinical trials on these 2 promising therapies (67,68).
Guazzi and colleagues demonstrated significant improvements in mean pulmonary artery pressure, right atrial pressure, pulmonary vascular resistance, tricuspid annular systolic excursion, and EF using invasive hemodynamics obtained in 44 patients with HFpEF randomized to placebo or sildenafil with benefits through 12 months of follow-up in patients with baseline evidence of chronically elevated left ventricular filling pressures (69). Guazzi’s work led to further investigation of sildenafil’s phosphodiesterase-5 inhibition in the RELAX clinical trial of 216 patients with endpoints closely related to hemodynamics: change in peak VO2 and 6-min walk distance (51). After 24 weeks, there were no significant changes in peak VO2 or 6-min walk distance; however, hemodynamic-related measurements of E/e’, left atrial volume index, and PASP were consistent with chronically elevated left ventricular filling pressures. Cardiovascular hemodynamics obtained from noninvasive and invasive measurements are very helpful for the confirmation or exclusion of patients who do not meet diagnostic criteria from the usual selection criteria. However, the actual impact added by hemodynamic measurements to driving event rates in clinical trials remains unknown and future inclusion in pilot studies and clinical trials are needed to verify their value.
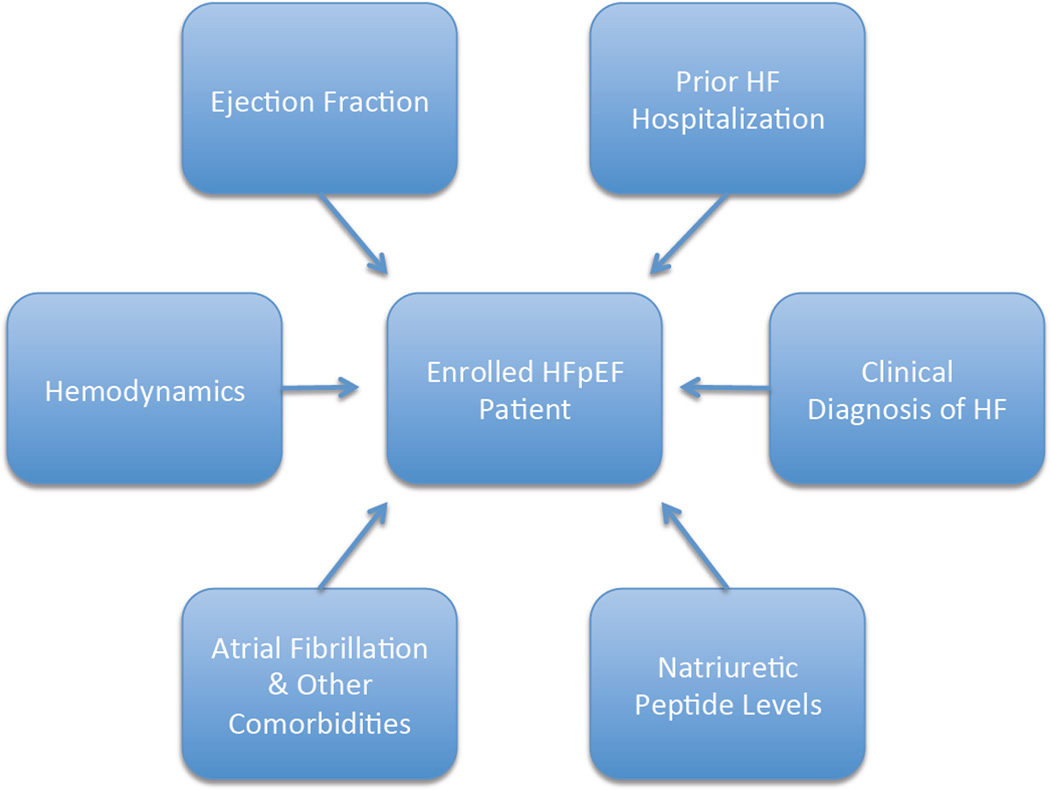
Lessons Learned
HF remains a clinical diagnosis that may be enhanced by weighing and/or limiting the patient selection criteria discussed herein including EF, prior HF hospitalization, NP levels, comorbidities such as atrial fibrillation, clinical diagnosis of HF, and hemodynamics (Figure 1). In addition to balancing patient selection criteria, clinical trial design, implementation, and integration of novel diagnostic techniques are paramount to discovering future therapies for HFpEF (Figure 2). Enrolling patients quickly prevents crossover to the treatment intervention, as evidenced by a restrictive analysis of PEP-CHF that trended towards clinical significance (p = 0.055) for the primary endpoint of all-cause mortality and unplanned HF hospitalization in the first year, with the secondary endpoint of unplanned hospitalization for HF significant (p = 0.033)(15). Implementing important patient-centered outcomes through the use of all hospitalizations, instead of only first hospitalizations, can drive important event rates (70). Factoring in the expected differences in event rates across geographical and international regions on the basis of past event rates related to differences in clinical and socioeconomic practices across the world (Table 2), as evidenced by post-hoc analyses from the TOPCAT trial demonstrating clinical benefit in the Americas (HR 0.82) compared with Russia/Republic of Georgia (HR 1.1) will allow for proper statistical power to detect meaningful differences (31,48).
Figure 1. Inclusion Criteria That Alter Event Rates in HFpEF Clinical Trials.
Representative inclusion criteria used in past, present, and future clinical trials of patients with HFpEF. HF = heart failure; HFpEF = heart failure with preserved ejection fraction.
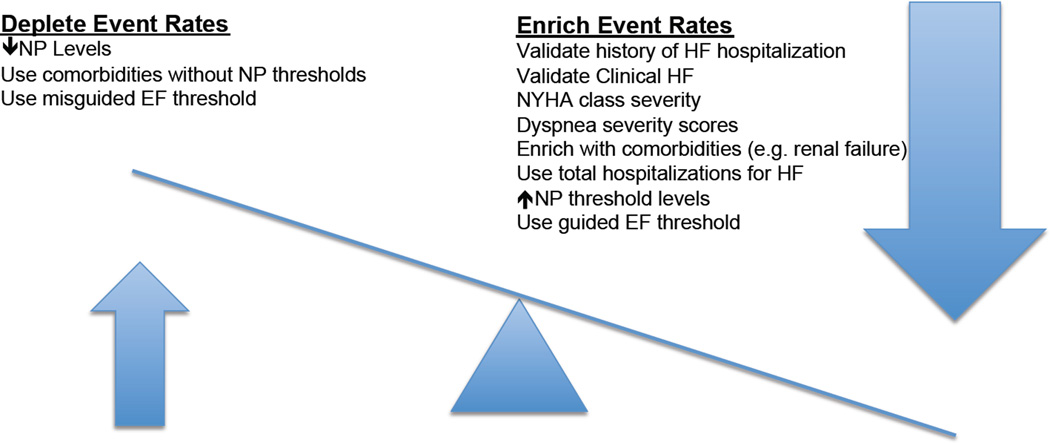
Figure 2. Strategies to enrich event rates in HFpEF Clinical Trials.
The appropriate use of specific inclusion criteria and targeted thresholds will facilitate the reduction or enrichment of event rates in well-designed clinical trials of patients with HFpEF. EF = ejection fraction; HF = heart failure; NP = natriuretic peptide. ↑= higher; ↓ = lower.
Recent studies evaluating new imaging techniques measuring impaired systolic function in patients with HFpEF through 3D speckle, left atrial, and longitudinal strain analyses are associated with mortality and may provide opportunities to enhance patient selection and event rates (71–76). Emerging and novel biomarkers such as cystatin C, galectin-3, and growth differentiation factor-15 may help phenotype, risk-stratify, and identify patients with or at risk for HFpEF (77–79). Evidence continues to mount from studies evaluating isolated comorbidities, such as coronary disease (80) and diabetes mellitus (81), in patients with HFpEF, demonstrating worse mortality, although splitting the heterogeneous HFpEF population into targeted groups with distinct phenotypes may lead to therapeutic advances (82,83). Shah and colleagues recently used statistical learning algorithms in 400 patients with HFpEF to perform an unbiased clustering analysis of dense phenotypic data to “phenomap” patients with HFpEF into more homogeneous subclasses (84). Combinations of “omics”, cluster analyses and phenomapping result in novel classifications of HFpEF that may simplify this heterogeneous population into discernable classifications that ultimately allow targeted pharmaceutical therapies (85,86). Integration of lessons learned from previous HFpEF clinical trials with current patient selection criteria, and emerging and novel imaging, biomarker, and phenotype classification schema provide a unique scaffold to advance HFpEF clinical trial success.
Conclusions
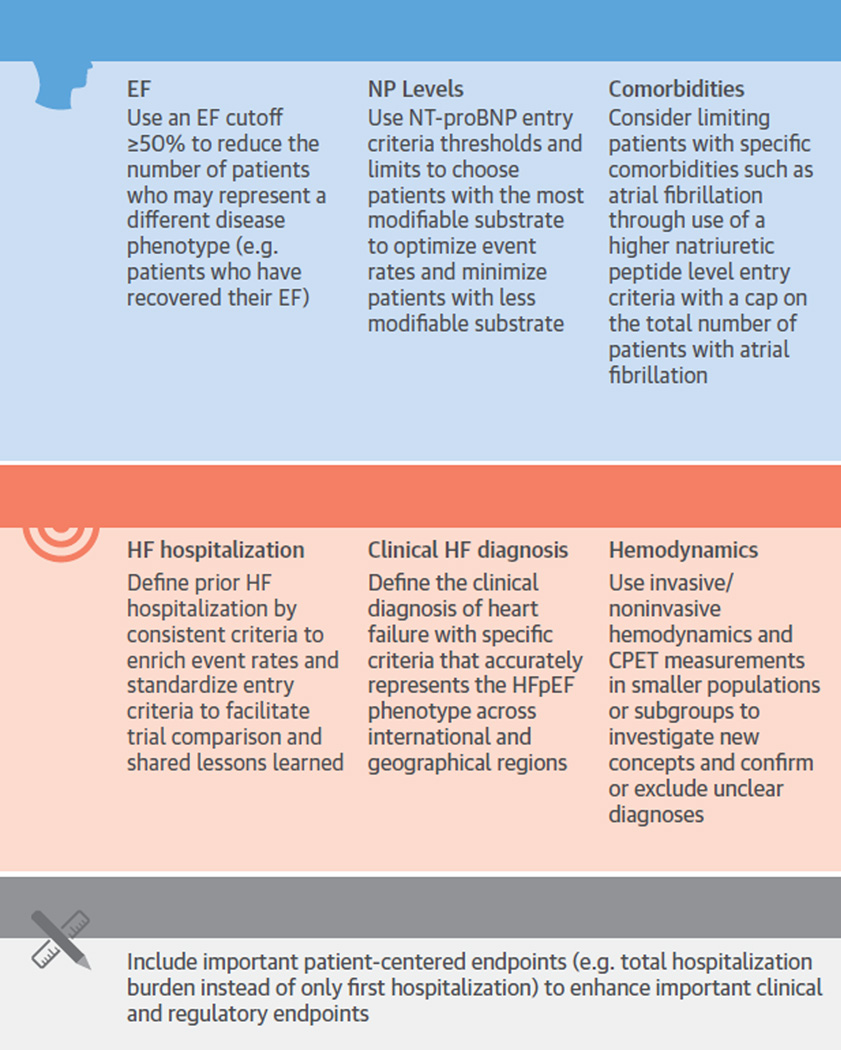
Promising new therapeutic options on the basis of sound scientific rationale and observational data, such as the recently published study on angiotensin-neprilysin inhibitors, may prove to benefit patients with HFpEF. However, clinical trials in HFpEF using standard, mortality-reducing therapies known to HFrEF have thus far been neutral. In order to optimize clinical trial effectiveness, trials in patients with HFpEF should consider inclusion of patients with the common comorbidities that drive HFpEF’s underlying pathophysiology through the balanced use of the following key inclusion and exclusion criteria: universal EF cutpoint; appropriate NP-level thresholds; limited number of patients with atrial fibrillation (with a higher NP cut-point); and use of a clearly defined history of HF and diagnosis of previous HF (Central Illustration). Attaining hemodynamic measurements related to HFpEF through use of echocardiography, cardiopulmonary exercise testing, and invasive hemodynamics may complement or validate challenging patients. Thoughtful clinical trial design that incorporates the lessons learned from previous and ongoing clinical trials in patients with HFpEF will provide the trial landscape necessary to determine if future therapies actually improve the outcomes and/or quality of life in patients with HFpEF.
Central Illustration. Methodological Recommendations to Enhance Clinical Trial Success Through Increased Event Rates.
Previous and ongoing clinical trial inclusion criteria and methodological considerations are presented with recommendations to highlight the complexity of clinical trial design with associated recommendations to enhance event rates and future clinical trial success. CPET = cardiopulmonary exercise testing; EF = ejection fraction; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; NP= natriuretic peptide; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Acknowledgments
Dr. Kelly receives funding from the NIH Ruth L. Kirschstein NRSA Institutional Research Training Grant 5 T32 HL 7101-39. Dr. Mentz has received research support from Gilead Sciences, AztraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Otsuka, Amgen, and ResMed; and honoraria from Thoratec. Dr. Mebazza has received speaker’s honoraria from Cardiorentis, Vifor, Edwards, Orion, and Bayer and is a consultant for Adrenomed and Cardiorentis. Dr. Voors has received consultancy fees and/or research grants from Alere, AstraZeneca, Bayer HealthCare, Boehringer Ingelheim, Cardio3Biosciences, Celladon, Johnson & Johnson, Merck/MSD, Novartis, Servier, Torrent, Trevena, and Vifor. Dr. Butler has served as a consultant to Amgen, BG Medicine, Celladon, Gambro, Ono Pharma, Trevena, Takada, Bayer HealthCare, Medtronic, CardioCell, Novartis, and GE Healthcare; and has received research support from the National Institutes of Health, European Union, and the Health Resources and Services Administration. Dr. Rossig is an employee of Bayer Pharma AG. Dr. Zannad has received grant funding from Novartis, BG Medicine, and Roche Diagnostics, served on a board for Boston Scientific, and served as a consultant for Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and ResMed. Dr. Pitt has received consultancy fees and/or stock options from Pfizer Inc., BayerHealthCare, Lilly, Stealth Peptides, SC Pharmaceuticals, Sarfez Pharmaceuticals, and Relypsa. Dr. O’Connor reports having received research support from Johnson & Johnson. Drs. Fiuzat and O’Connor have received research support from ResMed. A continuously updated list of disclosure information for Dr. O’Connor is available at https://www.dcri.org/about-us/conflict-of-interest. Dr. Lam has received research support from Boston Scientific, Medtronic, Vifor Pharma, and the National Medical Research Council of Singapore; and is a consultant for Bayer and Novartis.
Abbreviations
- EF
ejection fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LVEDP
left ventricular end diastolic pressure
- NP
natriuretic peptide
- PASP
pulmonary artery systolic pressure
- PCWP
pulmonary capillary wedge pressure
- VAS-AUC
visual analog scale area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics. Subcommittee Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Tate S, Griem A, Durbin-Johnson B, et al. Marked elevation of B-type natriuretic peptide in patients with heart failure and preserved ejection fraction. J Biomed Res. 2014;28:255–261. doi: 10.7555/JBR.28.20140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJ, Petrie MC, Murdoch DR, et al. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19(Suppl P):9–16. [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Authors/Task Force Members. Dickstein K, Cohen-Solal A, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 9.Authors/Task Force Members. McMurray JJV, Adamopoulos S, et al. ESC Committee for Practice Guidelines (CPG), Document Reviewers. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 10.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction. comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Pfeffer MA, Swedberg K, et al. CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 14.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 15.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 16.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–537. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 17.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 19.Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJ, O’Connor C. Lessons from the TOPCAT trial. N Engl J Med. 2014;370:1453–1454. doi: 10.1056/NEJMe1401231. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SD, Zile M, Pieske B, et al. Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 22.Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%) Eur J Heart Fail. 16:1049–1055. doi: 10.1002/ejhf.159. [DOI] [PubMed] [Google Scholar]
- 23.Fonarow GC, Stough WG, Abraham WT, et al. OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 24.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 26.Solomon SD, Anavekar N, Skali H, et al. Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 27.Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Veldhuisen DJ, Cohen-Solal A, Bohm M, et al. SENIORS Investigators. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure) J Am Coll Cardiol. 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Solomon SD, Dobson J, Pocock S, et al. Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer MA, Claggett B, Assmann SF, et al. Regional Variation in Patients and Outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 32.Mentz RJ, Kaski JC, Dan GA, et al. Implications of geographical variation on clinical outcomes of cardiovascular trials. Am Heart J. 2012;164:303–312. doi: 10.1016/j.ahj.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor CM, Fiuzat M, Swedberg K, et al. Influence of global region on outcomes in heart failure beta-blocker trials. J Am Coll Cardiol. 2011;58:915–922. doi: 10.1016/j.jacc.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 34.Kristensen SL, Kober L, Jhund PS, et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation. 2015;131:43–53. doi: 10.1161/CIRCULATIONAHA.114.012284. [DOI] [PubMed] [Google Scholar]
- 35.Davie AP, Francis CM, Caruana L, et al. Assessing diagnosis in heart failure: which features are any use? QJM. 1997;90:335–339. doi: 10.1093/qjmed/90.5.335. [DOI] [PubMed] [Google Scholar]
- 36.Gillespie ND, McNeill G, Pringle T, et al. Cross sectional study of contribution of clinical assessment and simple cardiac investigations to diagnosis of left ventricular systolic dysfunction in patients admitted with acute dyspnoea. BMJ. 1997;314:936–940. doi: 10.1136/bmj.314.7085.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madsen BK, Hansen JF, Stokholm KH, et al. Chronic congestive heart failure. Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J. 1994;15:303–310. doi: 10.1093/oxfordjournals.eurheartj.a060495. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444–450. doi: 10.1016/j.ahj.2005.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell SD, Saval MA, Robbins JL, et al. HF-ACTION Investigators. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158:S24–S30. doi: 10.1016/j.ahj.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mentz RJ, Mi X, Sharma PP, et al. Relation of dyspnea severity on admission for acute heart failure with outcomes and costs. Am J Cardiol. 2015;115:75–81. doi: 10.1016/j.amjcard.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins SP, Pang PS, Lindsell CJ, et al. International variations in the clinical, diagnostic, and treatment characteristics of emergency department patients with acute heart failure syndromes. Eur J Heart Fail. 2010;12:1253–1260. doi: 10.1093/eurjhf/hfq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mebazaa A, Pang PS, Tavares M, et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31:832–841. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 43.Pang PS, Collins SP, Sauser K, et al. Assessment of dyspnea early in acute heart failure: patient characteristics and response differences between likert and visual analog scales. Acad Emerg Med. 2014;21:659–666. doi: 10.1111/acem.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teerlink JR, Cotter G, Davison BA, et al. RELAXin in Acute Heart Failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 45.Steinhart B, Thorpe KE, Bayoumi AM, et al. Improving the diagnosis of acute heart failure using a validated prediction model. J Am Coll Cardiol. 2009;54:1515–1521. doi: 10.1016/j.jacc.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 46.McKelvie RS, Komajda M, McMurray J, et al. I-Preserve Investigators. Baseline plasma NT-proBNP and clinical characteristics: results from the irbesartan in heart failure with preserved ejection fraction trial. J Card Fail. 2010;16:128–134. doi: 10.1016/j.cardfail.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Komajda M, Carson PE, Hetzel S, et al. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) Circ Heart Fail. 2011;4:27–35. doi: 10.1161/CIRCHEARTFAILURE.109.932996. [DOI] [PubMed] [Google Scholar]
- 48.Pitt B, Pfeffer MA, Assmann SF, et al. TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 49.van Veldhuisen DJ, Linssen GC, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 50.Anand IS, Rector TS, Cleland JG, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4:569–577. doi: 10.1161/CIRCHEARTFAILURE.111.962654. [DOI] [PubMed] [Google Scholar]
- 51.Redfield MM, Chen HH, Borlaug BA, et al. RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edelmann F, Wachter R, Schmidt AG, et al. Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 53.Neeland IJ, Winders BR, Ayers CR, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. 2013;62:752–760. doi: 10.1016/j.jacc.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 55.Stavrakis S, Pakala A, Thomas J, et al. Obesity, brain natriuretic peptide levels and mortality in patients hospitalized with heart failure and preserved left ventricular systolic function. Am J Med Sci. 2013;345:211–217. doi: 10.1097/MAJ.0b013e318271c012. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Xiao Y, Chen X, et al. Association between plasma brain natriuretic peptide/N-terminal pro-brain natriuretic peptide levels and atrial fibrillation: evidence from a meta-analysis. Chin Med J (Engl) 2014;127:2824–2828. [PubMed] [Google Scholar]
- 57.Zheng LH, Wu LM, Yao Y, et al. Impact of body mass index on plasma N-terminal ProB-type natriuretic peptides in Chinese atrial fibrillation patients without heart failure. PloS One. 2014;9:e105249. doi: 10.1371/journal.pone.0105249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agoston I, Cameron CS, Yao D, et al. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol. 2004;94:1003–1007. doi: 10.1016/j.amjcard.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 59.Takase H, Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur J Clin Invest. 2014;44:303–308. doi: 10.1111/eci.12234. [DOI] [PubMed] [Google Scholar]
- 60.Zakeri R, Chamberlain AM, Roger VL, et al. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massie BM, O’Connor CM, Metra M, et al. PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 62.Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andersen MJ, Borlaug BA. Invasive hemodynamic characterization of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:435–444. doi: 10.1016/j.hfc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 66.Lainchbury JG, Richards AM. Exercise testing in the assessment of chronic congestive heart failure. Heart. 2002;88:538–5343. doi: 10.1136/heart.88.5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ponikowski P, Mitrovic V, Ruda M, et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J. 2014;35:431–441. doi: 10.1093/eurheartj/eht459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosmala W, Holland DJ, Rojek A, et al. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330–1338. doi: 10.1016/j.jacc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 69.Guazzi M, Vicenzi M, Arena R, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 70.Rogers JK, Pocock SJ, McMurray JJ, et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014;16:33–40. doi: 10.1002/ejhf.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowallick JT, Kutty S, Edelmann F, et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. 2014 Aug 12; doi: 10.1186/s12968-014-0060-6. [E-pub ahead of print];16:60; http://dx.doi.org/10.1186/s12968-014-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kraigher-Krainer E, Shah AM, Gupta DK, et al. PARAMOUNT Investigators Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo XX, Fang F, Lee AP, et al. What can three-dimensional speckle-tracking echocardiography contribute to evaluate global left ventricular systolic performance in patients with heart failure? Int J Cardiol. 2014;172:132–137. doi: 10.1016/j.ijcard.2013.12.314. [DOI] [PubMed] [Google Scholar]
- 74.Stampehl MR, Mann DL, Nguyen JS, et al. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography. 2015;32:71–78. doi: 10.1111/echo.12613. [DOI] [PubMed] [Google Scholar]
- 75.Wang J, Fang F, Wai-Kwok Yip G, et al. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol. 2014;178:131–135. doi: 10.1016/j.ijcard.2014.10.130. [DOI] [PubMed] [Google Scholar]
- 76.Izumiya Y, Hanatani S, Kimura Y, et al. Growth differentiation factor-15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. Can J Cardiol. 2014;30:338–344. doi: 10.1016/j.cjca.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Carrasco-Sanchez FJ, Galisteo-Almeda L, Paez-Rubio I, et al. Prognostic value of cystatin C on admission in heart failure with preserved ejection fraction. J Card Fail. 2011;17:31–38. doi: 10.1016/j.cardfail.2010.07.248. [DOI] [PubMed] [Google Scholar]
- 78.Gullestad L, Ueland T, Kjekshus J, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) Eur Heart J. 2012;33:2290–2296. doi: 10.1093/eurheartj/ehs077. [DOI] [PubMed] [Google Scholar]
- 79.de Boer RA, Edelmann F, Cohen-Solal A, et al. Galectin-3 in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:1095–1101. doi: 10.1093/eurjhf/hft077. [DOI] [PubMed] [Google Scholar]
- 80.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 81.Lindman BR, Davila-Roman VG, Mann DL, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64:541–549. doi: 10.1016/j.jacc.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maurer MS, Mancini D. HFpEF: is splitting into distinct phenotypes by comorbidities the pathway forward? J Am Coll Cardiol. 2014;64:550–552. doi: 10.1016/j.jacc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Greenberg B. Heart failure preserved ejection fraction with coronary artery disease: time for a new classification? J Am Coll Cardiol. 2014;63:2828–2830. doi: 10.1016/j.jacc.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 84.Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmad T, Pencina MJ, Schulte PJ, et al. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol. 2014;64:1765–1774. doi: 10.1016/j.jacc.2014.07.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:407–418. doi: 10.1016/j.hfc.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary Digitalis Investigation Group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conraads VM, Metra M, Kamp O, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto K, Origasa H, Hori M. J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF) Eur J Heart Fail. 2013;15:110–118. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 90.Edelmann F, Gelbrich G, Dungen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 91.Filippatos G, Teerlink JR, Farmakis D, et al. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur Heart J. 2014;35:1041–1050. doi: 10.1093/eurheartj/eht497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deswal A, Richardson P, Bozkurt B, et al. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Novartis Pharmaceuticals. [Accessed March 11, 2015];Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF) 2014 Available at: https://clinicaltrials.gov/ct2/show/NCT01920711.
- 94.Bayer. [Accessed March 11, 2015];Phase IIb Safety and Efficacy Study of Four Dose Regimens of BAY1021189 in Patients With Heart Failure and Preserved Ejection Fraction Suffering From Worsening Chronic Heart Failure (SOCRATES-PRESERVED) 2015 Available at: https://clinicaltrials.gov/ct2/show/NCT01951638.
- 95.Institut de Recherches Internationales Servier. [Accessed March 11, 2015];Effect of ivabradine versus placebo on cardiac function, exercise capacity, and neuroendocrine activation in patients with Chronic Heart Failure with Preserved left ventricular Ejection Fraction (EDIFY) 2013 Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-002742-20/HU.
- 96.Yancy CW, Lopatin M, Stevenson LW, et al. ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]