Dear Editor,
Refractory cytopenia of childhood (RCC) is the most common subtype of hypoplastic myelodysplastic syndrome (hMDS) in childhood, characterized by persistent cytopenia, dysplastic changes in erythroid precursors or megakaryocytes, and the presence of erythroid islands, defined as more than 10 erythroid precursors in a bone marrow (BM) biopsy section [1,2]. Since 75% of the patients with RCC show hypocellularity, it is challenging to differentiate RCC from other BM failure disorders such as pediatric aplastic anemia (AA). In contrast with its wild type, which has short half-life, the mutant p53 protein has a relatively long half-life and is thus detectable through p53 immunohistochemical (IHC) stain [3]. The clinical usefulness of p53 IHC on BM cells, which can be evidence of mutation in the TP53 gene, has been reported for discriminating hMDS from AA [4,5,6]. We evaluated the clinical relevance of p53 IHC stain in the differential diagnosis between RCC and pediatric AA.
Retrospectively, this study included 53 patients (28 men, 25 women; age 2-16 yr, median 8.5 yr) diagnosed as having pediatric AA in the Asan Medical Center, Seoul, Korea, from January 2002 to July 2012. The diagnosis of pediatric AA was reconfirmed following criteria described in the literature [7]. Clinical and laboratory data, including paroxysmal nocturnal hemoglobinuria (PNH) test results and karyotype at diagnosis, were obtained by reviewing the medical record. BM aspiration and biopsy slides at diagnosis were reviewed by two hematopathologists, and the diagnosis of possible RCC was assessed according to three morphologic findings: (1) the presence of erythroid islands consisting of at least 10 immature erythroid precursors in the BM biopsy or clot section; (2) the presence of at least three megakaryocytes in the BM aspiration, biopsy, or clot section (examined at least 30 fields under a low power field, ×100); and, if (2) verifies, (3) the presence of megakaryocytic dysplasia such as micromegakaryocytes and megakaryocytes with separated or round nuclei. In two cases with discrepant opinions about the presence of micromegakaryocytes, the CD61 IHC stain (1:100, Dako, Hamburg, Germany) was performed to confirm the presence of micromegakaryocytes. The diagnosis of possible RCC was confirmed when at least one of the three morphologic findings was observed consistently by both hematopathologists.
The p53 IHC stain was performed on the BM biopsy and clot section specimens of all 53 patients, with primary antibody against p53 (1:200; NCL-p53 DO-7; Novocastra, Newcastle upon Tyne, UK) and following the procedures described in the literature [4]. Known lymphoma specimens were included in every slide and stained as positive controls for p53 stain. Two hematopathologists examined p53 IHC stain positivity (defined as the presence of one or more nuclear stained BM cells) separately and, if there were discordant opinions, the slides were reexamined by them together using a multi-headed microscope. Morphologic findings and results for p53 IHC stain were compared between pediatric AA and possible RCC subgroups. This study was approved by the institutional review board of Asan Medical Center, Seoul, Korea.
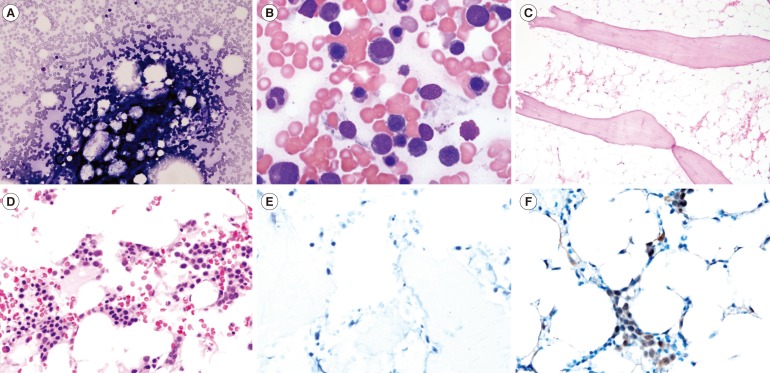
After morphological assessment, 39 (73.6%) patients remained as pediatric AA, and 14 (26.4%) patients were assessed as possible RCC. The patient with pediatric AA typically showed hypocellularity in the BM aspiration and biopsy, but the patient with possible RCC showed considerably variable cellularity (slightly hypo- or normocellular marrow) with occasional erythroid islands in the BM biopsy (Fig. 1A-D). Compared with the patients with pediatric AA, the patients with possible RCC showed a significantly higher proportion of RCC features, such as the presence of erythroid islands (33.3% vs. 100.0%, P< 0.001), the presence of at least three megakaryocytes (17.9% vs. 57.1%, P=0.005), and megakaryocytic dysplasia (14.3% vs. 75.0%, P=0.032). Notably, 11 (78.6%) patients with possible RCC showed positivity on the island of BM cells in the p53 IHC stain. However, all patients with pediatric AA were completely negative in the p53 IHC stain (Table 1, Fig. 1E, F). These results indicate that 26.4% of the patients previously diagnosed with pediatric AA had adequate histological evidence of RCC and that the p53 IHC stain positivity rate in these patients was 78.6%, which is high enough to be useful in the discrimination of RCC from pediatric AA. These results support the results of previous studies [4,5,6] and suggest the clinical relevance of p53 IHC stain for the discrimination between pediatric AA and RCC. Considering the limited number of patients, further studies with more patients are needed to confirm the conclusions of the present study.
Fig. 1. Comparison example of the bone marrow aspirates (A and B, Wright stain, ×200 and ×1,000, respectively), biopsies (C and D, Hematoxylin & Eosin stain, ×100 and ×400, respectively), and p53 immunohistochemical (IHC) stain results (E and F, ×400) between the patient with pediatric aplastic anemia (A, C, and E) and possible refractory cytopenia of childhood (B, D, and F).
Table 1. Comparison of the histological findings between patients with pediatric AA and possible RCC.
| Diagnosis | Histological findings (N of positive patients/total patients) | |||
|---|---|---|---|---|
| Presence of erythroid islands P<0.001 |
Presence of at least 3 megakaryocytes P=0.005 |
Presence of megakaryocytic dysplasia P=0.032 |
p53 IHC stain P<0.001 |
|
| AA (N=39) | 13/39 (33.3%) | 7/39 (17.9%) | 1/7 (14.3%) | 0/39 (0.0%) |
| Possible RCC (N=14) | 14/14 (100.0%) | 8/14 (57.1%) | 6*/8 (75.0%) | 11/14 (78.6%) |
P values were obtained from the Chi-square test or Fisher's exact test (for small numbers less than 5).
*These six cases with presence of megakaryocytic dysplasia included micromegakaryocytes (four cases) and megakaryocytes with separated nuclei or round nuclei (two cases).
Abbreviations: AA, aplastic anemia; RCC, refractory cytopenia of childhood; IHC, immunohistochemical.
In conclusion, the positivity rate of p53 IHC stain in possible RCC patients was 78.6%, which is significantly higher than 0% in pediatric AA patients. The p53 IHC stain could provide useful information in the discrimination of RCC from pediatric AA, suggesting RCC if the result is positive.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Passmore SJ, Chessells JM, Kempski H, Hann IM, Brownbill PA, Stiller CA. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia in the UK: a population-based study of incidence and survival. Br J Haematol. 2003;121:758–767. doi: 10.1046/j.1365-2141.2003.04361.x. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008. [Google Scholar]
- 3.Totzke G, Brüning T, Vetter H, Schulze-Osthoff K, Ko Y. P53 downregulation in myelodysplastic syndrome--a quantitative analysis by competitive RT-PCR. Leukemia. 2001;15:1663–1664. doi: 10.1038/sj.leu.2402233. [DOI] [PubMed] [Google Scholar]
- 4.Cha CH, Park CJ, Chi HS, Seo EJ, Jang S, Cho YU, et al. CD34 and p53 immunohistochemical stains differentiate hypocellular myelodysplastic syndrome (hMDS) from aplastic anemia and a CD34 immunohistochemical stain provides useful survival information for hMDS. Ann Lab Med. 2014;34:426–432. doi: 10.3343/alm.2014.34.6.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elghetany MT, Vyas S, Yuoh G. Significance of p53 overexpression in bone marrow biopsies from patients with bone marrow failure: aplastic anemia, hypocellular refractory anemia, and hypercellular refractory anemia. Ann Hematol. 1998;77:261–264. doi: 10.1007/s002770050455. [DOI] [PubMed] [Google Scholar]
- 6.Choi JW, Fujino M, Ito M. F-blast is a useful marker for differentiating hypocellular refractory anemia from aplastic anemia. Int J Hematol. 2002;75:257–260. doi: 10.1007/BF02982038. [DOI] [PubMed] [Google Scholar]
- 7.Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718–1721. [PubMed] [Google Scholar]