Abstract
Crustacean shellfish allergy is an important cause of food allergy and anaphylaxis in Asia. The major allergen in shellfish allergy is tropomyosin, a pan-allergen that is also found in house dust mites and cockroaches. Tropomyosins from house dust mites (HDMs) have a high sequence homology to shellfish tropomyosins, and cross-reactivity between HDM and shrimp tropomyosins has been demonstrated. Exposure to inhaled tropomyosins from house dust mites has been postulated to be the primary sensitizer for shellfish allergy, in a reaction analogous to the oral allergy (inhalant-food) syndrome. This notion is supported by indirect data from the effects of HDM immunotherapy on shellfish allergy, and strong correlations of shellfish and HDM sensitization. HDM immunotherapy has been reported to induce both shrimp allergy in non-allergic patients and shrimp tolerance in shrimp-allergic patients. Epidemiological surveys have also demonstrated a strong correlation between shellfish and HDM sensitization in both hospital-based and community-based studies. Unexposed populations have also been shown to develop sensitization-shellfish sensitization in orthodox Jews with no history of shellfish consumption was associated with HDM sensitization. Reciprocally, HDM sensitization in an Icelandic population living in a HDM-free environment was associated with shrimp sensitization. In vitro IgE inhibition studies on sera in shrimp-allergic Spanish patients indicate that mites are the primary sensitizer in shrimp-allergic patients living in humid and warm climates. Current data supports the hypothesis that tropomyosin is the link between HDM and shellfish allergies. The role of tropomyosin in HDM and shellfish allergies is a fertile field for investigation as it may provide novel immunotherapeutic strategies for shellfish allergy.
Keywords: House dust mite, Shellfish allergy, Tropomyosin, Asia
INTRODUCTION
In the last several decades, we have witnessed an allergy epidemic that documented a striking global rise in the prevalence of asthma, allergic rhinitis, and eczema. More recently, the prevalence of food allergy has been rising particularly in the Western world and has been considered the second wave of the allergy epidemic. Aside from egg and cow's milk allergies that are the most prevalent food allergies in infants and young Asian children, the pattern of food allergy shows significant differences from those of Western populations.
In the Asian region, fish and peanut allergies are not common1,2 Instead, crustacean shellfish allergy is an important cause of food allergy. A summary of epidemiological studies of shellfish allergy are presented in Table. Population surveys show that the prevalences in teenagers are 5.12% in the Philippines and 5.23% in Singapore.1 Hospital-based studies on anaphylaxis show that crustacean shellfish is a leading cause of food-induced anaphylaxis in Singapore,3,4 Thailand,5 Hong Kong,6 and Taiwan.7 One unique feature in the clinical manifestation of shellfish allergy in Asia is the predominance of a milder form of allergy with reactions localized to the oral mucosa, as reported by recent studies from Singapore8 and Thailand.9
Table. Population studies on shellfish allergy prevalence in Asia.
| Country | Shellfish (%) | Population (n) | Age (year) | Methodology | Year published | Reference |
|---|---|---|---|---|---|---|
| China | 0.17-0.42 | 1604 | 0-2 | Report, SPT, FE, DBPCFC | 2012 | 45 |
| Hong Kong, China | 0.9 | 3677 | 2-7 | Report/Doctor-diagnosed | 2009 | 46 |
| Hong Kong, China | 37.8 | 7393 | 0-14 | Report | 2012 | 47 |
| Philippines | 5.12 | 11,158 | 14-16 | Convincing history | 2010 | 1 |
| Singapore | 1.19 | 4115 | 4-6 | Convincing history | 2010 | 1 |
| 5.23 | 6342 | 14-16 | Convincing history | |||
| Taiwan | 1.1 | 813 | <3 | Convincing history +/- SPT/IgE | 2012 | 48 |
| 7.71 | 15,169 | 4-18 | Convincing history +/- SPT/IgE | |||
| 7.05 | 14,036 | >19 | Convincing history +/- SPT/IgE | |||
| Thailand | 0.88 | 452 | 3-7 | Report, SPT, OFC | 2005 | 49 |
Report: self/parent-reported based on symptoms provided only; Convincing history: symptoms occuring in less than 2 hours.
SPT, skin prick test; DBPCFC, double-blinded placebo-controlled food challenge; OFC, open food challenge; FE, food elimination.
The major allergen in shrimp and shellfish allergies is tropomyosin, a pan-allergen involved in muscle contraction in invertebrates. Tropomyosins from house dust mites (HDMs) and cockroaches share a high sequence homology to shellfish tropomyosins, with an 81% amino acid sequence similarity between prawns and HDMs, and 82% similarity between prawns and cockroach.10,11 Cross-reactivity between tropomyosins from HDMs and shellfish has been well demonstrated.12 House dust mites thrive in the humid tropical Asian environment, and there is a high prevalence of HDM allergy and IgE sensitization in our Asian population.13,14,15,16 In a recent report from Singapore on 39 shrimp-allergic patients, all subjects with shrimp allergy were also sensitized to HDMs.8 This review explores the hypothesis that inhalant exposure to tropomyosins from HDMs is the primary sensitizer for shellfish allergies, and IgE cross-reactivity with shellfish tropomyosins accounting for mild oral allergies upon consumption of shellfish.17 This may be similar to the oral allergy (inhalant-food) syndrome seen commonly in Europe, where birch pollen-allergic individuals react to cross-reactive allergens in fruits and nuts. The predominant clinical features are of lip itch and swelling, and are confined to the oral mucosa.18 Heat-labile pathogenesis-related (PR) proteins elicit IgE reactions from local tissue bound serum IgE in the oral mucosa, which are then rapidly denatured during digestion, halting the progression to systemic symptoms.19 Heat and proteolysis-stable Profilin and lipid transfer proteins have also been found to be involved in cross-reactivity, which likely accounts for the small subgroup of patients who do experience anaphylaxis.20 Tropomyosin is as a heat stable protein, and this may explain the spectrum of severity of reactions, although most reactions in tropical cohorts are confined to the oral mucosa. This hypothesis is supported by intriguing epidemiological, clinical, and immunochemical data that reinforces this HDM and shellfish allergy link and that tropomyosin plays a major role in these interactions.
MOLECULAR ASPECTS OF TROPOMYOSIN
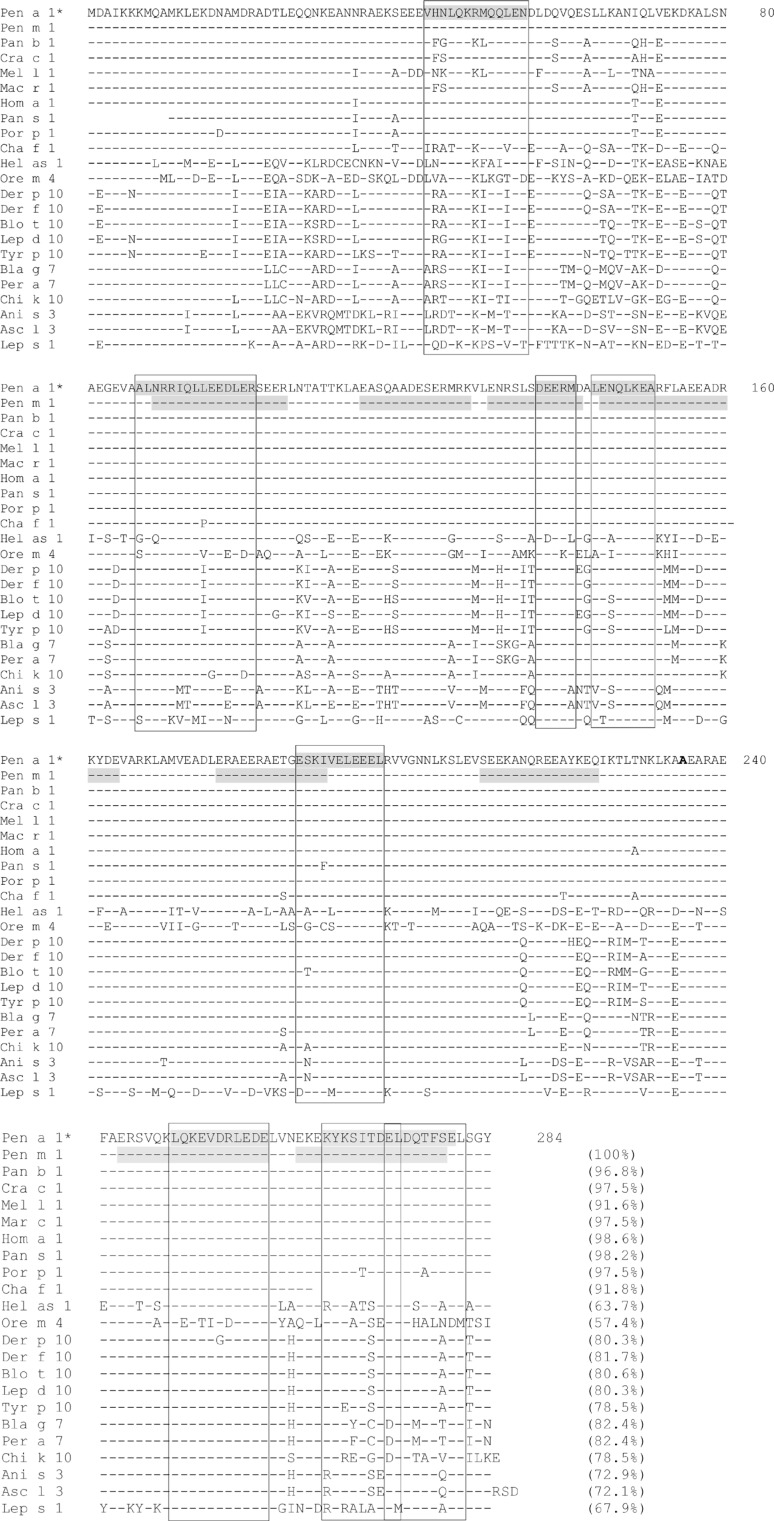
The amino acid sequences of allergic tropomyosin are compared in Figure. Tropomyosins of shrimps, prawns, lobsters, and crabs share sequence identity between 91% and 100%. The snail tropomyosin Hel as 1 and the fish tropomyosin Ore m 4 have 63.7% and 57.4% sequence identity to the shrimp tropomyosin Pen a 1, respectively. Tropomyosins of mites and cockroaches have 78.5%-81.7% and 82.4% sequence identity to Pen a 1, respectively. The sequence identity of Pen a 1 to tropomyosins of midge (chi k 10), parasite (Ani s 3) and silverfish (Lep s 1) are 78.5%, 72.9%, and 67.9%, respectively.
Figure. Comparison of identified Pen a 1 IgE-binding epitopes with homologous sequences in other allergic tropomyosins. Pen a 1 (Genbank accession number AAZ76743) from shrimp Penaeus aztecus, Pen m 1 (BAF47262) from shrimp Penaeus monodon, Pan b 1(CBY17558) from shrimp Pandalus borealis, Cra c 1 (ACR43473 ) from shrimp Crangon crangon, Mel l 1 (AGF86397) from prawn Melicertus latisulcatus, Mar c 1 (ADC55380) from prawn Macrobrachium rosenbergii, Hom a 1 (AAC48288) from lobster Homarus americanus, Pan s 1 (AAC38996) from Lobster Panulirus stimpsoni, Por p 1 (AGE44125) from crab Portunus pelagicus, Cha f 1 (AAF35431) from crab Charybdis feriatus, Hel as 1 (CAB38044.1) from snail Helix aspersa, Ore m 4 (AFV53352) from fish Oreochromis mossambicus, Der p 10 (CAA75141) from mite Dermatophagoides pteronyssinus, Der f 10 (BAA04557) from mite Dermatophagoides farinae, Blo t 10 (ABU97466) from mite Blomia tropicalis, Lep d 10 (CAB71342) from mite Lepidoglyphus destructor, Tyr p 10 (AAT40866) from mite Tyrophagus putrescentiae, Bla g 7 (AAF72534) from cockroach Blattella germanica, Per a 7 (AAD19606) from cockroach, Chi k 10 (CAA09938) from Midge Chironomus kiiensis, Ani s 3 (CAB93501) form parasite Anisakis simplex, Asc l 3 (ACN32322) from parasite Ascaris lumbricoides, Lep s 1 (CAC84590) from silverfish Lepisma saccharina. IgE epitopes on the studies of Pen a 1 and Pen m 1 are shaded. Sequences that are aligned with the identified Pen a 1 IgE epitopes are boxed. The numbers in the parentheses indicated the percentages of identity as compared to the sequence of Pen a 1. *Let v1 and Pen a 1 have identical sequence.
To investigate this homology, IgE-binding epitopes have been evaluated. The IgE epitopes of shrimp tropomyosin was first studied using the approach of peptide epitope mapping.21,22 Eight IgE-binding epitopes were identified in Pen a 1 from Penaeus aztecus.21 Further investigations showed that the 2 sequences 145-164 and 263-280 inhibited IgE-binding reactivity to tropomyosin of Penaeus monodon (Pen m 1) in all the shrimp-allergic sera tested, and these were proposed to be the major IgE epitopes.23
The multiple sequence alignment of tropomyosin from shrimp (Pen a 1, Pen m1), crab (Por p 1), lobster (Hom a 1), and HDM (Der p 10 and Blo t 10) revealed that the shrimp tropomysin Pen m 1 and the lobster tropomyocin Pro p 1 and Hom a 1 have almost identical sequences at all the 8 identified Pen a 1 IgE epitopes. The sequence identity of the HDM tropomyosins Der p 10 and Blo t 10 to Pen a 1 at these 8 IgE epitopes was also high (>80%), with the exception of the first epitope 43-55 for which the sequence identity is 58% (Figure). Pen a 1 epitopes and their homologous sequences in Hom a 1 (lobster tropomyosin) have similar IgE-binding reactivity to sera from shrimp-allergic subjects. In contrast, these sera showed similar IgE-binding reactivity to only 4 homologous sequences in Der p 10 (187-197, 249-259, 266-273, and 273-281) and had less or no IgE reactivity to the other 4 homologous sequences.24
An earlier study using Blomia tropicalis-allergic sera revealed that the IgE epitopes of Blo t 10 were mainly located at N- and C-terminal of the molecule.25 Only one-third of subjects had IgE reactivity to the middle region 125-214, indicating that the middle region of Blo t 10 is less allergenic. This may explain the reduced IgE cross-reactivity of shrimp-allergic sera to 2 IgE epitopes of Pen a 1 homologous sequences (137-141, 144-151) in Der p 10.
This data indicates the presence of IgE cross-reactivity between tropomyosins of shellfish and HDMs; however, the cross reactivity is stronger within shellfish (shrimp and lobster) than between shellfish and HDMs.
HDM AND SHELLFISH LINK
HDM immunotherapy and crustacean allergy
The effect of HDM immunotherapy on shellfish allergy provides evidence for the link between HDM and shellfish tropomyosins. The induction of shrimp allergy with positive skin prick tests and food challenge in previously non-allergic patients receiving HDM immunotherapy has been reported.26 Interestingly, clinical reactions were localized to the oral mucosa alone. On the other hand, sublingual HDM immunotherapy has also been reported to improve shrimp tolerance in a shrimp-allergic patient with previous anaphylaxis to shrimp-the same authors speculated that this effect may be related to the total dose of tropomyosin received, as this patient was treated with double the recommended dose of SLIT.27 Subcutaneous immunotherapy has also been reported to be associated with a decrease in specific serum IgE and resolution of shrimp and squid allergy.28 The effect of immunotherapy may be affected by the total dose of tropomyosin as well as the route of administration.
Some inference may also be drawn from the reported effects of HDM immunotherapy on snail allergy-although snails are molluscs and not crustaceans, inhibition experiments have confirmed the cross-reactivity between snail and HDM tropomyosins.29,30 HDM immunotherapy has been reported to worsen respiratory symptoms in snail-allergic patients,30 with induction of anaphylactic episodes in patients with previously mild symptoms.31
Conversely, the lack of neosensitization to shrimp-specific tropomyosin has also been reported in prospective studies of patients receiving SLIT HDM immunotherapy.32,33 These diverse observations suggest that the effect of HDM immunotherapy on induction of allergy or tolerance may depend on the level of tropomyosin in the immunotherapy extracts.
IgE sensitization to shrimp correlates with HDM sensitization
Shellfish sensitization has been shown to correlate with HDM sensitization and allergy in several different populations. A hospital-based study of atopic Singaporean children showed that 72.4 % of shellfish-sensitized patients are also sensitized to HDMs.34 Asthmatic children from the US also demonstrated a strong positive correlation between shrimp and HDM IgE levels.35
Studies of HDM and shellfish sensitization occurring in populations with little or no exposure to either HDMs or shellfish provide evidence to suggest primary sensitization with subsequent cross-reactivity. A study of unexposed Jews who observed strict Kosher dietary rules prohibiting consumption of shellfish showed that sensitization to shrimp was related to cross-reacting tropomyosin allergens in HDMs.36 They also showed that the IgE cross-reactivity translated into clinical allergy to shrimp in some of the subjects. Reciprocally, a population-based study of young adults from Iceland where exposure to HDMs is extremely rare showed that HDM sensitization is associated with sensitization to shrimp.37
Indirect in vivo data from a cohort of Singaporean shrimp-allergic patients revealed a subgroup of patients with mite sensitization and no evidence of shrimp sensitization, (negative skin prick tests to shrimp).8 Similar findings were also reported in a subgroup of patients from a Spanish study of shrimp-allergic patients living in the warm humid climate.38 Taken together, these findings suggest that HDMs are the likely primary sensitizer for shrimp allergy in these patients living in warm humid climates.
To further confirm this, sera of shrimp-allergic patients were investigated with IgE inhibition studies. A Spanish study investigating patients with both HDM and shrimp allergies found a strong correlation between sIgE to shrimp and HDM tropomyosins, with almost complete inhibition of shrimp extract by a mite (Chortoglyphus arcuatus) on immunoblot inhibition studies, suggesting that HDMs are the primary sensitizer39 A more recent study, also from Spain, demonstrated through reciprocal inhibition assays that Dermatophagoides pteronyssinus is the primary sensitizer in shrimp-allergic subjects living in a humid climate, while shrimp is the primary sensitizer in shrimp-allergic subjects living in a dry climate.38 These findings further substantiate the mite-shellfish link in populations living in tropical climates.
IS TROPOMYOSIN THE MOST IMPORTANT ALLERGEN IN SHELLFISH ALLERGY?
Tropomyosin remains the most thoroughly investigated and most prevalent allergen in shellfish allergy, with considerable evidence of cross-reactivity with HDMs, snails, and other molluscs. Sensitization to shellfish tropomyosins has been investigated through specific serum IgE measurement and has higher sensitivity and specificity in prediction of shellfish allergy than skin prick tests for the whole shrimp extract.40 Arginine kinase, a new class of invertebrate pan-allergens, has been shown to be cross-reactive in a Malaysian study of patients allergic to both red crab and blue crab.41 Myosin light chain kinase (Lit v 3)42 and sarcoplasmic calcium-binding protein (Lit v 4)43 have recently been identified as allergens in the Pacific white shrimp. In addition, α-actinin, β-actin, fructose biphosphate aldolase, and ubiquitin have been identified as allergens in the Solenocera melantho (red shrimp) species.38 A recent review of 40 shrimp-allergic patients from Italy showed that 10%-15% were sensitized to arginine kinase and Lit v4, suggesting that tropomyosin remains the primary allergen.44 However, only 1 case of co-sensitization with tropomyosin was recorded, suggesting that these minor allergens remained clinically relevant. Low rates of sensitization to other shrimp allergens, such as Lit v2, Lit v3, and Lit v4, have also been documented in a cohort of 39 shrimp-allergic patients from Singapore,8 indicating that in some populations, these non-tropomyosin allergens may be of less clinical importance.
CONCLUSIONS
We have reviewed the evidence to support the hypothesis that inhaled tropomyosins from HDMs are the primary sensitizer for shellfish allergy in warm and humid tropical climates. These findings open the possibility of novel therapeutic approaches with allergen immunotherapy for shellfish allergy.
The unique epidemiology of shellfish allergy in Asia raises intriguing questions: Is the high prevalence of shellfish allergy merely related to repeated exposure to inhaled HDM tropomyosins? Further studies on the temporal relationship between shellfish and HDM sensitization and the effect of genetic background on the development of shellfish allergy would provide insights into the mite-shellfish link.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331. 331.e1–331.e7. doi: 10.1016/j.jaci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Connett GJ, Gerez I, Cabrera-Morales EA, Yuenyongviwat A, Ngamphaiboon J, Chatchatee P, et al. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int Arch Allergy Immunol. 2012;159:384–390. doi: 10.1159/000338940. [DOI] [PubMed] [Google Scholar]
- 3.Thong BY, Cheng YK, Leong KP, Tang CY, Chng HH. Immediate food hypersensitivity among adults attending a clinical immunology/allergy centre in Singapore. Singapore Med J. 2007;48:236–240. [PubMed] [Google Scholar]
- 4.Liew WK, Chiang WC, Goh AE, Lim HH, Chay OM, Chang S, et al. Paediatric anaphylaxis in a Singaporean children cohort: changing food allergy triggers over time. Asia Pac Allergy. 2013;3:29–34. doi: 10.5415/apallergy.2013.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lertnawapan R, Maek-a-nantawat W. Anaphylaxis and biphasic phase in Thailand: 4-year observation. Allergol Int. 2011;60:283–289. doi: 10.2332/allergolint.10-OA-0256. [DOI] [PubMed] [Google Scholar]
- 6.Smit DV, Cameron PA, Rainer TH. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005;28:381–388. doi: 10.1016/j.jemermed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Hsin YC, Hsin YC, Huang JL, Yeh KW. Clinical features of adult and pediatric anaphylaxis in Taiwan. Asian Pac J Allergy Immunol. 2011;29:307–312. [PubMed] [Google Scholar]
- 8.Thalayasingam M, Gerez IF, Yap GC, Llanora GV, Chia IP, Chua L, et al. Clinical and immunochemical profiles of food challenge proven or anaphylactic shrimp allergy in tropical Singapore. Clin Exp Allergy. 2015;45:687–697. doi: 10.1111/cea.12416. [DOI] [PubMed] [Google Scholar]
- 9.Jirapongsananuruk O, Sripramong C, Pacharn P, Udompunturak S, Chinratanapisit S, Piboonpocanun S, et al. Specific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic children. Clin Exp Allergy. 2008;38:1038–1047. doi: 10.1111/j.1365-2222.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- 10.Ayuso R, Lehrer SB, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin) Int Arch Allergy Immunol. 2002;127:27–37. doi: 10.1159/000048166. [DOI] [PubMed] [Google Scholar]
- 11.Santos AB, Chapman MD, Aalberse RC, Vailes LD, Ferriani VP, Oliver C, et al. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104:329–337. doi: 10.1016/s0091-6749(99)70375-1. [DOI] [PubMed] [Google Scholar]
- 12.Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129:38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 13.Andiappan AK, Puan KJ, Lee B, Nardin A, Poidinger M, Connolly J, et al. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy. 2014;69:501–509. doi: 10.1111/all.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gendeh BS, Mujahid SH, Murad S, Rizal M. Atopic sensitization of children with rhinitis in Malaysia. Med J Malaysia. 2004;59:522–529. [PubMed] [Google Scholar]
- 15.Chew FT, Lim SH, Goh DY, Lee BW. Sensitization to local dust-mite fauna in Singapore. Allergy. 1999;54:1150–1159. doi: 10.1034/j.1398-9995.1999.00050.x. [DOI] [PubMed] [Google Scholar]
- 16.Goh DY, Chew FT, Quek SC, Lee BW. Prevalence and severity of asthma, rhinitis, and eczema in Singapore schoolchildren. Arch Dis Child. 1996;74:131–135. doi: 10.1136/adc.74.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klaewsongkram J. High prevalence of shellfish and house dust mite allergies in Asia-Pacific: probably not just a coincidence. Asian Pac J Allergy Immunol. 2012;30:247–248. [PubMed] [Google Scholar]
- 18.Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104:101–108. doi: 10.1016/j.anai.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106:27–36. doi: 10.1067/mai.2000.106929. [DOI] [PubMed] [Google Scholar]
- 20.Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, et al. Profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992;175:377–385. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayuso R, Lehrer SB, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin) Int Arch Allergy Immunol. 2002;127:27–37. doi: 10.1159/000048166. [DOI] [PubMed] [Google Scholar]
- 22.Reese G, Viebranz J, Leong-Kee SM, Plante M, Lauer I, Randow S, et al. Reduced allergenic potency of VR9-1, a mutant of the major shrimp allergen Pen a 1 (tropomyosin) J Immunol. 2005;175:8354–8364. doi: 10.4049/jimmunol.175.12.8354. [DOI] [PubMed] [Google Scholar]
- 23.Zheng LN, Lin H, Pawar R, Li ZX, Li MH. Mapping IgE binding epitopes of major shrimp (Penaeus monodon) allergen with immunoinformatics tools. Food Chem Toxicol. 2011;49:2954–2960. doi: 10.1016/j.fct.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129:38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 25.Yi FC, Cheong N, Shek LP, Wang DY, Chua KY, Lee BW. Identification of shared and unique immunoglobulin E epitopes of the highly conserved tropomyosins in Blomia tropicalis and Dermatophagoides pteronyssinus. Clin Exp Allergy. 2002;32:1203–1210. doi: 10.1046/j.1365-2745.2002.01449.x. [DOI] [PubMed] [Google Scholar]
- 26.van Ree R, Antonicelli L, Akkerdaas JH, Garritani MS, Aalberse RC, Bonifazi F. Possible induction of food allergy during mite immunotherapy. Allergy. 1996;51:108–113. [PubMed] [Google Scholar]
- 27.Cortellini G, Spadolini I, Santucci A, Cova V, Conti C, Corvetta A, et al. Improvement of shrimp allergy after sublingual immunotherapy for house dust mites: a case report. Eur Ann Allergy Clin Immunol. 2011;43:162–164. [PubMed] [Google Scholar]
- 28.Pevec B, Pevec MR, Markovic AS, Batista I. House dust mite subcutaneous immunotherapy does not induce new sensitization to tropomyosin: does it do the opposite? J Investig Allergol Clin Immunol. 2014;24:29–34. [PubMed] [Google Scholar]
- 29.van Ree R, Antonicelli L, Akkerdaas JH, Pajno GB, Barberio G, Corbetta L, et al. Asthma after consumption of snails in house-dustmite-allergic patients: a case of IgE cross-reactivity. Allergy. 1996;51:387–393. [PubMed] [Google Scholar]
- 30.Guilloux L, Vuitton DA, Delbourg M, Lagier A, Adessi B, Marchand CR, et al. ross-reactivity between terrestrial snails (Helix species) and house-dust mite (Dermatophagoides pteronyssinus). II. In vitro study. Allergy. 1998;53:151–158. doi: 10.1111/j.1398-9995.1998.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 31.Pajno GB, La Grutta S, Barberio G, Canonica GW, Passalacqua G. Harmful effect of immunotherapy in children with combined snail and mite allergy. J Allergy Clin Immunol. 2002;109:627–629. doi: 10.1067/mai.2002.122844. [DOI] [PubMed] [Google Scholar]
- 32.Asero R. Lack of de novo sensitization to tropomyosin in a group of mite-allergic patients treated by house dust mite-specific immunotherapy. Int Arch Allergy Immunol. 2005;137:62–65. doi: 10.1159/000085105. [DOI] [PubMed] [Google Scholar]
- 33.Rossi RE, Monasterolo G, Incorvaia C, Moingeon P, Frati F, Passalacqua G, et al. Lack of neo-sensitization to Pen a 1 in patients treated with mite sublingual immunotherapy. Clin Mol Allergy. 2010;8:4. doi: 10.1186/1476-7961-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang WC, Kidon MI, Liew WK, Goh A, Tang JP, Chay OM. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007;37:1055–1061. doi: 10.1111/j.1365-2222.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Calatroni A, Visness CM, Sampson HA. Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. J Allergy Clin Immunol. 2011;128:834–837. doi: 10.1016/j.jaci.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33:956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 37.Adalsteinsdottir B, Sigurdardottir ST, Gislason T, Kristensen B, Gislason D. What characterizes house dust mite sensitive individuals in a house dust mite free community in Reykjavik, Iceland? Allergol Int. 2007;56:51–56. doi: 10.2332/allergolint.O-06-447. [DOI] [PubMed] [Google Scholar]
- 38.Gámez C, Zafra M, Boquete M, Sanz V, Mazzeo C, Ibáñez MD, et al. New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol Nutr Food Res. 2014;58:1915–1925. doi: 10.1002/mnfr.201400122. [DOI] [PubMed] [Google Scholar]
- 39.Boquete M, Iraola V, Morales M, Pinto H, Francisco C, Carballás C, et al. Seafood hypersensitivity in mite sensitized individuals: is tropomyosin the only responsible allergen? Ann Allergy Asthma Immunol. 2011;106:223–229. doi: 10.1016/j.anai.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Yang AC, Arruda LK, Santos AB, Barbosa MC, Chapman MD, Galvão CE, et al. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J Allergy Clin Immunol. 2010;125:872–878. doi: 10.1016/j.jaci.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 41.Rosmilah M, Shahnaz M, Zailatul HM, Noormalin A, Normilah I. Identification of tropomyosin and arginine kinase as major allergens of Portunus pelagicus (blue swimming crab) Trop Biomed. 2012;29:467–478. [PubMed] [Google Scholar]
- 42.Ayuso R, Grishina G, Bardina L, Carrillo T, Blanco C, Ibáñez MD, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol. 2008;122:795–802. doi: 10.1016/j.jaci.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Ayuso R, Grishina G, Ibáñez MD, Blanco C, Carrillo T, Bencharitiwong R, et al. Sarcoplasmic calcium-binding protein is an EF-handtype protein identified as a new shrimp allergen. J Allergy Clin Immunol. 2009;124:114–120. doi: 10.1016/j.jaci.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Giuffrida MG, Villalta D, Mistrello G, Amato S, Asero R. Shrimp allergy beyond Tropomyosin in Italy: clinical relevance of Arginine Kinase, Sarcoplasmic calcium binding protein and Hemocyanin. Eur Ann Allergy Clin Immunol. 2014;46:172–177. [PubMed] [Google Scholar]
- 45.Chen J, Liao Y, Zhang HZ, Zhao H, Chen J, Li HQ. Prevalence of food allergy in children under 2 years of age in three cities in China. Zhonghua Er Ke Za Zhi. 2012;50:5–9. [PubMed] [Google Scholar]
- 46.Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009;20:339–346. doi: 10.1111/j.1399-3038.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 47.Ho MH, Lee SL, Wong WH, Ip P, Lau YL. Prevalence of self-reported food allergy in Hong Kong children and teens--a population survey. Asian Pac J Allergy Immunol. 2012;30:275–284. [PubMed] [Google Scholar]
- 48.Wu TC, Tsai TC, Huang CF, Chang FY, Lin CC, Huang IF, et al. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012;42:1310–1315. doi: 10.1111/j.1445-5994.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 49.Santadusit S, Atthapaisalsarudee S, Vichyanond P. Prevalence of adverse food reactions and food allergy among Thai children. J Med Assoc Thai. 2005;88(Suppl 8):S27–S32. [PubMed] [Google Scholar]