Abstract
High-grade B-cell lymphomas with recurrent chromosomal break points have been termed ‘double hit lymphoma’ (DHL). The most commonly seen DHL is diffuse large B-cell lymphoma (DLBCL) with t(14;18) and t(8;14) or t(8;22) resulting in overexpression of BCL2 and MYC, respectively. The increased proliferation due to MYC overexpression, without the ability for an apoptotic brake as a result of BCL2 overexpression, results in ‘the perfect storm of oncogenesis’. Thus this disease presents a number of diagnostic and therapeutic challenges for the hematologist. The first and foremost challenge is to recognize the DHL. As different morphological entities can be affected it is incumbent on pathologists and clinicians to maintain a high index of suspicion especially in disease that appears unusually aggressive or refractory to therapy. Diagnosis by fluorescence in situ hybridization (FISH) is a sensitive and specific method for detection of the disease but is time-consuming and expensive. While detection by immunohistochemistry (IHC) is sensitive and correlates with survival, standardized methods for this are not widely agreed upon. The second and equally important challenge in DHL is optimizing clinical outcome in a group of patients for whom the prognosis is widely regarded as poor. While improvements have been achieved by dose escalating standard chemotherapeutic regimens, many patients continue to do badly. Furthermore as a disease of aging many patients are unsuitable for dose-intensive chemotherapy regimens. There are now multiple novel targeted agents in various stages of clinical development that offer hope for better outcomes without undue toxicity. Among the most exciting of these developments include specific inhibitors of both BCL2 and MYC.
Keywords: BCL-2, diffuse large B-cell lymphoma, double hit lymphoma, MYC
Introduction: challenging lymphomas
Traditional pathological diagnosis of lymphoma has been based upon morphological appearance of involved tissue under light microscopy and definition of lineage by immunohistochemistry (IHC) or flow cytometry. Over recent years there has been increased recognition of the limitations of this approach in adequately differentiating disease entities in terms of clinical outcome, especially among aggressive B-cell lymphomas. Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma in Western countries [Friedberg and Fisher, 2008; Friedberg, 2012] and is defined by the World Health Organization (WHO) as a ‘neoplasm of large B lymphoid cells’ with a ‘diffuse growth pattern’ [Harris et al. 2008]. However, amongst patients with DLBCL, clinical behavior and responses to therapy can be heterogeneous [Barrans et al. 2010].
The mainstay of therapy for aggressive B-cell lymphoma is R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) or R-CHOP-like regimens. Chemoimmuno-therapy in DLBCL has improved 2-year overall survival (OS) to greater than 70% [Coiffier et al. 2002; Sehn et al. 2005; Pfreundschuh et al. 2006]. However, an ongoing challenge remains how to deal with early relapsing or primary refractory disease. These patients continue to have poor outcomes even after salvage with high-dose therapy (HDT) and autologous stem-cell transplant (SCT) and are candidates for augmentation of first-line therapy, including addition of novel agents with a view to improving outcomes [Gisselbrecht et al. 2010]. Understanding the genetic drivers of poor prognosis aggressive B-cell lymphomas is key to identifying high-risk individuals, and the prospective identification of patients at risk is a necessary first step.
Patients with high-grade clinically aggressive lymphomas and transformed disease are an enriched population for so-called ‘double hit’ lymphoma (DHL) [Pedersen et al. 2012]. Thangavelu and others in 1990 [Thangavelu et al. 1990] provided an early description of lymphoid malignancies characterized by both t(14;18) and t(8;14) or t(8;22) with resulting concurrent overexpression of both BCL2 and MYC. These lymphomas displayed aggressive biological behavior. The definition of DHL has been expanded over time to refer to high-grade B-cell lymphomas with recurrent chromosomal breakpoints resulting in activation of two or more oncogenes [Aukema et al. 2011]. It is therefore an historical quirk that we retain the name DHL even in an era where we recognize that multiple genetic events occur in the development of most B-cell lymphomas [Cigudosa et al. 1999; Lohr et al. 2012]. Although DH chromosomal changes are recognized in a number of B cell malignancies including follicular lymphoma (FL), acute lymphoblastic leukemia and B cell lymphoma unclassifiable, by far the most common histological subgroup affected is DLBCL. For the purposes of this review unless otherwise specified below DHL will refer to DLBCL with chromosomal translocations that result in overexpression of both BCL2 and MYC proteins.
DHL is important because many authors now believe that standard chemo-immunotherapy alone is insufficient therapy [Friedberg, 2012; Lin et al. 2012] with reports of a median OS of approximately one year [Green et al. 2012b]. Nonetheless, barriers to improving current outcomes are multiple and include: (1) defining a homogenous group of lymphomas to be included in the definition of this disease; (2) optimizing accurate, widely available and timely detection of this high-risk entity in clinical practice; and (3) identification of augmented regimens with meaningfully enhanced antilymphoma efficacy.
Defining DHL: genetics and phenotype
Overexpression of MYC and BCL2 proteins in the same B cell classically arises as a result of chromosomal translocations whereby the relevant genes are juxtaposed to the promoter regions of genes that are constitutively active during B-cell development, most typically the immunoglobulin heavy chain (IGH) gene. The 14;18 translocation juxtaposes the IGH and the BCL2 genes, resulting in overexpression of the anti-apoptotic protein BCL2. t(14;18) is the hallmark of follicular lymphoma, occurring in 85% of patients of European background and 70% of patients with Asian background [Biagi and Seymour, 2002]. The vast majority of cases of follicular lymphoma have high level of expression of BCL2 protein [Pezzella et al. 1990]. In contrast t(14;18) occurs less frequently in DLBCL where it is seen in between five and 30% of cases [Barrans et al. 2003]. BCL2 promotes the survival of cells in the face of major stressors such as DNA-damage and growth factor deprivation. Not only is avoidance of apoptosis through overexpression of BCL2 a recognized hallmark of cancer driving its development [Hanahan and Weinberg, 2000] it also mediates resistance to chemotherapy in experimental models of lymphoma [Schmitt and Lowe, 2001]. This latter attribute likely explains the association of BCL2 overexpression with poorer prognosis in patients with DLBCL treated with CHOP in the pre-rituximab era [Mounier et al. 2003]. It may also underpin the inability of chemotherapy alone to cure follicular lymphoma.
Overexpression of the MYC proto-oncogene due to its juxtaposition with IGH in the t(8;14) or with IGL or IGK in the t(8;22) and t(2;8) light chain variants respectively, influences a multitude of cellular functions including proliferation [Anderson et al. 2015], metabolism and protein synthesis. When a MYC translocation occurs in isolation or in the context of a simple karyotype, as in the case of Burkitt lymphoma, the increase in proliferation is accompanied by increased apoptosis [Fanidi et al. 1992; Murphy et al. 2008]. Morphologically this is recognized by the classical ‘starry sky’ pattern of Burkitt lymphoma. Detection of a MYC rearrangement is thought to confer inferior outcome in DLBCL [Savage et al. 2009; Barrans et al. 2010; Valera et al. 2013]. However in DLBCL, MYC translocations are infrequent as a single hit and are usually found as part of a complex karyotype [Boerma et al. 2009; Smith et al. 2010].
The combination of increased proliferation and reduced apoptosis, conferred by co-expression of MYC and BCL2, results in the ‘perfect storm’ of oncogenesis resulting in aggressive difficult to treat malignancies [Fanidi et al. 1992; Johnson et al. 2009; Li et al. 2012; Pedersen et al. 2012; Cheah et al. 2015]. Although MYC overexpression in isolation results in aggressive lymphoma morphology [Mohamed et al. 2001], at least two studies have found that MYC protein overexpression is clinically more meaningful when associated with BCL2 [Green et al. 2012b; Johnson et al. 2012]. In a study by Barrans and others, MYC rearrangements (often t(8;22)) occurred in approximately 14% of untreated de novo DLBCL, with the incidence of concurrent MYC and BCL2 rearrangements being approximately 10% [Barrans et al. 2010]. The incidence of DH status increases to around 20% amongst patients with aggressive lymphoma transformed from more indolent diseases such as follicular lymphoma [Pedersen et al. 2012]. This may represent acquisition of MYC during the process of transformation. Paradoxically, other studies suggest that the incidence of DHL is approximately the same amongst cohorts of relapsed versus newly diagnosed disease [Cuccuini et al. 2012].
While cytogenetically defined BCL2+/MYC+ DHL accounts for 60–90% of all DHL [Aukema et al. 2011; Petrich et al. 2014], other combinations of translocations seen include BCL6+/MYC+ (approximately 5%), BCL2+/BCL6+/MYC+ (also known as triple hit lymphoma [THL], approximately 8%) [Petrich et al. 2014] as well as rare translocations involving other genes including CCND1, BCL3 and PAX5 [Cheah et al. 2015]. The importance of DHL has been increasingly recognized because of its profound impact on clinical outcome, particularly in DLBCL where early relapse following rituximab-containing chemo-immunotherapy portends a poor prognosis [Gisselbrecht et al. 2010]. One study by Niitsu and others, demonstrated 2-year survival of 23% in MYC+/BCL2+, compared with 65% in MYC+/BCL2-, 81% in MYC-/BCL2+ and 84% in MYC-/BCL2- lymphomas [Niitsu et al. 2009].
DHL are associated with a high prevalence of independent adverse risk factors in DLBCL including B symptoms, high lactate dehydrogenase, advanced age, extra nodal disease, bone marrow involvement, higher Ki-67 (median 80%), Ann Arbor stages III and IV as well as central nervous system (CNS) disease [Niitsu et al. 2009; Savage et al. 2009; Barrans et al. 2010; Snuderl et al. 2010; Friedberg, 2012; Cheah et al. 2015]. Even accounting for high international prognostic index (IPI) scores, DHL status confers a poor prognosis [Le Gouill et al. 2007]. Unlike many conventional DLBCL risk factors the adverse prognosis portended by DHL status is not negated by chemo-immunotherapy with rituximab [Savage et al. 2009]. DHL affects adults of all ages [Cheah et al. 2015] and there is a male predominance [Oki et al. 2014]. Among 129 DHL patients from MD Anderson the most predictive adverse clinical features at diagnosis were poor performance status and stage four disease [Oki et al. 2014].
Optimizing diagnosis of DHL: practical versus purist
Accurate detection of adverse prognostic markers such as MYC and BCL2 as well as other genes conferring DH or TH status including BCL6, CCND1, BCL3 and PAX5 is key to identifying high-risk patients [Petrich et al. 2014; Cheah et al. 2015]. DHL patients are ideal candidates for clinical trials of novel agents and optimization of standard chemotherapeutic approaches, so early and accurate detection is essential.
In routine clinical practice there are two key methods available for detecting dysregulated MYC and BCL2: fluorescence in situ hybridization (FISH) to identify translocations and IHC to identify protein overexpression. FISH is the gold standard for detection of high-risk DH or TH translocation status. It is a reproducible technique that correlates with clinical outcome [Niitsu et al. 2009] and is used to define DHL in the majority of clinical trials. Some authors advocate FISH testing in all new diagnoses of DLBCL [Landsburg et al. 2014], as it is a both sensitive and specific test for DHL status (Figure 1). Over time FISH has become cheaper and hence more widely available, with establishment of standardized reference laboratories. However it can be time-consuming meaning that results may not always be available to prospectively use in planning treatment decisions. In addition, optimal tissue samples are required for this technique.
Figure 1.
Detection of DHL by FISH. The G-banded karyotype was 49,XY,+8,t(8;14)(q24;q32),+12, t(14;18)(q32;q21),+r[11]/46,XY[18]. (A) FISH using the LSI MYC (8q24) dual color break apart rearrangement probe (Vysis) showed two intact copies of MYC and one rearranged (split) MYC signal. In metaphases the rearranged 5’MYC signal (red) was located on the derivative chromosome 8 [der(8)der(8;14)] and the 3’MYC signal (green) was located on a derivative chromosome 14 [the der(14)t(8;14)]. (B) FISH using the LSI IGH/BCL2 t(14;18) dual color dual fusion translocation probe (Vysis) identified two IGH/BCL2 fusion signals: one on a derivative chromosome 14 [der(14)t(14;18)] and one on the derivative chromosome 18 [der(18)t(14;18)]. Additional IGH signals (green) were seen on the der(8)t(8;14) and the der(14)t(8;14). A BCL2 signal (red) was present on the intact chromosome 18.
DHL, double hit lymphoma; FISH, fluorescence in situ hybridization.
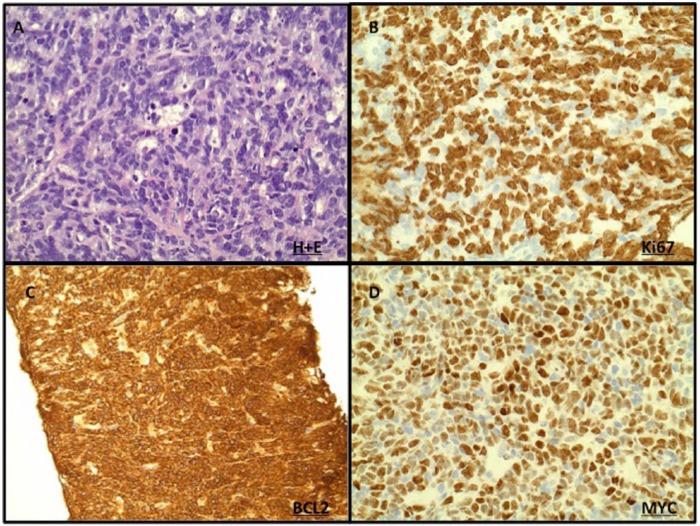
IHC to detect double protein expression is often used clinically to rapidly screen for DHL and when positive every effort should be made to confirm DHL status using FISH. IHC lacks standardization with no reliable cut off for positive used between clinical trials [De Jong et al. 2007]. Furthermore there is currently a paucity of evidence to support changing clinical practice based on the results of IHC alone. It also lacks sensitivity and may miss translocation-positive cases where the translocation is present in less than 40% of cells [Horn et al. 2013]. However, IHC to detect double protein expression is an inexpensive and widely available technique that is commonly utilized in clinical practice (Figure 2). More recently, adoption of standardized IHC methods has been associated with greater predictive value for this technique. With many current trials prospectively examining outcome in patients both by standardized IHC and FISH, the role of IHC in the detection of DHL is likely to become clearer.
Figure 2.
Detection of DHL by IHC. IHC of DH DLBCL is characterized by (A) diffuse growth of large B lymphoid cells on H+E with (B) high Ki-67, (C) high BCL2 and (D) high MYC expression.
DH, double hit; DHL, double hit lymphoma; DLBCL, diffuse large B-cell lymphoma; IHC, immunohistochemistry.
Using a cut off for positive of ⩾40% MYC positive cells, concurrent MYC and BCL2 were detected by IHC in 21% of DLBCL [Johnson et al. 2012]. Using a cut off for positive of ⩾50%, it was shown that among 77 cases of DLBCL, all patients with a MYC translocation were detected by IHC [Kluk et al. 2012]. Furthermore, in this study 4 out of 77 patients who were positive for MYC by IHC but negative by FISH demonstrated increased MYC transcription by gene expression testing and all IHC MYC positive cases demonstrated a poor prognosis [Kluk et al. 2012]. Others suggest that IHC MYC ⩾ 70% is the optimal cut off giving a sensitivity of 100% and a specificity of 93% for MYC expression as measured by quantitative real-time polymerase chain reaction (QRT-PCR) [Green et al. 2012a]. Among 193 patients with B-cell lymphoma treated with R-CHOP, 29% demonstrated increased MYC and BCL2 expression by IHC: all of whom had a poor outcome [Green et al. 2012b]. In this study only 11% had a detectable MYC rearrangement by FISH and only 6% were DHL by FISH [Green et al. 2012b]. The apparent incidence of DH status appears to increase from around 10% of DLBCL when defined by FISH to between 20% and 30% with IHC [Green et al. 2012b]. The higher incidence of DH status with IHC over FISH likely pertains to detection of protein overexpression driven by mechanisms other than translocations e.g. microRNA overexpression, amplification or mutation [Cheah et al. 2015] (Table 1). Similar issues arise in detection of BCL2 abnormalities by IHC.
Table 1.
Detection of MYC and BCL2. Comparison of FISH and IHC for detecting MYC and BCL2.
| Sensitivity | Specificity | References |
|---|---|---|
| 167 DLBCL patients; IHC positive for MYC at 40%; BCL2 and MYC detected in 21%. MYC IHC and MYC FISH strongly correlated (p < 0.01) | Johnson et al. [2012] | |
| 193 DLBCL patients treated with R-CHOP; IHC MYC positive at >40%; BCL2 and MYC detected in 29%; patients with IHC BCL2 and MYC had worse outcome | Only 6% were DHL by FISH. | Green et al. [2012] |
| 77 DLBCL patients; MYC IHC positive >50%; all MYC translocation positive cases detected by IHC | 4/77 cases had positive IHC but were negative by FISH | Kluk et al. [2012] |
| 219 DLBCL + Burkitt lymphoma patients; MYC IHC graded weak to strong; 93% of subjects with MYC translocations had ⩾80% MYC positive cells on IHC; ROC analysis ⩾ 70% optimal cut off with sensitivity of 100% | Only 3% of non MYC rearranged cases had ⩾80% MYC staining on IHC (p < 0.0001); specificity of a MYC cut off of ⩾70% is 93% | Green et al. [2012] |
DLBCL, diffuse large B-cell lymphoma; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; DHL, double hit lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; ROC, receiver operating characteristic
All new diagnoses of DLBCL should have IHC for MYC and BCL2 at a minimum [Friedberg, 2012] and when one or both is positive FISH should be performed for MYC, BCL2 and BCL6 to confirm DHL status. However, the cut off for positive IHC remains controversial in the literature, and in cases that are borderline positive, confirmatory testing with FISH is necessary. A standardized approach to the diagnosis of this entity will facilitate assessment of incidence and optimized management approaches.
Improving prognosis in DHL: incremental versus paradigm shifts
Given the poor outcome seen in DLBCL that relapses early post-chemo-immunotherapy [Gisselbrecht et al. 2010] optimizing therapy remains the key clinical challenge in DHL [Klapper et al. 2008; Oki et al. 2014; Pedersen et al. 2014]. The challenges here are twofold: (1) optimizing chemo-immunotherapy regimens to minimize the incidence of primary refractory and early relapsing disease (collectively, early treatment failure) and (2) salvaging individuals with poor response to initial therapy.
Approaches to optimizing traditional chemotherapeutic regimens, to minimize the incidence of early treatment failure, is critical, as outcomes in these patients remain poor even with HDT followed by autologous SCT [Gisselbrecht et al. 2010]. Variations on R-CHOP, including R-CHOP administered every 14 instead of every 21 days or the addition of etoposide to the regimen, have resulted in incremental benefits in survival of questionable significance, often at the expense of increased toxicity [Pfreundschuh et al. 2004]. Given the poor results with traditional chemo-immunotherapeutic approaches in the early treatment failure setting, much of the current research effort is directed towards evaluation of the safety and efficacy of a multitude of novel agents in the treatment of high-risk lymphoma such as DH DLBCL.
The key challenges in the field of novel agents for DHL and DLBCL in general are (1) defining which combinations of agents optimize clinical outcome while minimizing toxicity and (2) ordering the utilization of new agents and standard chemotherapeutics for example sequential therapy versus maintenance therapy [Anderson et al. 2015]. These challenges are compounded by the fact that DHL, like many cancers, is a disease of aging with a median age of diagnosis in the seventh decade of life [Green et al. 2012b; Johnson et al. 2012]. While the optimal therapeutic approach for this difficult group of patients remains unknown, ongoing clinical trials particularly with novel agents offer the hope of better outcomes for patients in the future [Friedberg, 2012]. Given that DHL patients are often poorly recognized and represent only a subset of poor prognosis DLBCL, much of the available data regarding optimizing treatment in DHL must be inferred from studies in a wider group of relapsed and refractory DLBCL patients.
Optimizing traditional chemotherapeutic approaches for DHL
Despite the prognosis in DHL, R-CHOP chemotherapy remains the backbone of treatment given the good outcomes achieved in DLBCL patients as a whole. Multiple studies have addressed the question of 14- versus 21-day treatment cycles and the addition of etoposide to the regimen [Pfreundschuh et al. 2004]. To date the numbers of patients with DHL included in these studies are too small to define whether this group of patients does better with increased dose intensity or the addition of etoposide, and evidence in favor of such approaches is anecdotal at best. Furthermore the incremental clinical benefit derived from modified R-CHOP-like regimens is not sufficient to justify the additional toxicity. Future clinical trials must prospectively look at outcomes in this high-risk subgroup.
DA-R-EPOCH (dose adjusted rituximab, etoposide, prednisolone, vincristine, cyclophosphamide and doxorubicin) is another variation on the traditional chemo-immunotherapy approach and has demonstrated efficacy and tolerability in patients, median age 50, with untreated de novo DLBCL [Wilson et al. 2008]. However, in this study the impact of MYC status was not assessed. The MD Anderson group looked at 129 cases of DHL treated with R-CHOP, R-EPOCH or R-HyperCVAD/MA (hyper-fractionated cyclophosphamide, vincristine, doxorubicin alternating with cytarabine plus methotrexate) [Oki et al. 2014]. R-HyperCVAD/MA had significantly more complete remissions (CRs) than R-CHOP (p = 0.011) and R-EPOCH was significantly better than R-CHOP in terms of event-free survival (EFS) (p = 0.004) [Oki et al. 2014]. Given the success of R + CODOX-M/IVAC (rituximab plus cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate, ifosfamide, etoposide and high dose cytarabine) in MYC positive Burkitt lymphoma, some authors have considered its use in high-grade large B-cell lymphomas characterized by MYC and BCL2 expression [Corazzelli et al. 2012]. However, tolerability is poor in older age groups and there remains a paucity of data to support a survival benefit.
The traditional role of SCT in DLBCL has been to salvage patients with chemo-sensitive disease on relapse after CHOP [Gisselbrecht et al. 2010]. However in the R-CHOP era, patients with treatment failure within 6–12 months have a poor prognosis, even with HDT and SCT [Gisselbrecht et al. 2010]. This has led some groups to investigate the utility of autograft in first remission as well as allograft for patients at high risk of relapsed and refractory disease, in particular the DHL group. The MD Anderson group looked at SCT in first CR (CR1) among 23 DHL patients and compared them directly to 48 patients who did not receive a transplant [Oki et al. 2014]. Their findings suggest that transplant in CR1 has no effect on OS [Oki et al. 2014]. Furthermore, the utility of transplant is limited by poor tolerability in the elderly and those with multiple comorbidities [Sorror et al. 2005]. Thus SCT in CR1 cannot be recommended based on available evidence.
Finally, DHL status is a recognized risk factor for CNS relapse in DLBCL [Snuderl et al. 2010; Oki et al. 2014] and most authors recommend the use of CNS prophylaxis as part of routine therapy for these patients. Given the poor outcomes associated with traditional therapeutic approaches to DHL and the toxicities associated with many of the dose escalated regimens proposed to mitigate the risk of lymphoma related death, there is no current universally accepted standard of care approach for this condition. Thus, wherever possible patients with DHL should be managed as part of a clinical trial.
Novel targeted approaches to the treatment of DHL
Given the poor prognosis associated with DHL status, it is an area of active preclinical and early phase clinical research for exploring novel approaches to the treatment of difficult lymphomas. As DHL is characterized by overexpression of both BCL2 and MYC, approaches targeting these specific pathways are particularly exciting areas of exploration (Table 2). Due to the relative rarity of DHL, there are few studies looking at this population in isolation. However many lessons pertaining to the optimization of outcomes in DHL may be drawn from studies in the broader group of relapsed or refractory DLBCL.
Table 2.
Targeted therapy for DHL. The two approaches involve (a) inhibition of BCL2 or (b) inhibition of MYC. Ideally the combination of both approaches would be tested in clinical trials along with chemo-immunotherapy.
| Targeted therapy for DHL | Proposed mechanism | Preliminary clinical results |
|---|---|---|
| BCL2 inhibitors | ||
| Navitoclax | Inhibition of BCL2, BCLxL and BCLw | 4/4 evaluable patients with R/R DLBCL progressed [Wilson et al. 2010] |
| Venetoclax | Selective inhibition of BCL2 | 28% ORR in R/R DLBCL [Davids et al. 2014] |
| MYC inhibition | ||
| Alisertib | Inhibitor of aurora A kinase, a downstream target of myc, which when blocked results in apoptosis [den Hollander et al. 2010] | 3/21 patients with R/R DLBCL responded [Friedberg et al. 2014] |
| JQ1 | BET bromodomain (BRD4) inhibitor resulting in down regulation of MYC transcription [Delmore et al. 2011] | Not in clinical trials |
| GSK525762 | BET bromodomain inhibitor | Results awaited [ClinicalTrials.gov identifier: NCT01943851] |
| CPI0610 | BET bromodomain inhibitor | Results awaited [ClinicalTrials.gov identifier: NCT01949883] |
DLBCL, diffuse large B-cell lymphoma; DHL, double hit lymphoma; R/R, relapsed/refractory; ORR, overall response rate.
Targeting BCL2 and MYC in DHL: the Holy Grail
BCL2 inhibition
BCL2 overexpression impairs a malignant cell’s ability to undergo apoptotic death and underpins the development of malignancy as well as chemo-resistance in many hematological malignancies [Hanahan and Weinberg, 2000; Anderson et al. 2015]. The first extensively validated potent BCL2 inhibitor was the BH3 mimetic, ABT-737 [Oltersdorf et al. 2005]. As a single agent ABT-737 had no single-agent activity in a robust in vivo murine model of BCL2 and MYC overexpressing DHL. However, the combination of ABT-737 with cyclophosphamide demonstrated significant synergy, resulting in better outcomes than achieved with either agent alone, and apparent cures in this model [Mason et al. 2008]. ABT-737 did not have suitable pharmacokinetic properties for clinical development. Consequently, an orally available analogue, ABT-263 [Tse et al. 2008] (now known as navitoclax), was generated and this showed activity with in vitro DHL cell lines [Sasaki et al. 2011].
In addition to targeting BCL2, navitoclax and ABT-737 also targeted the BCL2-family members, BCLxL and BCLw [Anderson et al. 2014]. Very early in its clinical development navitoclax was associated with a dose-dependent thrombocytopenia [Roberts et al. 2012], due to circulating platelet dependence on BCLxL for survival in the periphery [Mason et al. 2007; Zhang et al. 2007]. Thus, in clinical trials of navitoclax dose escalation was limited by on-target thrombocytopenia and the full anti-BCL2 potential of the drug at higher doses may not have been realized. Among four evaluable patients with refractory or multiply relapsed DLBCL on the phase I trial of single agnet navitoclax in NHL, none achieved a partial response (PR) [Wilson et al. 2010], in line with what had been observed in the murine model.
The clinical imperative for dose escalation unencumbered by BCLxL-mediated thrombocytopenia led to the development of a BCL2 selective inhibitor: ABT-199 [Souers et al. 2013]. ABT-199, (now known as venetoclax), demonstrated preclinical efficacy in MYC driven murine lymphoma models [Vandenberg and Cory, 2013] and in DHL cell lines where it appears to produce a cytotoxic effect, especially when used in combination with either traditional chemotherapy or other novel agents [Johnson-Farley et al. 2015]. Venetoclax is currently in early phase clinical trials in patients with relapsed or refractory NHL including DLBCL [ClinicalTrials.gov identifier: NCT01328626]. Preliminary results from the phase I single agent study suggest greater activity than seen with navitoclax, with an 28% overall response rate (ORR) observed among 18 patients with DLBCL [Davids et al. 2014]. Given the success in preclinical studies of combination therapies of BH3 mimetics (ABT-737, venetoclax) and conventional DNA-damaging chemotherapy approaches, the results of ongoing clinical trials with venetoclax in combination with chemo-immunotherapy in broad populations of patients with NHL and related diseases are awaited with interest.
MYC inhibition
Inhibition of MYC is likely pivotal to the development of truly novel approaches to treatment of DHL. Although targeting transcription factors with small-molecule inhibitors has proved technically challenging, there are several agents currently being developed to modulate MYC expression or activity indirectly in vivo. To date, indirect targeting of signaling cascades downstream of MYC has yet to bear fruit. For example, Aurora kinase A and B are upregulated by MYC, and when blocked cells undergo apoptosis [den Hollander et al. 2010]. Yet, a clinical trial of a selective aurora kinase A inhibitor in patients with relapsed or refractory DLBCL saw responses in only three out of 21 patients [Friedberg et al. 2014]. Progress is being made in the targeting of the regulation of MYC activity. Inhibition of the BET bromodomain mitigates the effect of MYC overexpression by preventing signal transduction, important in regulating MYC transcriptional initiation and elongation [Delmore et al. 2011]. JQ1 inhibits the bromodomain BRD4, thereby reducing MYC transcription [Filippakopoulos et al. 2010]. While this agent has demonstrated preclinical activity, including specifically in DHL cell lines [Johnson-Farley et al. 2015], it has not been suitable for clinical development. However, other BET bromodomain inhibitors are currently in phase I clinical trials for patients with relapsed or refractory lymphomas including GSK525762 [ClinicalTrials.gov identifier: NCT01943851] and CPI-0610 [ClinicalTrials.gov identifier: NCT01949883]. Results are eagerly anticipated.
Other novel approaches to DHL treatment: lessons from relapsed and refractory DLBCL
Approaches to modifying the immune system as a way of treating relapsed and refractory DLBCL have been very popular after the stunning success of rituximab in improving outcomes [Coiffier et al. 2010]. While these agents have not been specifically tested in DHL a number are proving promising in relapsed or refractory DLBCL more generally. Approaches currently being explored include optimized anti-CD20 antibodies [Cillessen et al. 2007; Morschhauser et al. 2013], anti CD40 antibodies [Advani et al. 2006], anti-PD1 monoclonal antibodies [ClinicalTrials.gov identifier: NCT02362997] and vascular endothelial growth factor (VEGF) antibodies [Willett et al. 2004; Stopeck et al. 2012]. In the search for efficacy, some monoclonal antibodies have been conjugated to a variety of drugs and radioactive molecules [Bartlett et al. 2013; Advani et al. 2010; Ribrag et al. 2014; Morschhauser et al. 2004]. More recently, bispecific T-cell engager (BiTE) antibodies with specificity to both B and T cells have been developed in order to enhance T-cell-mediated tumor cytotoxicity [Viardot et al. 2011]. Modified autologous T cells engineered to recognize CD19, also known as chimeric antigen receptor T cells (CART cells) have shown promise in clinical trials of relapsed or refractory DLBCL [Kochenderfer et al. 2013; Kochenderfer et al. 2015]. Finally drugs such as lenalidomide, which have immunomodulatory effects on lymphoma, have also shown some efficacy [Witzig et al. 2011].
Kinase inhibitors may also play a role in future attempts to better treat DHL. Data to date in B-cell lymphomas indicate activity for inhibitors of signaling downstream of the B-cell receptor. What role drugs such as ibrutinib [Wilson et al. 2012] or idelalisib [Lannutti et al. 2011; Furman et al. 2014] may play in new combination approaches to DHL is unclear. Currently ibrutinib is one of the few agents in DHL specific trials [ClinicalTrials.gov identifier: NCT02272686].
Conclusions: towards a brighter future
DHL remains a challenging disease on a number of levels. To ensure appropriate diagnosis, there needs to be widespread availability of fast, reliable and affordable detection methods. Once diagnosed, specific treatment strategies are needed, but as yet there is no universally accepted therapeutic approach. While it is widely acknowledged that standard R-CHOP chemotherapy is insufficient to cure many of these cases, alternatives are not clear. Likely ways forward for this disease will come in two guises: (1) intensified regimens using standard agents such as DA-R-EPOCH and (2) inclusion of novel agents that either target the malignant B cell generally (e.g. immunotherapy) or the roots of the DHL problem, overexpression of MYC and BCL2 proteins. The greatest challenge going forward in this disease will be finding new agents that work to ameliorate the poor prognosis conferred by DH status. Wherever possible every effort should be made to accurately diagnose DHL using FISH and confirmed cases should be enrolled in clinical trials both in the upfront and relapsed/refractory setting.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MAA is supported by a Jill and Ross Webster Bequest Fellowship. DCHS and AWR are supported by Fellowships from the National Health and Medical Research Council of Australia (grant numbers 1043149 and 1079560, respectively). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Research funding from AbbVie and Genentech; Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone payments related to venetoclax.
Contributor Information
Mary Ann Anderson, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade Parkville, Victoria 3052, Australia.
Alpha Tsui, Department of Pathology, Royal Melbourne Hospital, Parkville, Australia.
Meaghan Wall, Victorian Cancer Cytogenetics Service, St Vincent’s Hospital, Fitzroy, Australia; Department of Medicine, St Vincent’s Hospital, University of Melbourne, Fitzroy, Australia.
David C. S. Huang, Departments of Medical Biology and Medicine, Faculty of Medicine, University of Melbourne, Parkville, Australia Division of Cancer and Haematology, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Australia.
Andrew W. Roberts, Department of Clinical Hematology and Bone Marrow Transplant, Royal Melbourne Hospital, Parkville, Australia Departments of Medical Biology and Medicine, Faculty of Medicine, University of Melbourne, Parkville, Australia; Division of Cancer and Haematology, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Australia.
References
- Advani A., Coiffier B., Czuczman M., Dreyling M., Foran J., Gine E., et al. (2010) Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab czogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol 28: 2085–2093. [DOI] [PubMed] [Google Scholar]
- Advani R., Forero-Torres A., Furman R., Rosenblatt J., Younes A., Shankles B., et al. (2006) SGN-40 (Anti-huCD40 mAb) monotherapy induces durable objective responses in patients with relapsed aggressive non-Hodgkin’s lymphoma: evidence of antitumor activity from a phase I study. Blood 108: abstract 695. [Google Scholar]
- Anderson M., Huang D., Roberts A. (2014) Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol 51: 219–227. [DOI] [PubMed] [Google Scholar]
- Anderson M., Huang D., Roberts A. (2015) BCL2 inhibition in double hit lymphoma. Leuk Lymphoma 56: 1928–1929. [DOI] [PubMed] [Google Scholar]
- Aukema S., Siebert R., Schuuring E., van Imhoff G., Kluin-Nelemans H., Boerma E., et al. (2011) Double-hit B-cell lymphomas. Blood 117: 2319–2331. [DOI] [PubMed] [Google Scholar]
- Barrans S., Crouch S., Smith A., Turner K., Owen R., Patmore R., et al. (2010) Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 28: 3360–3365. [DOI] [PubMed] [Google Scholar]
- Barrans S., Evans P., O’Connor S., Kendall S., Owen R., Haynes A., et al. (2003) The T(14;18) is associated with germinal center-derived diffuse large B-cell lymphoma and is a strong predictor of outcome. Clin Cancer Res 9: 2133–2139. [PubMed] [Google Scholar]
- Bartlett N., Sharman J., Oki Y., Advani R., Bello C., Winter J., et al. (2013) A phase 2 study of brentuximab vedotin in patients with relapsed or refractory CD30 - positive non-Hodgkin lymphomas: interim results in patients with DLBCL and other B-cell lymphomas. In ASH Annual Meeting and Exposition, New Orleans, LA, December 10. [Google Scholar]
- Biagi J., Seymour J. (2002) Insights into the molecular pathogenesis of follicular lymphoma arising from analysis of geographic variation. Blood 99: 4265–4275. [DOI] [PubMed] [Google Scholar]
- Boerma E., Siebert R., Kluin P., Baudis M. (2009) Translocations involving 8q24 in Burkitt lymphoma and other malignant lymphomas: a historical review of cytogenetics in the light of todays knowledge. Leukemia 23: 225–234. [DOI] [PubMed] [Google Scholar]
- Cheah C., Oki Y., Westin J., Turturro F. (2015) A clinician’s guide to double hit lymphomas. Br J Haematol 168: 784–795. [DOI] [PubMed] [Google Scholar]
- Cigudosa J., Parsa N., Louie D., Filippa D., Jhanwar S., Johansson B., et al. (1999) Cytogenetic analysis of 363 consecutively ascertained diffuse large B-cell lymphomas. Genes Chromosomes Cancer 25: 123–133. [PubMed] [Google Scholar]
- Cillessen S., Mackus W., Castricum K., Vos W., Kortman P., van de Winkel J., et al. (2007) Chemotherapy-refractory diffuse large B-cell lymphomas (DLBCL) are effectively killed by ofatumumab-induced complement-mediated cytoxicity. ASH Annual Meeting Abstracts 110: abstract 2346. [Google Scholar]
- Coiffier B., Lepage E., Briere J., Herbrecht R., Tilly H., Bouabdallah R., et al. (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346: 235–242. [DOI] [PubMed] [Google Scholar]
- Coiffier B., Thieblemont C., Van Den Neste E., Lepeu G., Plantier I., Castaigne S., et al. (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the groupe d’Etudes des Lymphomes de l’Adulte. Blood 116: 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazzelli G., Frigeri F., Russo F., Frairia C., Arcamone M., Esposito G., et al. (2012) RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol 156: 234–244. [DOI] [PubMed] [Google Scholar]
- Cuccuini W., Briere J., Mounier N., Voelker H., Rosenwald A., Sundstrom C., et al. (2012) MYC+ diffuse large B-cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood 119: 4619–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids M., Seymour J., Gerecitano J., Kahl B., Pagel J., Wierda W., et al. (2014) Phase I study of ABT-199 (GDC-0199) in patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL): responses observed in diffuse large B-cell (DLBCL) and follicular lymphoma (FL) at higher cohort doses. J Clin Oncol (Meeting Abstracts) 32 (Suppl. 5s): abstract 8522. [Google Scholar]
- De Jong D., Rosenwald A., Chhanabhai M., Gaulard P., Klapper W., Lee A., et al. (2007) Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications–a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol 25: 805–812. [DOI] [PubMed] [Google Scholar]
- Delmore J., Issa G., Lemieux M., Rahl P., Shi J., Jacobs H., et al. (2011) Bet bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146: 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander J., Rimpi S., Doherty J., Rudelius M., Buck A., Hoellein A., et al. (2010) Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood 116: 1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanidi A., Harrington E., Evan G. (1992) Cooperative Interaction between c-myc and bcl-2 proto-oncogenes. Nature 359: 554–556. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W., Fedorov O., et al. (2010) Selective inhibition of bet bromodomains. Nature 468: 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg J. (2012) Double-hit diffuse large B-cell lymphoma. J Clin Oncol 30: 3439–3443. [DOI] [PubMed] [Google Scholar]
- Friedberg J., Fisher R. (2008) Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am 22: 941–952, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg J., Mahadevan D., Cebula E., Persky D., Lossos I., Agarwal A., et al. (2014) Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol 32: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman R., Sharman J., Coutre S., Cheson B., Pagel J., Hillmen P., et al. (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht C., Glass B., Mounier N., Singh Gill D., Linch D., Trneny M., et al. (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28: 4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T., Nielsen O., De Stricker K., Xu-Monette Z.Y., Young K.H., Moller M.B. (2012a) High levels of nuclear MYC protein predict the presence of MYC rearrangement in diffuse large B-cell lymphoma. Am J Surg Pathol 36: 612–619. [DOI] [PubMed] [Google Scholar]
- Green T., Young K., Visco C., Xu-Monette Z., Orazi A., Go R., et al. (2012b) Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30: 3460–3467. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. (2000) The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- Harris N., Swerdlow S., Campo E., Jaffe E., Stein H., Pileri S., et al. (2008) The World Health Organization (WHO) classification of lymphoid neoplasms: what’s new? Annals of Oncology 19: 119. [Google Scholar]
- Horn H., Ziepert M., Becher C., Barth T., Bernd H., Feller A., et al. (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 121: 2253–2263. [DOI] [PubMed] [Google Scholar]
- Johnson N., Savage K., Ludkovski O., Ben-Neriah S., Woods R., Steidl C., et al. (2009) Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 114: 2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N., Slack G., Savage K., Connors J., Ben-Neriah S., Rogic S., et al. (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30: 3452–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Farley N., Veliz J., Bhagavathi S., Bertino J. (2015) ABT-199, a BH3 mimetic that specifically targets Bcl-2, enhances the antitumor activity of chemotherapy, bortezomib and JQ1 in “double hit” lymphoma cells. Leuk Lymphoma 56: 2146–2152. [DOI] [PubMed] [Google Scholar]
- Klapper W., Stoecklein H., Zeynalova S., Ott G., Kosari F., Rosenwald A., et al. (2008) Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Leukemia 22: 2226–2229. [DOI] [PubMed] [Google Scholar]
- Kluk M., Chapuy B., Sinha P., Roy A., Dal Cin P., Neuberg D., et al. (2012) Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One 7: e33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J., Dudley M., Carpenter R., Kassim S., Rose J., Telford W., et al. (2013) Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122: 4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J., Dudley M., Kassim S., Somerville R., Carpenter R., Stetler-Stevenson M., et al. (2015) Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsburg D., Nasta S., Svoboda J., Morrissette J., Schuster S. (2014) ‘Double-hit’ cytogenetic status may not be predicted by baseline clinicopathological characteristics and is highly associated with overall survival in B cell lymphoma patients. Br J Haematol 166: 369–374. [DOI] [PubMed] [Google Scholar]
- Lannutti B., Meadows S., Herman S., Kashishian A., Steiner B., Johnson A., et al. (2011) CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 117: 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gouill S., Talmant P., Touzeau C., Moreau A., Garand R., Juge-Morineau N., et al. (2007) The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 92: 1335–1342. [DOI] [PubMed] [Google Scholar]
- Li S., Lin P., Fayad L., Lennon P., Miranda R., Yin C., et al. (2012) B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol 25: 145–156. [DOI] [PubMed] [Google Scholar]
- Lin P., Dickason T., Fayad L., Lennon P., Hu P., Garcia M., et al. (2012) Prognostic value of MYC rearrangement in cases of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Cancer 118: 1566–1573. [DOI] [PubMed] [Google Scholar]
- Lohr J., Stojanov P., Lawrence M., Auclair D., Chapuy B., Sougnez C., et al. (2012) Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A 109: 3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason K., Carpinelli M., Fletcher J., Collinge J., Hilton A., Ellis S., et al. (2007) Programmed anuclear cell death delimits platelet life span. Cell 128: 1173–1186. [DOI] [PubMed] [Google Scholar]
- Mason K., Vandenberg C., Scott C., Wei A., Cory S., Huang D., et al. (2008) In vivo efficacy of the BCL-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci U S A 105: 17961–17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A., Palutke M., Eisenberg L., Al-Katib A. (2001) Chromosomal analyses of 52 cases of follicular lymphoma with t(14;18), including blastic/blastoid variant. Cancer Genet Cytogenet 126: 45–51. [DOI] [PubMed] [Google Scholar]
- Morschhauser F., Huglo D., Martinelli G., Paganelli G., Zinzani P., Hadjiyiannakis D., et al. (2004) Yttrium-90 ibritumomab tiuxetan (Zevalin) for patients with relapsed/refractory diffuse large B-cell lymphoma not appropriate for autologous stem cell transplantation: results of an open-label phase II trial. Blood 104: 107. [DOI] [PubMed] [Google Scholar]
- Morschhauser F., Cartron G., Thieblemont C., Solal-Celigny P., Haioun C., Bouabdallah R., et al. (2013) Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large B-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol 31: 2912–2919. [DOI] [PubMed] [Google Scholar]
- Mounier N., Briere J., Gisselbrecht C., Emile J., Lederlin P., Sebban C., et al. (2003) Rituximab plus CHOP (R-CHOP) overcomes bcl-2–associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood 101: 4279–4284. [DOI] [PubMed] [Google Scholar]
- Murphy D., Junttila M., Pouyet L., Karnezis A., Shchors K., Bui D., et al. (2008) Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell 14: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu N., Okamoto M., Miura I., Hirano M. (2009) Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 23: 777–783. [DOI] [PubMed] [Google Scholar]
- Oki Y., Noorani M., Lin P., Davis R., Neelapu S., Ma L., et al. (2014) Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 166: 891–901. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Elmore S., Shoemaker A., Armstrong R., Augeri D., Belli B., et al. (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435: 677–681. [DOI] [PubMed] [Google Scholar]
- Pedersen M., Gang A., Poulsen T., Knudsen H., Lauritzen A., Nielsen S., et al. (2012) Double-hit BCL2/MYC translocations in a consecutive cohort of patients with large B-cell lymphoma - a single centre’s experience. Eur J Haematol 89: 63–71. [DOI] [PubMed] [Google Scholar]
- Pedersen M., Gang A., Poulsen T., Knudsen H., Lauritzen A., Nielsen S., et al. (2014) MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. Eur J Haematol 92: 42–48. [DOI] [PubMed] [Google Scholar]
- Petrich A., Gandhi M., Jovanovic B., Castillo J., Rajguru S., Yang D., et al. (2014) Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 124: 2354–2361. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A., Cordell J., Pulford K., Gatter K., Mason D. (1990) Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol 137: 225–232. [PMC free article] [PubMed] [Google Scholar]
- Pfreundschuh M., Trumper L., Kloess M., Schmits R., Feller A., Rube C., et al. (2004) Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 104: 634–641. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M., Trumper L., Osterborg A., Pettengell R., Trneny M., Imrie K., et al. (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MinT) Group. Lancet Oncol 7: 379–391. [DOI] [PubMed] [Google Scholar]
- Ribrag V., Dupuis J., Tilly H., Morschhauser F., Laine F., Houot R., et al. (2014) A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 20: 213–220. [DOI] [PubMed] [Google Scholar]
- Roberts A., Seymour J., Brown J., Wierda W., Kipps T., Khaw S., et al. (2012) Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol 30: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N., Kuroda J., Nagoshi H., Yamamoto M., Kobayashi S., Tsutsumi Y., et al. (2011) Bcl-2 is a better therapeutic target than c-Myc, but attacking both could be a more effective treatment strategy for B-cell lymphoma with concurrent Bcl-2 and c-Myc overexpression. Exp Hematol 39: 817–828. [DOI] [PubMed] [Google Scholar]
- Savage K., Johnson N., Ben-Neriah S., Connors J., Sehn L., Farinha P., et al. (2009) MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 114: 3533–3537. [DOI] [PubMed] [Google Scholar]
- Schmitt C., Lowe S. (2001) Bcl-2 mediates chemoresistance in matched pairs of primary E(Mu)-myc lymphomas in vivo. Blood Cells Mol Dis 27: 206–216. [DOI] [PubMed] [Google Scholar]
- Sehn L., Donaldson J., Chhanabhai M., Fitzgerald C., Gill K., Klasa R., et al. (2005) Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23: 5027–5033. [DOI] [PubMed] [Google Scholar]
- Smith S., Anastasi J., Cohen K., Godley L. (2010) The impact of MYC expression in lymphoma biology: beyond Burkitt lymphoma. Blood Cells Mol Dis 45: 317–323. [DOI] [PubMed] [Google Scholar]
- Snuderl M., Kolman O., Chen Y., Hsu J., Ackerman A., Dal Cin P., et al. (2010) B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol 34: 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror M., Maris M., Storb R., Baron F., Sandmaier B., Maloney D., et al. (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106: 2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers A., Leverson J., Boghaert E., Ackler S., Catron N., Chen J., et al. (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19: 202–208. [DOI] [PubMed] [Google Scholar]
- Stopeck A., Unger J., Rimsza L., Leblanc M., Farnsworth B., Iannone M., et al. (2012) A phase 2 trial of standard-dose cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) and rituximab plus bevacizumab for patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: SWOG 0515. Blood 120: 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavelu M., Olopade O., Beckman E., Vardiman J., Larson R., Mckeithan T., et al. (1990) Clinical, morphologic, and cytogenetic characteristics of patients with lymphoid malignancies characterized by both t(14;18)(q32;q21) and t(8;14)(q24;q32) or t(8;22)(q24;q11). Genes Chromosomes Cancer 2: 147–158. [DOI] [PubMed] [Google Scholar]
- Tse C., Shoemaker A., Adickes J., Anderson M., Chen J., Jin S., et al. (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68: 3421–3428. [DOI] [PubMed] [Google Scholar]
- Valera A., Lopez-Guillermo A., Cardesa-Salzmann T., Climent F., Gonzalez-Barca E., Mercadal S., et al. (2013) MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica 98: 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg C., Cory S. (2013) ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood 121: 2285–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viardot A., Goebeler M., Noppeney R., Krause S., Kallert S., Ferstl B., et al. (2011) Blinatumomab monotherapy shows efficacy in patients with relapsed diffuse large B cell lymphoma. In ASH Annual Meeting Abstracts, San Diego, CA, December 10. [Google Scholar]
- Willett C., Boucher Y., di Tomaso E., Duda D., Munn L., Tong R., et al. (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W., Dunleavy K., Pittaluga S., Hegde U., Grant N., Steinberg S., et al. (2008) Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol 26: 2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W., Gerecitano J., Goy A., De Vos S., Kenkre V.P., Barr P.M., et al. (2012) The Bruton’s Tyrosine Kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/refractory de novo diffuse large B-cell lymphoma (DLBCL): interim results of a multicenter, open-label, phase 2 study. ASH Annual Meeting Abstracts, Atlanta, GA, 10 December. [Google Scholar]
- Wilson W., O’Connor O., Czuczman M., Lacasce A., Gerecitano J., Leonard J., et al. (2010) Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 11: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig T., Vose J., Zinzani P., Reeder C., Buckstein R., Polikoff J., et al. (2011) An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol 22: 1622–1627. [DOI] [PubMed] [Google Scholar]
- Zhang H., Nimmer P., Tahir S., Chen J., Fryer R., Hahn K., et al. (2007) Bcl-2 family proteins are essential for platelet survival. Cell Death Differ 14: 943–951. [DOI] [PubMed] [Google Scholar]