Abstract
Background
Interstitial tonicity increases vascular endothelial growth factor-C (VEGF-C), a lymphangiogenic factor in salt-induced hypertension. Therefore, it can be assumed that changes of serum VEGF-C level may be associated with increasing blood pressure. However, there is no report about the changes of serum VEGF-C levels in patients with chronic kidney disease (CKD). The aims of this study were to investigate the changes of serum and urine VEGF-C levels in patients with CKD stage 3–4 and to evaluate the relationship between blood pressure and serum VEGF-C levels in the patients with CKD stage 5 and hemodialysis.
Methods
Glomerular filtration rate (GFR) was assessed by the Modification of Diet in Renal Disease equation. Blood pressure and VEGF-C levels (serum and urine) were measured by enzyme-linked immunosorbent assay (ELISA) in nine patients with stage 3–4 CKD, 41 hemodialysis patients, and eight healthy individuals.
Results
The median serum level of VEGF-C in patients with stage 3–4 CKD and stage 5 hemodialysis significantly decreased in comparison with healthy individuals. Urinary VEGF-C excretion increased in patients with stage 3–4 CKD compared with healthy control patients. For 41 hemodialysis patients, the serum level of VEGF-C in patients with stage 1 or stage 2 hypertension with hemodialysis did not significantly increase when compared with prehypertension hemodialysis patients.
Conclusion
We demonstrated that circulating levels of VEGF-C were decreased in patients with CKD, and the decrease of VEGF-C in patients with stage 3–4 CKD coincided with an increase in the urinary excretion of VEGF-C.
Keywords: Chronic kidney disease, Hemodialysis, Hypertension, VEGF-C
Introduction
Vascular endothelial growth factor (VEGF) is a key molecule for angiogenesis and lymphangiogenesis [1]. The VEGF families are composed of six different proteins: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor. The VEGF binds three highly related receptor tyrosine kinases (RTKs), VEGFR-1, VEGFR-2, and VEGFR-3. One of the members of the VEGF family, VEGF-C is known as a strong lymphangiogenic growth factor [2]. VEGF-C has high affinity for both VEGFR-2 and VEGFR-3 [2]. The VEGF-C through VEGFR-2 or VEGFR-3 has a critical role in mitogenesis, migration, and survival of lymphatic endothelial cells [3].
Recently, it was suggested that the mononuclear phagocyte system is involved in the control of interstitial volume and blood pressure(BP) homeostasis. High salt diet feeding activates the tonicity-responsive enhancer binding protein (TonEBP) in mononuclear phagocyte cells that are present in the skin [4], [5]. Activation of TonEBP increases VEGF-C production and increases lymphangiogenesis [6], [7]. Thus, VEGF-C can be linked to maintain a normal blood pressure by regulating interstitial sodium accumulation state. Dysregulation of this physiological extra renal mechanism may lead to salt-sensitive hypertension [4], [5].
VEGF inhibitors such as bevacizumab [8], sunitinib [9], sorafenib [10], vatalanib [11], and cediranib [12] have been used in the treatment of patients with tumor metastasis and mass growth in lung, colorectal, and skin cancer. The adverse effect of these antiangiogenesis therapies has been demonstrated. Patients with metastatic renal cell carcinoma treated with inhibitor of VEGF have high incidence of intracerebral hemorrhage due to uncontrolled hypertension [13]. The mechanisms of hypertension induced by angiogenic inhibitor are related to an increase of systemic vascular resistance as a result of vascular rarefaction, dysregulation of renal endothelial cells, and podocyte VEGF expression [13]. Therefore, inhibition of VEGFR axis may increase BP in cancer patients. It was demonstrated that VEGF-C levels were higher in patients with preeclampsia than those with gestational hypertension [14], and VEGF-C has a role in salt homeostasis in humans [15]. These results suggest that changes of VEGF-C expression may be associated with hypertension.
However, there is currently no report about the changes of serum VEGF-C levels in patients with chronic kidney disease (CKD) and the relationship between the degree of hypertension and serum VEGF-C level in these patients. We assumed that the changes of serum VEGF-C level in patients with CKD may be associated with increases in BP, and there is a relationship between degree of hypertension and serum VEGF-C level. The aims of this study were to investigate the changes of serum and urine level of VEGF-C levels in patients with CKD stage 3–4 and to evaluate the relationship between BP and serum VEGF-C levels in the patients with CKD stage 5 and hemodialysis.
Methods
Study populations
The Chonbuk National University Hospital Ethical Committee approved the protocol. For analyses of serum and urine levels of VEGF-C between healthy patients and predialysis CKD stage 3–4 and CKD stage 5 hemodialysis (HD) patients, we enrolled eight healthy control patients, nine patients with CKD stage 3–4, and 41 patients with CKD stage 5 and HD. For analysis, correlation between circulating VEGF-C levels and blood pressure stage in HD patients, we grouped the HD patients as seven with prehypertension, 17 with stage 1 hypertension, and 17 with stage 2 hypertension.
The serum and urine for this study were provided by the Biobank of Chonbuk National University Hospital, and all samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols. The patients with CKD stage 3–4 had a stable serum creatinine level within 10% for at least 6 months prior to inclusion. Exclusion criteria for the CKD study population were previous transplantation, medication with Oriental medicine, extremities amputation, or acute inflammation. Patients with CKD stage 3 or 4 were defined by the Modification of Diet in Renal Disease (MDRD) estimated glomerular filtration rate (eGFR) between 30–59 mL/min/1.73 m2 and 15–29 mL/min/1.73 m2, respectively. The dialysis patients had maintained hemodialysis three times a week and no medical events in the 6 months prior to inclusion in this study. The number of anuric HD patients was 38. In all of these patients, a detailed history and laboratory examination data were extracted by electronic medical records. Eight healthy patients were included as control patients. Edema was defined by depressing the skin with a finger by applying pressure to the pretibial area. Cerebrovascular disease included cardiovascular events such as myocardial infarction and/or revascularization procedures, stroke, new-onset or worsening congestive heart failure, and peripheral arterial disease.
BP measurement
BP was measured oscillometrically in the supine position in a quiet environment with an automated device (Dinamap, Critikon Co., Tampa, FL, USA) in healthy control patients and those with CKD stages 3 and 4. The mean of three consecutive measurements, taken 5 minutes apart after a rest of at least 20 minutes, was taken for analysis. In the HD patients, BP was assessed noninvasively (Phoenix Hemodialysis System, Gambro, Lund, Sweden) prior to and after hemodialysis in the supine position. We also measured BP every hour during hemodialysis. Mean value of at least four measurements was used for analysis. The patients were classified as having prehypertension (BP 120–139/80–89 mmHg), stage 1 hypertension (BP 140-159/90–99 mmHg), and stage 2 hypertension (BP ≥ 160/100 mmHg) [16].
Antihypertensive medication
Antihypertensive medication was evaluated by electrical medical records. Medication was quantified using the Anatomical Therapeutic Chemical (ATC) classification system. According to the World Health Organization recommendation, the defined daily dose was used as a measuring unit [17].
Blood VEGF-C measurement
After 2 hours of hemodialysis, blood samples for measurement of VEGF–C were drawn with the patient in a supine position. The serum level of VEGF-C was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's guidelines (eBioscience, San Diego, CA, USA).
Measurement of urinary VEGF–C
VEGF–C urine levels were measured simultaneously with blood levels. The urine level of VEGF-C was measured by ELISA according to the manufacturer's guidelines (eBioscience). Urine VEGF-C (pg) per urine creatinine (mg) was calculated by the urine level of VEGF-C (pg/mL) divided by urine creatinine (mg/dL).
Fractional excretion of VEGF–C
Fractional excretion (FE) of VEGF-C is the percentage of the VEGF-C filtered by the kidney that is excreted in the urine. This formula is represented mathematically as:
Biochemical parameters
All measures were taken at the same clinical visit; prior to a midweek session of HD or at clinic review for patients with CKD stage 3–4. Serum and urine creatinine and serum cholesterol were measured using a Hitachi 7180 autoanalyzer (Hitachi High-Technologies Corporation, Tokyo, Japan). Serum samples from patients with hemodialysis were taken prehemodialysis.
Statistical analyses
Data are presented as median (25–75 percentiles) or as mean ± standard deviation (SD). Normality tests and other analyses were performed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The stage 3–4 CKD subgroups, HD patients, and the healthy control patients were analyzed using the Student t test. The Chi-square test (or Fisher's exact test) was used for comparing categorical variables. Nonparametric Mann-Whitney U tests were used for non-normally distributed variables (VEGF-C levels, urine VEGF-C/urine creatinine ratio, and FE of VEGF-C). The degree of clinical correlation was estimated by variable Pearson method. Statistical significance was accepted at a two-sided P value of < 0.05.
Results
Patient demographics
The clinical characteristics of the study participants are described in Table 1. The mean age of control patients was significantly lower than those of patients with CKD stage 3–4 and HD patients (31.3 ± 6.7 years in healthy control patients, 62.2 ± 14.4 years in patients with CKD stage 3–4 CKD, and 61.3 ± 13.3 years in HD patients; P < 0.01). The BPs and serum creatinine levels were significantly higher in patients with CKD stage 3–4 and HD patients compared with normal control patients (BP 141.8 ± 14.4/78.8 ± 14.5 mmHg in patients with CKD stage 3–4, 152.8 ± 12.3/83.1 ± 10.1 mmHg in HD patients, and 122.5 ± 8.1/76.2 ± 6.4 mmHg in healthy control patients, P < 0.01; mean serum creatinine level, 3.98 ± 2.06 mg/dL in patients with CKD stage 3–4, 7.37 ± 2.80 mg/dL in HD patients, and 0.82 ± 0.20 mg/dL in healthy control patients, P < 0.001). The mean eGFR was 16.1 ± 8.2 mL/min/1.73m2 in patients with CKD stage 3–4, 5.9 ± 1.9 mL/min/1.73m2 in HD patients, and 107.1 ± 22.4 mL/min/1.73m2 in healthy control patients (P < 0.01). There were no statistically significant differences in male-to-female ratio (2/7 in patients with CKD stage 3–4, 19/22 in HD patients, and 6/2 in healthy control patients) and percentage of smokers (3/9 in patients with CKD stage 3–4, 21/41 in HD patients, and 6/2 in healthy control patients) between the study groups. There was no significant difference in mean total cholesterol level between the CKD groups and control patients (181.4 ± 64.1 mg/dL for patients with CKD stage 3–4, 198.3 ± 56.9 mg/dL for HD patients, and 187.5 ± 37.8 mg/dL for healthy control patients). Antihypertensive drugs were more frequently used in patients with CKD stage 3–4 and HD patients than healthy control patients (Table 2).
Table 1.
Baseline characteristics of healthy control subjects, chronic kidney disease stage 3–4 and hemodialysis patients
| Parameters | Controls (n = 8) | CKD stage 3–4 (n = 9) | CKD stage 5 with HD (n =41) |
|---|---|---|---|
| Age (y) | 31.3 ± 6.7 | 62.2 ± 14.4a | 61.3 ± 13.3a |
| Sex (male/female) | 6/2 | 2/7 | 19/22 |
| Smokers (n, %) | 3 (37.5) | 3 (33.3) | 21 (51.2) |
| SBP (mmHg) | 122.5 ± 8.1 | 141.8 ± 14.4a | 152.8 ± 12.3a |
| DBP (mmHg) | 76.2 ± 6.4 | 78.8 ± 14.5 | 83.1 ± 10.1b |
| Serum Cr (mg/dL) | 0.8 ± 0.2 | 3.9 ± 2.1a | 7.4 ± 2.8a |
| eGFR (mL/min/1.73m2) | 107.1 ± 22.4 | 16.1 ± 8.2a | 5.9 ± 1.9a |
| Total cholesterol (mg/dL) | 187.5 ± 37.8 | 181.4 ± 64.1 | 198.3 ± 56.9 |
| Diabetes mellitus (n, %) | 0 (0) | 2 (22.2) | 11(26.8) |
| Cerebrovascular disease (n, %) | 0 (0) | 1 (11.1) | 8 (19.5) |
| Cancer (n, %) | 0 (0) | 0 (0) | 8 (19.5) |
| Diuretic (n, %) | 0 (0) | 4 (44.4) | 7 (17.0) |
| Edema (n, %) | 0 (0) | 2 (22.2) | 4 (9.7) |
| No. of patients with antihypertensive drug (n, %) | 0 (0) | 9 (100)* | 38 (92.6)a |
CKD, chronic kidney disease; Cr, creatinine; CVA, cerebrovascular accident; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate by the Modification of Diet in Renal Disease equation; HD, hemodialysis; SBP, systolic blood pressure.
P < 0.01 versus control subjects.
P < 0.05 versus control subjects.
Table 2.
The number of concomitant total antihypertensive drugs (The number of antihypertensive drugs/person)
| Antihypertensive drugs | CKD stage 3–4 (n = 9) | CKD stage 5 with HD (n = 41) |
|---|---|---|
| Calcium antagonist | 9 (1.00) | 37 (0.97) |
| β-blocker | 1 (0.11) | 4 (0.11) |
| α-blocker | 0 (0) | |
| α- and β-blocker | 4 (0.44) | 14 (0.37) |
| ACE inhibitor | 0 (0) | |
| ARB | 8 (0.89) | 22 (0.58) |
| Vasodilator | 0 (0) | 12 (0.32) |
| Diuretic | 4 (0.44) | 7 (0.18) |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker.
Decreased serum VEGF-C levels in patients with CKD stage 3–4
As shown in Table 3, median serum VEGF-C levels decreased in patients with CKD stage 3–4 compared with healthy control patients [114.6 (49.0–353.1) vs. 2341.1 (1679.1–7952.1) pg/mL; P < 0.05]. Median serum VEGF-C levels of the HD patients were also decreased in comparison with healthy control patients [146.4 (98.6–245.8) vs. 2341.1 (1679.1–7952.1) pg/mL; P < 0.05].
Table 3.
The median serum levels of vascular endothelial growth factor-C in chronic kidney disease stage 3–4, hemodialysis patients, and healthy controls
| Parameters | Controls (n = 8) | CKD stage 3–4 (n = 9) | CKD stage 5 with HD (n = 41) |
|---|---|---|---|
| Serum VEGF-C (pg/ml) | 2341.1 (1679.1–7952.1) | 114.6a (49.0–353.1) | 146.4a (98.6–245.8) |
CKD, chronic kidney disease; VEGF-C, vascular endothelial growth factor-C.
P < 0.05 versus control subjects.
Increased urinary excretion of VEGF-C in patients with CKD stage 3–4
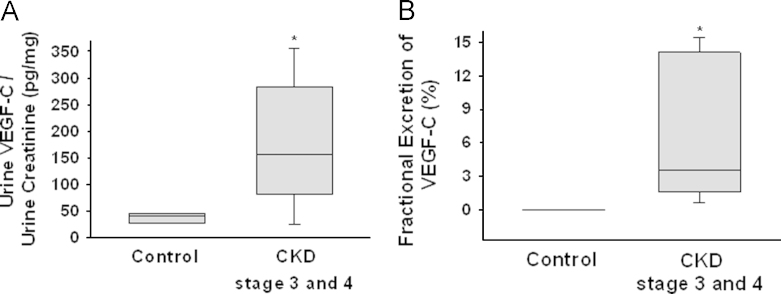
To evaluate whether urinary VEGF-C excretion was increased, we measured urine VEGF-C per urine creatinine and FE of VEGF-C in patients with CKD stage 3–4. The urine VEGF-C (pg) per urine creatinine (mg) were significantly increased in patients with CKD stage 3–4 compared with healthy control patients [40.1 (27.7–44.1) ng of urine VEGF-C per mg of urine creatinine to healthy control patients, 156.4 (82.0–283.0) ng of urine VEGF-C per mg of urine creatinine in patients with CKD stage 3–4; P < 0.01] (Fig. 1A). The FE of VEGF-C was also increased in patients with CKD stage 3–4 compared with healthy control patients [3.53 (1.60–14.11)% in patients with CKD stage 3–4 and 0.01 (0.00–0.03)% in healthy control patients; P < 0.01] (Fig. 1B). These data suggested that urinary excretion of VEGF-C was increased in patients with CKD stage 3–4.
Figure 1.
Urinary excretions of VEGF-C in patients with CKD stage 3–4 (CKD stages 3 and 4) and healthy control subjects (Control). (A) Urine levels of VEGF-C per urine creatinine in patients with CKD stage 3–4 and healthy control subjects. (B) Fractional excretion of VEGF-C in patients with CKD stage 3–4 and healthy control subjects. *P < 0.05 versus control. CKD, chronic kidney disease; VEGF-C, vascular endothelial growth factor.
No relationship between serum VEGF-C and degree of hypertension
The concentrations of serum VEGF-C in patients with stage 1 hypertension or stage 2 hypertension tended to be higher than those in healthy control patients, but the differences did not reach statistical significance (536.5 ± 982.2 pg/mL to stage 1 hypertension; 386.8 ± 991.4 pg/mL to stage 2 hypertension, and 116.2 ± 30.6 pg/mL prehypertension; P > 0.05). Thus, our data showed that no significant differences in the serum concentrations of VEGF-C were detected between prehypertension, stage 1 hypertension, and stage 2 hypertension group in HD patients. We also evaluated the correlation between serum levels of VEGF-C and the number of antihypertensive drugs in HD patients. Our data showed that calcium antagonists were more frequently used with concomitant drugs in patients with stage 1 and stage 2 hypertension than in healthy control patients. However, other antihypertensive concomitant drugs were similar in all groups except calcium antagonists. The serum levels of VEGF-C were not correlated with the number of antihypertensive drugs used in patients with CKD stage 5. CKD stage 5. We demonstrated that serum VEGF-C levels decreased in the patients with CKD stage 3–4 and stage 5 and HD compared to healthy controls, and urinary excretion of VEGF-C was increased in patients with CKD stage 3–4.
Discussion
In this study, our results also demonstrated that the serum levels of VEGF-C in patients with CKD stage 5 were not correlated with the number of hypertensive drugs used.
A diet high in salt has been implicated in hypertension. However, individual variations exist in the sensitivity of the BP to salt and various mechanisms for salt-sensitive hypertension [18]. The kidney is an organ that regulates total body sodium for homeostasis of extracellular fluid volume [19]. The extracellular volume consists of two distinct body fluid compartments, including the interstitial and the intravascular spaces. Because extracellular body fluids are in equilibrium, excess interstitial sodium is readily moved into the bloodstream for external renal sodium excretion. It has been reported that lymphatic vessels drain the interstitial fluid [20]. Therefore, one of the critical functions of lymphatic system is regulation of the interstitial fluid in sodium excessive state.
Machnik et al [5] demonstrated that leukocyte in the skin interstitial space is involved in the development of hypertension. Mononuclear phagocyte cells such as macrophages participated in the pathogenesis of hypertension. A diet high in salt leads to interstitial hypertonic Na+ accumulation and activation of TonEBP in mononuclear phagocyte cells in the skin [4], [5]. These authors suggested that TonEBP increases VEGF-C production and increases lymphangiogenesis [6], [7]. Therefore, VEGF-C can be associated with sodium excessive state and activation of high TonEBP in high salt diet-induced hypertension.
Because VEGF-C is involved in salt-induced hypertension and hypertension in patients with CKD is linked to volume expansion, we assumed that serum levels of VEGF-C increase in the patients with hypertension and CKD. However, our data demonstrated that serum levels of VEGF-C in patients with CKD stage 3–4 and HD patients had significantly lower levels of VEGF-C than control patients. Therefore, the extra renal fluid homeostasis mechanism via the VEGF-C system in patients with CKD and hypertension can be evaluated in the future.
There is a tendency that the mean level of serum VEGF-C increased in stage 1 and stage 2 hypertension groups compared with prehypertension in HD patients. However, the differences did not reach statistical significance. Because there are various factors that can be involved in blood pressure regulation in patients with end-stage kidney disease, we also evaluated the relationship between the number of anti-hypertensive drugs used and stage of hypertension. Our results showed that there was no relationship between the number of antihypertensive drugs used and stage of hypertension. There may be many factors that can affect serum levels of VEGF-C in normal patients or those with CKD. However, to our knowledge, there are few reports about the regulating factors and metabolism of serum VEGF-C. Therefore, further studies are needed in this field in the future.
Lely et al [14] demonstrated that serum levels of VEGF-C increased in patients with preeclampsia. However, our results demonstrated that blood levels of VEGF-C decreased in patients with CKD stage 3–4 with hypertension. Also, our data regarding serum VEGF-C level in patients with CKD were different from those of patients with preeclampsia. We also found that urinary excretion of VEGF-C was increased in patients with CKD stage 3–4 compared with healthy control patients. Thus, all of these data suggested that decreased serum levels of VEGF-C may be associated with increased excretion of VEGF-C in patients with CKD stage 3–4. To our knowledge, these are the first data in the English literature about increased urinary excretion of VEGF-C in patients with CKD. However, further investigation is needed regarding the exact mechanism of VEGF-C kidney excretion.
There was evidence that increased serum levels of VEGF-C are associated with cancer and cancer metastasis [21], [22], [23], [24], [25]. Thus, we evaluated the prevalence of cancer in each group. Our demographic data showed that cancer prevalence in patients with CKD stage 3–4 and HD patients was not different compared with that in healthy control patients (Table 1). Although healthy control patients in this study were younger than other patients, the prevalence of diabetes mellitus and cerebrovascular disease was not different between groups. The patients with CKD stage 3–4 tended to take more diuretics compared with those in the healthy control group. However, statistical significance was not reached (P = 0.08).
Recently, Slagman et al [15] demonstrated that VEGF-C levels in patients with CKD and high salt intake are higher than in patients with CKD and low salt intake. By comparison, the serum levels of VEGF-C were low in our study of patients with CKD stage 3 and 4. The difference in serum VEGF-C levels between the study by Slagman et al and this study may be due to the difference in kidney function: creatinine clearance in the study by Slagman et al was 82 ± 6 mL/min and eGFR in this study was 16.1 mL/min/1.7m2.
The clinical significance of this study was to develop the role of VEGF-C in patients with hypertension and CKD. Our data demonstrate that serum VEGF-C levels were decreased and urinary VEGF-C excretion was increased in patients with CKD stage 3 and 4. Our results also showed that serum VEGF-C levels were decreased in HD patients. We suggest that decreased serum VEGF-C levels may be associated with increased urinary excretion of VEGF-C. Furthermore, we suggest that the decreased serum levels of VEGF-C may be associated with hypertension in patients with CKD. However, we could not explain about the exact mechanism of decreased serum VEGF-C level in HD patients.
The current study has several limitations. Patients with CKD stages 3 and 4 and the normal control population are a relatively small group. There were fundamental differences of the three studied populations in age and the number of patients using antihypertensive drugs. Thus, these findings should be considered carefully in interpreting the results.
Our results demonstrate that circulating levels of VEGF-C decreased in patients with CKD and the decreased circulating VEGF-C levels may be associated with increased excretion of VEGF-C in patients with CKD stage 3–4. Additional studies are needed to evaluate the clinical relevance of the decreased lymphangiogenic factor, VEGF-C, in patients with CKD.
Conflict of interest
No conflict of interest.
Acknowledgments
This study was supported by a grant (CUHBRI-2012-02-003) of the CNUH-BRI. The biospecimens for this study were provided by the Biobank of Chonbuk National University Hospital, a member of the National Biobank of Korea which is supported by the Ministry of Health, Welfare and Family Affairs. This study was partially supported by a grant from the Korea Healthcare Technology Research and Development Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A102065).
References
- 1.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 3.Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 4.Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, Eckardt KU, Muller DN, Park JK, Luft FC, Kerjaschki D, Titze J. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2006;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 5.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 6.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 2009;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 7.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115:2316–2319. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heitz F, Harter P, Barinoff J, Beutel B, Kannisto P, Grabowski JP, Heitz J, Kurzeder C, du Bois A. Bevacizumab in the treatment of ovarian cancer. Adv Ther. 2012;29:723–735. doi: 10.1007/s12325-012-0041-9. [DOI] [PubMed] [Google Scholar]
- 9.Wood L. Sunitinib malate for the treatment of renal cell carcinoma. Expert Opin Pharmacother. 2012;13:1323–1336. doi: 10.1517/14656566.2012.689130. [DOI] [PubMed] [Google Scholar]
- 10.Strumberg D. Sorafenib for the treatment of renal cancer. Expert Opin Pharmacother. 2012;13:407–419. doi: 10.1517/14656566.2012.654776. [DOI] [PubMed] [Google Scholar]
- 11.Los M, Roodhart JM, Voest EE. Target practice: Lessons from phase iii trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist. 2012;12:443–450. doi: 10.1634/theoncologist.12-4-443. [DOI] [PubMed] [Google Scholar]
- 12.Sahade M, Caparelli F, Hoff PM. Cediranib: A VEGF receptor tyrosine kinase inhibitor. Future Oncol. 2012;8:775–781. doi: 10.2217/fon.12.73. [DOI] [PubMed] [Google Scholar]
- 13.Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, Khayat D, Spano JP. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2012;20:807–815. doi: 10.1093/annonc/mdn713. [DOI] [PubMed] [Google Scholar]
- 14.Lely AT, Salahuddin S, Holwerda KM, Karumanchi SA, Rana S. Circulating lymphangiogenic factors in preeclampsia. Hypertens Pregnancy. 2013;32:42–49. doi: 10.3109/10641955.2012.697953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slagman MC, Kwakernaak AJ, Yazdani S, Laverman GD, van den Born J, Titze J, Navis G. Vascular endothelial growth factor C levels are modulated by dietary salt intake in proteinuric chronic kidney disease patients and in healthy subjects. Nephrol Dial Transplant. 2012;27:978–982. doi: 10.1093/ndt/gfr402. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC-7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.Official version of the German Anatomical Therapeutic Chemical (ATC) Classification with defined daily doses (DDD) 2009: Deutsches Institut für Medizinische Dokumentation und Information, Cologne, 2009.
- 18.Guyton AC, Coleman TG, Cowley AW, Jr., Liard JF, Norman RA, Jr., Manning RD., Jr Systems analysis of arterial pressure regulation and hypertension. Ann Biomed Eng. 1972;1:254–281. doi: 10.1007/BF02584211. [DOI] [PubMed] [Google Scholar]
- 19.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 20.Go WY, Liu X, Roti MA, Liu F, Ho SN. Nfat5/tonebp mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura H, Kato H, Tanaka N, Inose T, Faried A, Sohda M, Nakajima M, Fukai Y, Miyazaki T, Masuda N, Manda R, Fukuchi M, Ojima H, Tsukada K, Kuwano H. Preoperative serum vascular endothelial growth factor-C (VEGF-C) levels predict recurrence in patients with esophageal cancer. Anticancer Res. 2008;28:165–169. [PubMed] [Google Scholar]
- 22.Tamura M, Ohta Y. Serum vascular endothelial growth factor-C level in patients with primary nonsmall cell lung carcinoma: A possible diagnostic tool for lymph node metastasis. Cancer. 2003;98:1217–1222. doi: 10.1002/cncr.11529. [DOI] [PubMed] [Google Scholar]
- 23.Vihinen PP, Hilli J, Vuoristo MS, Syrjanen KJ, Kahari VM, Pyrhonen SO. Serum VEGF-C is associated with metastatic site in patients with malignant melanoma. Acta Oncol. 2007;46:678–684. doi: 10.1080/02841860600965020. [DOI] [PubMed] [Google Scholar]
- 24.Wang TB, Deng MH, Qiu WS, Dong WG. Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol. 2007;13:1794–1797. doi: 10.3748/wjg.v13.i12.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu XM, Lo CY, Lam AK, Leung P, Luk JM. Serum vascular endothelial growth factor C correlates with lymph node metastases and high-risk tumor profiles in papillary thyroid carcinoma. Ann Surg. 2008;247:483–489. doi: 10.1097/SLA.0b013e31815fa447. [DOI] [PubMed] [Google Scholar]