Abstract
Aims
The aims of this study were to compare the diagnostic test characteristics of ultrasound alone, metal artefact reduction sequence MRI (MARS-MRI) alone, and ultrasound combined with MARS-MRI for identifying intra-operative pseudotumours in metal-on-metal hip resurfacing (MoMHR) patients undergoing revision surgery.
Methods
This retrospective diagnostic accuracy study involved 39 patients (40 MoMHRs). The time between imaging modalities was a mean of 14.6 days (0 to 90), with imaging performed at a mean of 5.3 months (0.06 to 12) before revision. The prevalence of intra-operative pseudotumours was 82.5% (n = 33).
Results
Agreement with the intra-operative findings was 82.5% (n = 33) for ultrasound alone, 87.5% (n = 35) for MARS-MRI alone, and 92.5% (n = 37) for ultrasound and MARS-MRI combined. The diagnostic characteristics for ultrasound alone and MARS-MRI alone reached similar sensitivities (90.9% vs 93.9%) and positive predictive values (PPVs; 88.2% vs 91.2%), but higher specificities (57.1% vs 42.9%) and negative predictive values (NPVs; 66.7% vs 50.0%) were achieved with MARS-MRI. Ultrasound and MARS-MRI combined produced 100% sensitivity and 100% NPV, whilst maintaining both specificity (57.1%) and PPV (91.7%).
For the identification of a pseudotumour, which was confirmed at revision surgery, agreement was substantial for ultrasound and MARS-MRI combined (κ = 0.69), moderate for MARS-MRI alone (κ = 0.54), and fair for ultrasound alone (κ = 0.36).
Discussion
These findings suggest that ultrasound and/or MARS-MRI have a role when assessing patients with a MoMHR, with the choice dependent on local financial constraints and the availability of ultrasound expertise. However in patients with a MoMHR who require revision, combined imaging was most effective.
Take home message: Combined imaging with ultrasound and MARS-MRI always identified intra-operative pseudotumours if present. Furthermore, if neither imaging modality showed a pseudotumour, one was not found intra-operatively.
Cite this article: Bone Joint J 2016;98-B:40–8.
Keywords: Metal-on-metal; hip resurfacing; pseudotumour; MARS MRI; ultrasound; revision
More than one million patients worldwide have received large-diameter metal-on-metal (MoM) arthroplasties of the hip.1 High short-term rates of failure owing to pseudotumour formation have been reported with many MoM hip designs.2,3 Furthermore, poor outcomes from revision surgery following the development of a pseudotumour have been reported,4,5 which is a concern given most patients with these articulations are young and active.6,7
Currently most patients with MoM hip arthroplasties are reviewed regularly as recommended by worldwide regulatory authorities, as it is perceived that early detection and revision surgery for pseudotumour may improve the outcome.8-10 All authorities recommend either ultrasound or metal artefact reduction sequence magnetic resonance imaging (MARS- MRI) for assessing the periprosthetic soft-tissues around MoM hips.8-10 However, no preference between these modalities has been identified, with the choice being left to the surgeon. This is because many studies have shown that both these forms of investigation are acceptable for identifying pseudotumour formation in MoM hips.11-13 The relative merits of both modalities have been described.14 The drawbacks of ultrasound are that it is operator dependent and can be difficult to undertake in large patients, while MARS-MRI is more costly (MARS-MRI estimated cost £216 vs £49 for a hip ultrasound),15 time-consuming, and subject to prosthetic artifact.
Four studies, including between 19 and 83 MoM hips, have recently compared ultrasound and MARS-MRI for assessing the status of MoM hips.14,16-18 Three showed that ultrasound was effective,16-18 while the authors of a smaller cohort study reported that ultrasound was inferior to MARS-MRI for detecting pseudotumours.14 However, these studies have important limitations, including delays of up to one-year between performing ultrasound and MRI in the same patient. Furthermore, studies have compared these two imaging modalities either by using MRI as the “gold standard” or by assuming agreement between modalities was an acceptable result.14,16-18 The benchmark for diagnostic accuracy in such a study should be correlation with findings at the time of revision surgery. One study did validate imaging findings but only in five patients undergoing revision, with good correlation reported between both modalities and the operative findings.14
We first reported pseudotumours as a serious complication associated with MoM hip resurfacings (MoMHRs).19 We have performed revision surgery for this indication since 2007, with many patients undergoing both ultrasound and MARS-MRI before revision. The aims of this study were to compare the diagnostic test characteristics of ultrasound alone, MARS-MRI alone, and ultrasound combined with MARS-MRI for identifying intra-operative pseudotumours in MoMHR patients undergoing revision surgery.
Patients and Methods
This retrospective diagnostic accuracy study was performed at the Nuffield Orthopaedic Centre in Oxford. Between December 2000 and November 2014, 216 consecutive MoMHR revisions (199 patients) were performed for all indications. The details of each patient were prospectively recorded in our institutional database. Most of the MoMHRs (174; 81%) had been performed at this institution. The remainder (42; 19%) were referred from elsewhere. Since 2007, most of the revisions of MoMHRs we have conducted have been for pseudotumour formation (133 of 216 revisions; 62%).
At this centre, MoMHR has been performed as previously described.20 Patients have subsequently been recalled and reviewed according to recommendations from regulatory authorities.8 Symptomatic patients have undergone comprehensive investigation, including measurement of the levels of metal ions in the blood and ultrasound examination, which is our preferred cross-sectional imaging modality.19,20 Asymptomatic patients have been followed-up according to local protocols,8 although some have also been investigated in the same manner as the symptomatic cohort for the purposes of research.11
The indications for performing MARS-MRI were: patients in whom ultrasound was equivocal but a pseudotumour or other hip pathology was suspected; or those in whom ultrasound confirmed a pseudotumour or other hip pathology, but more detailed anatomical information was required before revision. The indications for revision have evolved. Initially revisions were performed for pseudotumours in symptomatic patients with large lesions. However, as short-term outcomes following revision were poor,4 the indications were broadened to include mildly symptomatic patients with smaller pseudotumours.
Patients undergoing revision of MoMHRs for any indication with both an ultrasound and MARS-MRI scan performed of the same hip before revision were eligible for inclusion in the study (53 hips in 51 patients). Revisions did not necessarily have to be performed for a pseudotumour as we also wanted to investigate the ability of both forms of imaging to exclude a pseudotumour.
Imaging was performed at least one-year following primary MoMHR, because of difficulties in distinguishing post-surgical changes from soft-tissue abnormalities within this period; no hips were excluded for this reason.13 Furthermore, both scans had to be performed within one year before revision surgery, in acknowledgement of recent observations that MARS-MRIs performed more than one year before revision have poor sensitivity (29%) for detecting a pseudotumour found at operation.21 Five hips were excluded for this reason. Ultrasound and MARS-MRI had to be performed in each patient within three months of each other for inclusion in the study, as this interval has been considered acceptable in previous studies.14,16-18,21 Eight hips were excluded for this reason. The final cohort included 40 MoMHRs in 39 patients undergoing imaging with both ultrasound and MARS-MRI before revision surgery (Table I).
Table I.
The demographics of the patients in the study
| 40 hips in 39 patients | |
|---|---|
| Gender (M/F) (%) | 17 (42.5)/23 (57.5) |
| Mean age at primary (yrs) (range) | 52.8 (28.7 to 71.8) |
| Mean BMI (kg/m2) (range) | 29.9 (22.2 to 50.1) |
| Unilateral /bilateral metal-on-metal hips (%) | 23 (57.5)/17 (42.5) |
| Hip resurfacing design (%) | |
| Birmingham Hip Resurfacing (Smith & Nephew, Warwick, UK) | 21 (52.5) |
| Conserve Plus (Wright Medical, Memphis, Tennessee) | 15 (37.5) |
| Recap (Biomet, Bridgend, UK) | 3 (7.5) |
| Articular Surface Replacement (DePuy, Leeds, UK) | 1 (2.5) |
| Mean component sizes (mm) (range) | Femoral 47 (40 to 54) |
| Acetabular 53 (46 to 60) | |
| Acetabular component position (o) | |
| Mean inclination (range) | 49.6 (34.0 to 74.1) |
| Mean anteversion (range) | 10.8 (2.0 to 33.0) |
| Abnormal radiographic features (%) | |
| Acetabular loosening | 7 (17.5) |
| Femoral loosening | 2 (5.0) |
| Acetabular osteolysis | 9 (22.5) |
| Femoral osteolysis | 4 (10.0) |
| Femoral neck thinning | 14 (35.0) |
| Femoral neck fracture | 2 (5.0) |
| Heterotopic ossification | 3 (7.5) |
| Median blood metal ion concentration in µg/l (IQR) | Cobalt: 12.9 (2.4 to 61.5) |
| Chromium: 11.4 (3.4 to 54.9) | |
| Median pre-revision Oxford hip score (0 to 48 scale) (IQR) | 40.5 (27.0 to 47.0) |
| Mean pre-revision UCLA score (1 to 10 scale) (range) | 5.7 (2 to 9) |
| Pre-revision symptoms (%) | |
| Symptomatic | 34 (85) |
| Asymptomatic | 6 (15) |
| Systemic symptoms | 1 (2.5) |
| Mean time between US and MARS-MRI (days) (range) | 14.6 (0 to 90) |
| Mean time between imaging and revision (mths) (range) | 5.3 (0.06 to 12) |
| Mean time from primary to revision (yrs) (range) | 6.3 (1.7 to 11.5) |
| Mean age at revision (yrs) (range) | 59.1 (40.0 to 77.7) |
| Indication for revision (%) | |
| Pseudotumour | 33 (82.5) |
| ARMD | 5 (12.5) |
| Cup loosening | 1 (2.5) |
| Femoral loosening | 1 (2.5) |
BMI, body mass index; IQR, interquartile range; MARS-MRI, metal artefact reduction sequence MRI; US, ultrasound; UCLA, University of California, Los Angeles activity score; ARMD, adverse reaction to metal debris
All ultrasound examinations were performed using a high linear array transducer (Sonoline Antares, Siemens, New York: 4-12 MHz and GE Logic E9 Wauwatosa, Wisconsin: 5-15 MHz) after obtaining consent from the patients. Probes of varying frequencies were used based on the size of the patient. The scans were all performed by an experienced musculoskeletal radiologist (SO: performed over 500 ultrasound examinations for suspected problems with MoMHRs), which has been recommended for achieving results comparable with MRI.16 The examinations were performed with the patients supine initially and then in the lateral decubitus position. All ultrasound examinations were performed using a standard technique recommended by the European Society of Skeletal Radiology,22 which encompasses a systematic approach to assess the anterior, medial, lateral and posterior regions of the hip. Standard sagittal oblique images were acquired by positioning the probe parallel to the long axis of the femoral neck. The probe was placed anteriorly, posteriorly, and directly lateral to the femoral neck to obtain dynamic views of these regions. Sagittal views along the axis of the femoral neck, anteriorly and posteriorly, and coronal views over the greater trochanter were stored. Any abnormalities were examined in multiple planes, with images subsequently stored. All stored static and dynamic images were available on the hospitals electronic picture archiving and communication system (PACS, GE Healthcare, Barrington, Illinois). The ultrasound examinations typically took no longer than 15 minutes.
All MRI images were obtained using a 1.5 tesla magnet scanner (Symphony, Siemens, Erlangen, Germany) with an optimised MARS as previously described.23 This included short tau inversion recovery (STIR) coronal (field of view (FOV) = 265 mm; time to resonate (TR) = 3500 ms; time to echo (TE) = 32 ms; bandwidth = 305 Hz/pixel) and STIR axial sequences (FOV = 240 mm; TR = 3810 ms; TE = 23 ms; bandwidth = 326 Hz/pixel), and T1-weighted coronal (FOV = 265 mm; TR = 692 ms; TE = 6.6 ms; bandwidth = 326 Hz/pixel) and axial sequences (FOV = 240 mm; TR = 634 ms; TE = 7.2 ms; bandwidth = 326 Hz/pixel). The thickness of the slices was 4 mm for all coronal and axial sequences. The MARS-MRI examination typically took no longer than 12 minutes.
In April 2015, all ultrasound and MARS-MRI images were assessed by one experienced musculoskeletal radiologist (RM). This radiologist was blinded to all clinical information, including that patients subsequently underwent a revision. Furthermore, images were reviewed in a random sequence to ensure that the radiologist was blinded to the results from the other modality when assessing the same MoMHR.
Each scan was assessed using a standard proforma. The radiologist categorically stated whether the scan was normal or abnormal, and whether or not a pseudotumour was present. The definition used for a pseudotumour for both imaging modalities was a cystic, solid, or mixed mass in continuity with the hip joint.19,23 For all pseudotumours, the volume (product of the maximum recorded dimension in each of three orthogonal planes in cm), consistency (solid, cystic, or mixed) and location in relation to the prosthesis were recorded. Any other abnormalities observed on ultrasound and/or MARS-MRI were also recorded, including effusions (defined as a distance of > 4 mm between the femoral neck and capsule),13 isolated distension and/or thickening of either the trochanteric or iliopsoas bursa, muscle atrophy (involving gluteus medius, gluteus minimus, and iliopsoas), and abnormalities of tendons (avulsion, atrophy, ossification).
Revision procedures were performed by one of six arthroplasty surgeons. All operation notes were retrospectively reviewed by an independent observer (GM), who was blinded to the results of the imaging. Findings were extracted from the operation notes to determine the presence or absence of a pseudotumour. When pseudotumours were present, their location and consistency (solid, cystic, or mixed) were recorded with the latter being determined by the surgeon’s observations of the thickness of the wall and the content of the lesion.21 The same definition for a pseudotumour at revision was used as that described for imaging, with the additional caveats that the diagnosis was confirmed on histopathological analysis,24,25 and microbiological analysis confirmed an aseptic process. Similar to the review of the imaging, all abnormalities other than pseudotumours documented by the surgeon in the operation notes at the revision procedure were also recorded. Hips were considered to have been revised for adverse reaction to metal debris (ARMD) rather than pseudotumour when no pseudotumour was present but features such as metallosis, synovitis, tissue damage and/or necrosis were observed with histopathology confirming a reaction to metal debris.21,24-26
Statistical analysis
All statistical analysis was performed using Stata Version 13.1 (StataCorp., College Station, Texas). Either the median and interquartile range (IQR) or the mean and range were used depending on the distribution of the data. The characteristics of the diagnostic test (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio and negative likelihood ratio, all with 95% confidence intervals (CIs)) for identifying MoMHRs with intra-operative pseudotumours were calculated for: ultrasound alone, MARS- MRI alone, and a combination of ultrasound and MARS- MRI. The reference standard was defined as the presence or absence of a histologically proven pseudotumour at revision surgery. The agreement of the imaging modalities (both alone and in combination) with the operative findings was measured using the kappa statistic (κ) with these values considered to represent slight (0 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), substantial (0.61 to 0.80) and almost perfect (0.81 to 1.00) agreement.27
Results
Revision surgery was performed for 40 MoMHRs in 39 patients (Table I). A total of 33 pseudotumours (82.5%; 95% CI 67.2 to 92.7) were found intra-operatively and confirmed histologically. The other seven MoMHRs without a pseudotumour were revised for ARMD (five), acetabular (one) and femoral component loosening (one). Intra-operative pseudotumours were most commonly cystic (23; 70%) or mixed (eight; 24%), and were most frequently located anteriorly (19; 58%) followed by posteriorly (five; 15%).
The abnormalities detected in the 40 MoMHRs on ultrasound and MARS-MRI are summarised (Table II). The prevalence of pseudotumour detected by both imaging modalities alone was identical (85.0%; 95% CI 70.2 to 94.3). The findings on imaging agreed with the intra-operative presence or absence of a pseudotumour as follows (these values also represent the accuracy of imaging): 82.5% (n = 33) for ultrasound alone, 87.5% (n = 35) for MARS- MRI alone, and 92.5% (n = 37) for ultrasound and MARS- MRI combined. There were nine hips (22.5%) in which the findings from either ultrasound (four; Fig. 1), or MARS-MRI (two), or both (three; Fig. 2) did not agree with the intra-operative findings (Table III).
Figs. 1a - 1b.
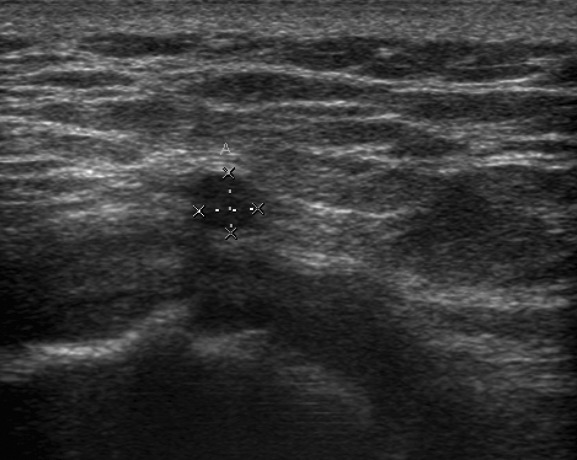
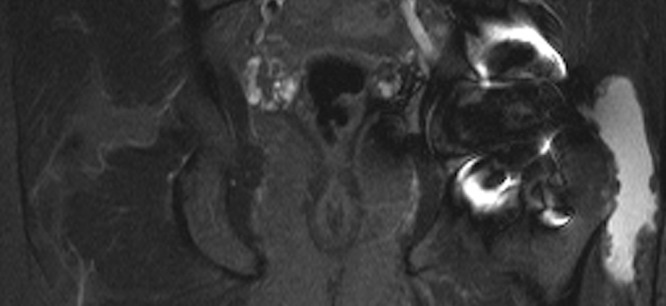
Disagreement of ultrasound with the presence of an intra-operative pseudotumour which was also confirmed on metal artefact reduction sequence magnetic resonance imaging (MARS-MRI). A 60-year-old male with a Conserve hip resurfacing underwent revision two years later; a large cystic psedotumour was identified intra-operatively and confirmed histologically; a) ultrasound imaging showed a small effusion and a separate 4 mm fluid collection laterally; however there was no pseudotumour. b) MARS- MRI was performed 61 days following ultrasound and confirmed a predominantly lateral cystic pseudotumour of 216 cm3 (coronal image shown).
Figs. 2a - 2b.
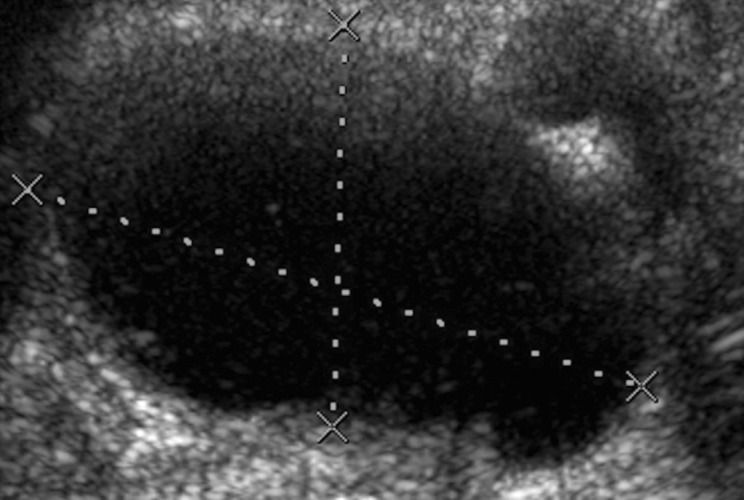
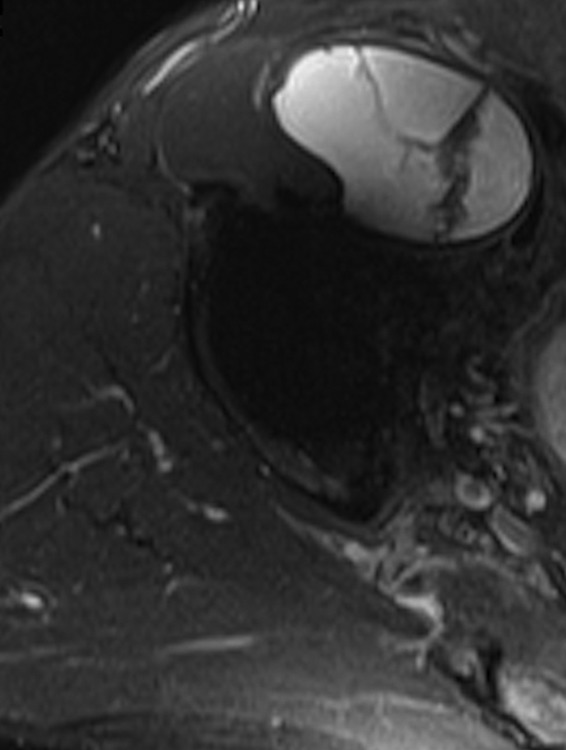
Disagreement of both ultrasound and metal artefact reduction sequence magnetic resonance imaging (MARS-MRI) with the absence of a pseudotumour intra-operatvely. A 59-year-old male with a Recap hip resurfacing underwent revision 4.9 years later. At revision there was significant metallosis but no collection of fluid or pseudotumour. Histology confirmed a reaction to metal debris. Ultrasound demonstrated separate a) anterior, and posterolateral cystic pseudotumours with a total volume of 288 cm3. MARS-MRI was performed 47 days following ultrasound and also demonstrated separate b) anterior, and posterior mixed pseudotumours with a total volume of 260 cm3.
Table II.
Findings identified on imaging performed in 40 metal-on-metal hip resurfacings before revision surgery
| Ultrasound | MARS-MRI | |
|---|---|---|
| Hips with 1 or more imaging abnormality (%) | 38 (95) | 38 (95) |
| Hips with any pseudotumour on imaging (%) | 34 (85) | 34 (85) |
| Median pseudotumour volume (cm3; IQR) | 22.6 (6.6 to 111.2) | 42.8 (6.9 to 131.7) |
| Pseudotumour consistency (%) | 9 (26) Cystic | 16 (48) Cystic |
| 16 (48) Mixed | 13 (38) Mixed | |
| 9 (26) Solid | 5 (15) Solid | |
| Pseudotumour location (%) | 18 (53) Anterior only | 14 (41) Anterior only |
| 8 (24) Anterior + posterior, lateral, or medial | ||
| 10 (29) Anterior + posterior, lateral, or medial | 8 (24) Posterior only, or postero-lateral | |
| 5 (15) Posterior only, or postero-lateral | 3 (9) Lateral only | |
| 1 (3) Medial only | 1 (3) Medial only | |
| Details of non-pseudotumour abnormalities (%) | 15 (38) joint effusions (1 cm3 to 8.1 cm3) | 25 (63) trochanteric and/or iliopsoas bursitis/distension |
| 14 (35) trochanteric and/or iliopsoas bursitis/distension | 19 (48) muscle atrophy (gluteus medius, gluteus minimus, iliopsoas, iliacus, obturator) | |
| 2 (5) trochanteric bursal thickening | 8 (20) tendon abnormalities (including tears, avulsions and/or ossification of gluteus medius, gluteus minimus, and hamstrings) | |
| 1 (3) subgluteus medius bursitis | 6 (15) bony erosions (acetabulum, proximal femur, femoral neck, pubic symphysis) | |
| 1 (3) heterotopic ossification | 4 (10) joint effusions (1 cm3 to 13.2 cm3) | |
IQR, interquartile range; MARS-MRI, metal artefact reduction sequence MRI
Table III.
Details of the nine metal-on-metal hip resurfacings where either or both imaging modalities disagreed with the presence or absence of an intra-operative pseudotumour
| Patient age at revision (yrs)/ gender | Primary HR/time in-situ (yrs) | Time between ultrasound and MARS-MRI (days) | Time between imaging and revision (yrs) | Ultrasound findings | MARS-MRI findings | Intra-operative revision + histology findings |
|---|---|---|---|---|---|---|
| 50.6 Female | Conserve/1.7 | 29 | 0.5 | Solid pseudotumour (anterior/31 cm3) | Fluid in trochanteric bursa. No pseudotumour | Large cystic pseudotumour (lateral) |
| 33.0 Female | BHR/4.3 | 37 | 0.4 | Mixed pseudotumour (anterior/9 cm3) | Mixed pseudotumour (lateral/33 cm3) | ARMD with metallosis & granulation tissue. No pseudotumour |
| 72.1 Female | Conserve/3.6 | 17 | 1.0 | No pseudotumour. Small effusion | Cystic pseudotumour (lateral/103 cm3) | Large mixed pseudotumour (postero-lateral) |
| 74.3 Male | BHR/2.5 | 16 | 0.3 | No pseudotumour. Trochanteric bursal distension | Cystic pseudotumour (anterior/114 cm3) | Large cystic pseudotumour (anterior) |
| 65.6 Female | ASR/4.0 | 40 | 0.3 | Mixed pseudotumour (anterior/50 cm3) | Normal scan | ARMD with joint effusion, synovitis & metallosis. No pseudotumour |
| 54.7 Male | Recap/7.0 | 0 | 0.3 | Mixed pseudotumour (anterior/23 cm3) | Cystic pseudotumour (anterior/5.4 cm3) | ARMD with acetabular osteolysis, synovitis & metallosis. No pseudotumour |
| 59.3 Male | Recap/4.9 | 47 | 0.4 | Cystic pseudotumour (anterior & postero-lateral/288 cm3) | Mixed pseudotumour (anterior & posterior / 260 cm3) | ARMD with metallosis. No pseudotumour |
| 59.7 Male | Conserve/2.0 | 61 | 0.8 | No pseudotumour. Small effusion and 4 mm fluid collection laterally | Cystic pseudotumour (anterior & lateral/216 cm3) | Large cystic pseudotumour (lateral) |
| 55.4 Female | BHR/10.2 | 0 | 0.6 | Solid pseudotumour (anterior/1 cm3) | Normal scan | Small cystic pseudotumour (anterior) |
ARMD, adverse reaction to metal debris; BHR, Birmingham Hip Resurfacing; HR, hip resurfacing, MARS-MRI, metal artefact reduction sequence MRI; ASR, Articular Surface Replacement
The characteristics of the diagnostic tests for identifying the presence or absence of a pseudotumour for ultrasound alone and MARS-MRI alone reached similar sensitivities (90.9% and 93.9%, respectively) and PPVs (88.2% and 91.2%, respectively). Higher specificities (57.1% vs 42.9%) and NPVs (66.7% vs 50.0%) were achieved with MARS-MRI alone compared with ultrasound alone (Table IV). When the findings from ultrasound and MARS-MRI were combined, the sensitivity and NPV both reached 100% with maintenance of the specificity (57.1%) and PPV (91.7%) (Table IV). Similar findings were observed for agreement between the imaging modalities and the presence or absence of a pseudotumour at revision. Agreement was substantial (κ = 0.69) for ultrasound and MARS-MRI combined, moderate for MARS-MRI alone (κ = 0.54), and fair for ultrasound alone (κ = 0.36) (Table IV).
Table IV.
Diagnostic test characteristics for imaging identifying an intra-operative pseudotumour in 40 revised metal-on-metal hip resurfacings
| Ultrasound alone | MARS-MRI alone | Ultrasound and MARS-MRI combined | |
|---|---|---|---|
| Pseudotumour prevalence on imaging* | 85.0 (70.2 to 94.3) | 85.0 (70.2 to 94.3) | 90.0 (76.3 to 97.2) |
| Sensitivity | 90.9 (75.7 to 98.1) | 93.9 (79.8 to 99.3) | 100.0 (89.4 to 100.0) |
| Specificity | 42.9 (9.9 to 81.6) | 57.1 (18.4 to 90.1) | 57.1 (18.4 to 90.1) |
| PPV | 88.2 (72.5 to 96.7) | 91.2 (76.3 to 98.1) | 91.7 (77.5 to 98.2) |
| NPV | 50.0 (11.8 to 88.2) | 66.7 (22.3 to 95.7) | 100.0 (39.8 to 100.0) |
| LR+ | 1.6 (0.8 to 3.1) | 2.2 (0.9 to 5.2) | 2.3 (1.0 to 5.5) |
| LR- | 0.2 (0.1 to 0.8) | 0.11 (0.02 to 0.5) | NA |
| Kappa statistic κ | 0.36 (-0.02 to 0.74) | 0.54 (0.19 to 0.90) | 0.69 (0.36 to 1.0) |
* Prevalence of pseudotumours confirmed intra-operatively and on histology (i.e. the gold standard) = 82.5% (95% confidence intervals 67.2 to 92.7) PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR-, negative likelihood ratio; MARS-MRI, metal artefact reduction sequence MRI All prevalence and diagnostic test characteristic values are provided as percentages with 95% confidence intervals provided in brackets NA, not able to calculate
Discussion
Ultrasound and MARS-MRI have previously been shown to be useful for identifying problems associated with MoM hips,11-13 with regulatory authorities recommending the use of either modality during patient follow-up.8-10 More recently studies have compared the use of these modalities for assessing MoM hips, however these studies had important limitations coupled with the lack of an international consensus on the ideal modality to use.14,16-18
Our study is the first to compare the characteristics of ultrasound alone, MARS-MRI alone, and ultrasound combined with MARS-MRI against the reference standard of pseudotumours confirmed intra-operatively and histologically, in 40 revised MoMHRs. The characteristics and κ agreement of the imaging modalities suggest that all three options can be used to identify the presence or absence of a pseudotumour. However, there was a trend supporting the combination of ultrasound and MARS-MRI as the most effective, followed by MARS-MRI alone, then ultrasound alone. Given the significant costs associated with these forms of imaging28 in MoMHR patients and the requirement for experienced radiologists to perform ultrasound of the hip,11,13 we recommend that institutions choose the imaging option which best suits their circumstances.
One previous study attempted to validate ultrasound and MARS-MRI findings with revision findings. Although good correlation was reported, only five patients were revised with delays of up to one year between performing ultrasound and MARS-MRI in the same patient.14 Lainiala et al validated findings from ultrasound29 and MARS- MRI21 in MoMHR patients who subsequently underwent revision for pseudotumour. The authors reported better agreement for ultrasound (κ = 0.52 to 0.64)29 than MARS- MRI (κ = 0.40),21 which is in contrast to our findings of MARS-MRI alone (κ = 0.54) providing better agreement than ultrasound alone (κ = 0.36). These differences may be explained by the fact that all patients in the previous studies had a MoM hip system which has subsequently been withdrawn, which included stemmed devices, and also that patients were not imaged with both modalities.21,29
We found that the combination of ultrasound and MARS-MRI provided substantial agreement (κ = 0.69) for the detection of intra-operative pseudotumours, with 100% sensitivity and NPV while maintaining the specificity (57.1%) and PPV (91.7%) compared with MARS-MRI or ultrasound alone. Therefore, all patients with a pseudotumour were correctly identified by combined imaging, and those without a pseudotumour on combined imaging were correctly confirmed as having no such lesions intra-operatively. Although combined imaging did not miss any pseudotumours, both ultrasound and MARS-MRI results taken in isolation missed large pseudotumours. This provides support for using combined imaging in MoMHR patients who are thought to require revision when necessary resources are available.
There were three hips in which ultrasound and MARS- MRI both identified a pseudotumour but no such lesion was found at revision surgery. Although this may represent incorrect diagnosis at imaging, it could alternatively reflect surgeons inaccurately reporting the findings at revision, given the retrospective design of the study. This could have resulted in an underestimation of the diagnostic characteristics of the imaging modalities for identifying the presence or absence of a pseudotumour. Excluding these three hips improved the characteristics of the diagnostic tests and agreement for ultrasound alone (sensitivity 90.9%, specificity 75.0%, PPV 96.8%, NPV 50.0%, κ = 0.54 representing moderate agreement), MARS-MRI alone (sensitivity 93.9%, specificity 100%, PPV 100%, NPV 66.7%, κ = 0.77 representing substantial agreement), and both modalities combined (sensitivity 100%, specificity 100%, PPV 100%, NPV 100%, κ = 1.0 representing perfect agreement).
The best characteristics of cross-sectional imaging identifying pseudotumours in MoM hips were reported by Garbuz et al.16 They assessed ultrasound against MRI and reported 100% sensitivity, 96% specificity, 92% PPV, and 100% NPV with consensus between modalities used as the reference standard.16 Our results for ultrasound and MARS-MRI combined produced similar results to Garbuz et al16 for ultrasound alone, although it is important to consider the methodological differences between the studies. Garbuz et al16 concluded that ultrasound should be used initially to screen patients with a MoM hip for pseudotumour. However, our data suggest that if this approach is used and the ultrasound is normal, MARS-MRI is required to exclude a pseudotumour. Our data also suggest that the converse is true. For institutions preferring to use MARS- MRI initially, a normal MARS-MRI requires a subsequently normal ultrasound to exclude a pseudotumour beyond all doubt.
It has recently been reported that the guidance for the follow-up of patients with a MoM hip around the world is neither evidence-based nor financially sustainable, with most protocols lacking the sensitivity to detect asymptomatic pseudotumours.28 Regulatory authorities currently recommend the use of either ultrasound or MARS-MRI as first-line for imaging patients with a MoMHR.8-10 Although our data support this recommendation, we observed that combined imaging produced optimal diagnostic characteristics and substantial agreement with the intra-operative findings. Despite being more costly, the use of both ultrasound and MARS-MRI to assess these patients has the advantage of identifying non-pseudotumour pathologies, which can be the source of symptoms.13,14 Given the time interval between imaging and revision in our study, these recommendations only apply to the first year following the initial imaging, after which time repeat imaging may be required.
Importantly, our findings are based on patients requiring revision surgery, and therefore may not apply to the many well-functioning MoMHRs under surveillance. In this large group of patients the cost of combined imaging is a more pertinent issue. Screening with ultrasound or MARS-MRI alone, both of which have been shown to be effective in identifying the development and progression of pseudotumours,11,12,30-32 may be more suitable for the purposes of surveillance. Ultrasound also has the advantage of detecting smaller lesions close to the prosthesis which may require serial monitoring.17
This study has limitations. Ultrasound is a dynamic investigation, therefore the retrospective review of ultrasound reports and images could have affected interpretation. However, all original ultrasound imaging was performed by an experienced musculoskeletal radiologist as recommended,16 with all retrospective reviews performed by a different but similarly experienced and blinded musculoskeletal radiologist. In addition, dynamic images were saved by the original radiologist if abnormalities were identified and were also available for review. Furthermore, in all cases the reports and images categorically confirmed the presence or absence of a pseudotumour which was the primary outcome of interest. Another limitation relates to the selection bias introduced by our follow-up protocol. Ultrasound was used as the first-line investigation with MARS-MRI subsequently performed only in more problematic cases. The data presented may therefore underestimate the diagnostic characteristics for ultrasound. A superior study design would involve all patients who undergo a MoMHR having both an ultrasound and MARS-MRI regardless of the results, preferably on the same day.
The potential limitations of surgeon reporting has already been recognised, and this may explain some of the discrepancies between the imaging and intra-operative findings. However, the presence or absence of a pseudotumour was always clearly stated in the operation notes. Surgeons were not blinded to the imaging findings, which is an unavoidable limitation of this type of study and has been encountered elsewhere.21 We applied a strict definition of pseudotumour, which included communication with the joint and incorporated histological findings. Earlier definitions of pseudotumour were broader,12,19 and this must be considered when interpreting our results.The time between performing ultrasound and MARS-MRI in our patients was a maximum of three months, and similar or shorter than in previous reports.14,17,18,21 However, this delay may explain some discrepancies between the imaging and intra-operative findings. Finally, our findings may not be applicable to stemmed MoM hip replacements.
We conclude that there remains a role for ultrasound and/or MARS-MRI when assessing patients who have undergone MoMHR. The choice of imaging will depend on many factors, including the local financial constraints and the available expertise with ultrasound. However, in patients who have a MoMHR requiring revision, a combination of ultrasound and MARS-MRI was most effective in detecting and excluding pseudotumours. Combined imaging always identified intra-operative pseudotumours if present. Furthermore, if neither imaging modality detected a pseudotumour, one was not found at revision.
References
- 1.Lombardi AV Jr, Barrack RL, Berend KR, et al. The Hip Society: algorithmic approach to diagnosis and management of metal-on-metal arthroplasty. J Bone Joint Surg [Br] 2012;94-B:14–18. [DOI] [PubMed] [Google Scholar]
- 2.Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW. National Joint Registry of England and Wales. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet 2012;379:1199–1204. [DOI] [PubMed] [Google Scholar]
- 3.Smith AJ, Dieppe P, Howard PW, Blom AW. National Joint Registry for England and Wales. Failure rates of metal-on-metal hip resurfacings: analysis of data from the National Joint Registry for England and Wales. Lancet 2012;380:1759–1766. [DOI] [PubMed] [Google Scholar]
- 4.Grammatopoulos G, Pandit H, Kwon YM, et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg [Br] 2009;91-B:1019–1024. [DOI] [PubMed] [Google Scholar]
- 5.Matharu GS, Pynsent PB, Dunlop DJ. Revision of metal-on-metal hip replacements and resurfacings for adverse reaction to metal debris: a systematic review of outcomes. Hip Int 2014;24:311–320. [DOI] [PubMed] [Google Scholar]
- 6.Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg [Br] 2012;94-B:315–321. [DOI] [PubMed] [Google Scholar]
- 7.Matharu GS, McBryde CW, Pynsent WB, Pynsent PB, Treacy RB. The outcome of the Birmingham Hip Resurfacing in patients aged 50 years up to 14 years post-operatively. Bone Joint J 2013;95-B:1172–1177. [DOI] [PubMed] [Google Scholar]
- 8.No authors listed. Medical and Healthcare products Regulatory Agency (MHRA) (2012). Medical Device Alert: all metal-on-metal (MoM) hip replacements. MDA/2012/036. (date last accessed 16 September 2015).
- 9.No authors listed. European Federation of National Associations of Orthopaedics and Traumatology (2012). Consensus statement “Current Evidence on the Management of Metal-on-Metal Bearings.” (last accessed 16 September 2015).
- 10.No authors listed. U.S. Food and Drug Administration (2013). Medical Devices. Metal-on-Metal Hip Implants. Information for Orthopaedic Surgeons. (last accessed 16 September 2015).
- 11.Kwon YM, Ostlere SJ, McLardy-Smith P, et al. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty 2011;26:511–518. [DOI] [PubMed] [Google Scholar]
- 12.Hart AJ, Satchithananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg [Am] 2012;94-A:317–325. [DOI] [PubMed] [Google Scholar]
- 13.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Ultrasound screening of periarticular soft tissue abnormality around metal-on-metal bearings. J Arthroplasty 2012;27:895–900. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui IA, Sabah SA, Satchithananda K, et al. A comparison of the diagnostic accuracy of MARS MRI and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop 2014;85:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd J, Starks I, Wainwright T, Middleton R. Metal-on-metal resurfacing and the cost to the nation: a conservative estimate of the unexpected costs required to implement the new metal-on-metal follow-up programme in the UK. Total Hip Arthroplasty 2013;45–52. [Google Scholar]
- 16.Garbuz DS, Hargreaves BA, Duncan CP, et al. The John Charnley Award: diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res 2014;472:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Is ultrasound screening reliable for adverse local tissue reaction after hip arthroplasty? J Arthroplasty 2014;29:2239–2244. [DOI] [PubMed] [Google Scholar]
- 18.Muraoka K, Naito M, Nakamura Y, Hagio T, Takano K. Usefulness of ultrasonography for detection of pseudotumors after metal-on-metal total hip arthroplasty. J Arthroplasty 2015;30:879–884. [DOI] [PubMed] [Google Scholar]
- 19.Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg [Br] 2008;90-B:847–851. [DOI] [PubMed] [Google Scholar]
- 20.Murray DW, Grammatopoulos G, Pandit H, et al. The ten-year survival of the Birmingham hip resurfacing: an independent series. J Bone Joint Surg [Br] 2012;94-B:1180–1186. [DOI] [PubMed] [Google Scholar]
- 21.Lainiala O, Elo P, Reito A, et al. Comparison of extracapsular pseudotumors seen in magnetic resonance imaging and in revision surgery of 167 failed metal-on-metal hip replacements. Acta Orthop 2014;85:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beggs I, Bianchi S, Bueno A, et al. European Society of Musculoskeletal Radiology. Musculoskeletal Ultrasound Technical Guidelines IV. Hip. (last accessed 16 September 2015).
- 23.Hauptfleisch J, Pandit H, Grammatopoulos G, et al. A MRI classification of periprosthetic soft tissue masses (pseudotumours) associated with metal-on-metal resurfacing hip arthroplasty. Skeletal Radiol 2012;41:149–155. [DOI] [PubMed] [Google Scholar]
- 24.Campbell P, Ebramzadeh E, Nelson S, et al. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res 2010;468:2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grammatopoulos G, Pandit H, Kamali A, et al. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg [Am] 2013;95-A:81. [DOI] [PubMed] [Google Scholar]
- 26.Langton DJ, Jameson SS, Joyce TJ, et al. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg [Br] 2010;92-B:38–46. [DOI] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 28.Matharu GS, Mellon SJ, Murray DW, Pandit HG. Follow-up of metal-on-metal hip arthroplasty patients is currently not evidence based or cost effective. J Arthroplasty 2015;30:1317–1323. [DOI] [PubMed] [Google Scholar]
- 29.Lainiala O, Elo P, Reito A, et al. Good sensitivity and specificity of ultrasound for detecting pseudotumors in 83 failed metal-on-metal hip replacements. Acta Orthop 2015;86:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almousa SA, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res 2013;471:3814–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reito A, Elo P, Puolakka T, et al. Repeated magnetic resonance imaging in 154 hips with large-diameter metal-on-metal hip replacement. Acta Orthop 2014;85:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matharu GS, Janardhan S, Brash L, et al. Follow-up of metal-on-metal hip arthroplasty patients: the utility of repeat ultrasound imaging. Ann R Coll Surg Engl 2016. (In Press). [DOI] [PMC free article] [PubMed]


