SUMMARY
Four distinct subgroups of cerebellar medulloblastomas (MBs) differ in their histopathology, molecular profiles, and prognosis. c-Myc (Myc) or MycN overexpression in granule neuron progenitors (GNPs) induces Group 3 (G3) or Sonic Hedgehog (SHH) MBs, respectively. Differences in Myc and MycN transcriptional profiles depend, in part, on their interaction with Miz1, which binds strongly to Myc but not MycN, to target sites on chromatin. Myc suppresses ciliogenesis and “reprograms” the transcriptome of SHH-dependent GNPs through Miz1-dependent gene repression to maintain “stemness”. Genetic disruption of the Myc/Miz1 interaction inhibited G3 MB development. Target genes of Myc/Miz1 are repressed in human G3 MBs, but not in other subgroups. Therefore, the Myc/Miz1 interaction is a defining hallmark of G3 MB development.
INTRODUCTION
Members of the MYC family of proto-oncogenes (c-MYC (MYC), MYCN and MYCL) play important roles in a wide range of cellular processes including proliferation, differentiation, apoptosis, and tumorigenesis (Dang, 2012; Roussel and Robinson, 2013). MYC family genes encode closely related basic helix-loop-helix (bHLH)/leucine-zipper transcription factors that form heterodimers with MAX to drive transcriptional activation upon binding to Enhancer Box sequences (E-boxes) (Conacci-Sorrell et al., 2014). Whereas a dimeric Myc/Max complex activates gene transcription, Myc represses transcription when in complex with Miz1. Miz1, a Pox virus and zinc finger (POZ) domain transcription factor, is ubiquitously expressed during mouse embryonic development and activates transcription upon binding to its cognate binding site (Adhikary et al., 2003; Wolf et al., 2013). When Myc is induced in response to supra physiological mitogenic stimuli, Miz1 is recruited to the Myc/Max complex to repress transcription (Walz et al., 2014; Wolf et al., 2014). For example, a ternary Myc/Max/Miz1 complex represses the transcription of negative cell cycle regulator genes CDKN1A and CDKN2B to antagonize TGF-β-dependent activation of these two cyclin-dependent kinase (CDK) inhibitors (Seoane et al., 2001; Staller et al., 2001).
Medulloblastoma (MB), the most common malignant pediatric brain tumor, arises in the cerebellum and is characterized by four molecularly distinct subgroups: Wingless-Int (WNT), Sonic Hedgehog (SHH), Group 3 (G3) and G4 (Taylor et al., 2012). MYC genes are often overexpressed or amplified in MB (Roussel and Robinson, 2013). Among the four subgroups, MYC and MYCN are differentially expressed. While WNT and G3 MBs overexpress MYC with or without amplification, SHH and G4 MBs express MYCN (Robinson et al., 2012; Roussel and Robinson, 2013). Mycn is a target of SHH signaling (Kenney et al., 2003) and is required for the proliferation of mouse GNPs and SHH MB development (Zindy et al., 2006). Enforced expression of MycN in granule neuron progenitors (GNPs) from the cerebella of Trp53−/−;Cdkn2c−/− mice induces SHH MB; remarkably, overexpression of Myc in the same GNPs induces G3 MBs (Kawauchi et al., 2012). This was unexpected since Myc and MycN have similar structures and are partially functionally redundant during mouse development (Malynn et al., 2000).
Previous studies found that the Myc/Miz1 interaction is a prerequisite for Myc-induced lymphoma development (van Riggelen et al., 2010) and that Myc enhances self-renewal of neural progenitor cells (NPCs) via binding to Miz1 (Kerosuo et al., 2008). Interestingly, mouse G3 MB cells express many markers of “stemness”, suggesting that Miz1 might be required for the development of more stem-like G3 MBs in response to Myc overexpression (Kawauchi et al., 2012). We hypothesized that the difference between Myc- and MycN-induced MBs might be due to their interaction with different partner proteins in GNPs.
RESULTS
Myc/Miz1 interaction is required for G3 MB development in vivo
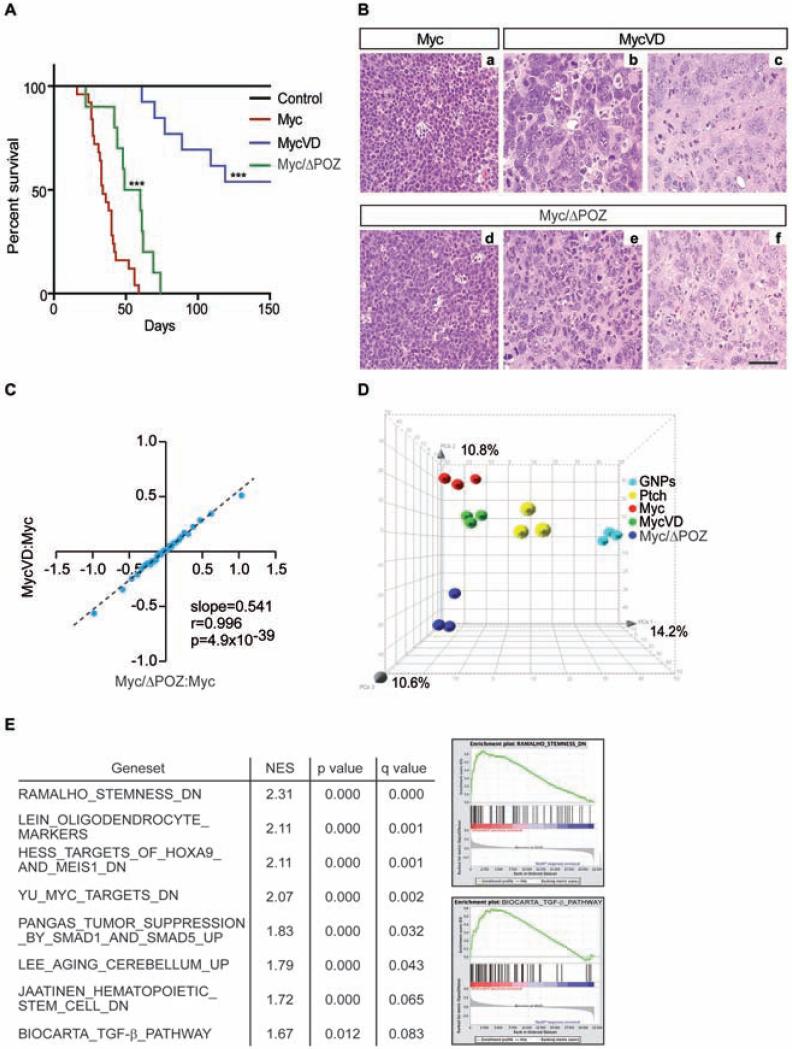
To investigate whether the interaction of Myc with Miz1 contributes to the development of G3 MBs, we used a Myc mutant, MycV394D (termed MycVD thereafter), that has a greatly reduced affinity to Miz1 (Herold et al., 2002). We enforced the expression of Myc or MycVD proteins inGNPs, purified from the cerebella of postnatal Trp53−/−;Cdkn2c−/− mice, by retroviral transduction, and implanted the transduced GNPs into the cortices of naïve recipient animals. The percentage of infected cells with retroviruses encoding Myc and MycVD was comparable (53.3 ± 8% for Myc vs. 49.0 ± 7% for MycVD). Whereas mice transplanted with Myc-infected GNPs developed tumors with complete penetrance, as previously shown (Kawauchi et al., 2012), only 40% of the mice developed tumors by MycVD-infected GNPs. Mice bearing MycVD-infected GNPs developed brain tumors later than mice bearing Myc-infected GNPs (Figure 1A) despite comparable Myc protein levels in Myc- versus MycVD-driven tumors (Figure S1A).
Figure 1. Characterization of Myc, MycVD and Myc/ΔPOZ engineered tumors.
(A) Kaplan-Meier survival curves of mice transplanted with cerebellar cells purified from (i) Trp53−/−;Cdkn2c−/− mice infected with Myc (red line), MycVD (blue line), or empty vector (black line) and (ii) Myc/ΔPOZ mice infected with Myc (green line). ***, p<0.0001 MycVD or Myc/ΔPOZ versus Myc. (B) H&E staining of sections from mouse Myc, MycVD and Myc/ΔPOZ tumors: (a) An example of Myc tumors showing anaplastic morphology with cell molding and abundant mitotic figures and apoptotic bodies. (b, c) Examples of MycVD tumors showing large, pleomorphic nuclei with abundant cytoplasm. (d-f) Examples of Myc/ΔPOZ tumors showing a proliferating cell population with anaplastic/large cell features (d) or a population of cells comprised of anaplastic cells with pleomorphic nuclei with a scattering of large and bizarre nuclei and abundant cytoplasm (e, f). Morphology of the cell population in (e, f) shows an unclassifiable type. Scale bar = 100 μm. (C) Microarray analysis of gene expression induced by MycVD versus Myc and Myc/ΔPOZ versus Myc. The plot shows values of all genes by grouping them into 30 equally sized bins. The x-axis shows change in expression between tumors that arise in a Myc/ΔPOZ-background in comparison to control G3 MB, both of which are induced by overexpression of wild-type Myc. The y-axis shows changes in expression between MycVD induced tumors in comparison to Myc/G3 MB. Slope: regression coefficient, r: Pearson correlation coefficient, p value (two-tailed t-test). (D) Principal component analysis (PCA) for GNPs (n = 3), SHH MB (Ptch1+/−;Cdkn2c−/−) (n = 3), Myc (n = 3), MycVD (n = 3), and Myc/ΔPOZ (n = 3) tumors. Percentages represent the proportion of variance in each vector. (E) Gene Set Enrichment Analysis (GSEA) of gene expression in four Myc/ΔPOZ tumors and four MycVD tumors in comparison to three Myc tumors. List and examples of selected gene sets of up-regulated genes shown. See also Figure S1 and Table S1.
To confirm that the delay in tumor formation observed in mice transplanted with MycVD-expressing GNPs was due to lack of complex formation with Miz1, we expressed Myc in GNPs from Miz1ΔPOZ/ΔPOZ;Trp53fl/fl;Nestin-Cre mice (termed Trp53 fl/fl-ΔPOZ mice thereafter). Trp53 fl/fl-ΔPOZ mice express a Miz1 allele, which maintains binding to the Myc/Max complex but is strongly compromised in its function (Kosan et al., 2010). Mice bearing Myc-infected GNPs from Trp53 fl/fl-ΔPOZ mice developed tumors (called Myc/ΔPOZ tumors thereafter) with complete penetrance and an intermediate time of onset (Figure 1A). Myc proteins levels in Myc- and MycVD-driven tumors were comparable (Figure S1A) whereas Myc/ΔPOZ tumors were variable between tumors (Figure S1B).
Immunopathology analysis revealed that Myc-induced tumors were large cell/anaplastic (LC/A) similar to G3 MB, as previously shown (Kawauchi et al., 2012; Pei et al., 2012) (Figure 1B). In contrast, MycVD-induced tumors and Myc/ΔPOZ tumors were no longer identified as G3 MB. Instead both tumor types showed a range of mixed pathologies classified as neuroepithelial of unclassifiable type; Myc/ΔPOZ tumors also included regions of primitive neuroectodermal tumors (PNETs) (Figure 1B, Table 1). Global gene expression profiles of both MycVD and Myc/ΔPOZ tumors were similar to each other but distinct from Myc/G3 MBs (Figures 1C and S1C). Consistent with their morphology and pathology, principle component analysis (PCA) of gene expression profiles showed that MycVD and Myc/ΔPOZ tumors segregate away from G3 and SHH MBs and from GNPs, indicating that they acquire identities along lineages not observed in MB (Figure 1D). Gene set enrichment analysis (GSEA) showed that previously identified sets of Myc/Miz1-repressed genes were upregulated in both MycVD and Myc/ΔPOZ tumors including target genes of the TGF-β pathway (Figure 1E) (Seoane et al., 2001; Staller et al., 2001). In addition, several sets of genes that are repressed in hematopoietic and potentially also in embryonic and neuronal stem cells were upregulated in both MycVD and Myc/ΔPOZ tumors (Figure 1E). These observations underscore that Myc/Miz1-mediated repression was compromised in both MycVD and Myc/ΔPOZ tumors and suggest that complex formation of Myc with Miz1 is required to maintain a stem-like phenotype. This notion is supported by the decrease in MycVD and Myc/ΔPOZ tumors of the expression of genes that are characterized by bivalent chromatin marks in neuronal progenitor cells (Figure S1D).
Table 1.
Summary of Myc, MycVD, and Myc/APOZ Engineered Tumors.
| Number of Mice with Tumor / Total Number of Mice | Tumor Latency (Days) | Median Survival (Days) | Pathology | |
|---|---|---|---|---|
| Myc | 25/25 | 16, 24, 26, 26, 27, 27, 28, 31, 32, 33, 33, 33, 34, 36, 38, 40, 40, 40, 41, 42, 43, 52, 56, 56, 59 | 34 | LC/A, Myc/G3 MB |
| MycVD | 6/13 | 61, 70, 77, 89, 109, 119 | 83 | Neuroepithelial proliferation of unclassified type |
| Myc/ΔPOZ | 10/10 | 22, 42, 44, 48, 49, 60, 61, 62, 69, 74 | 54.5 | PNET-like tumor with LC/A; unclassified type |
LC/A = Large cell/anaplastic, G3 MB = Group3 medulloblastoma, PNET = Primitive neuroectodermal tumor, median survival was calculated based on the number of mice that develop one tumor per animal.
MycVD and Myc/ΔPOZ tumors form tumorspheres that can be passaged continuously
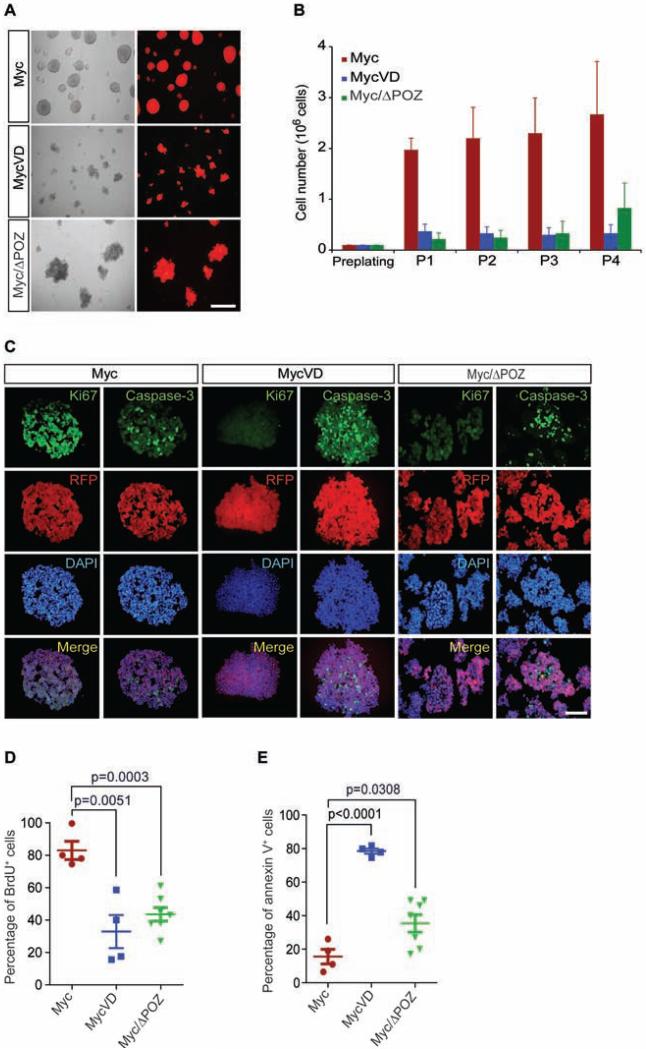
We previously reported that tumorspheres from G3 MBs could be passaged continuously with the cell number increasing up to 25-fold; under these conditions, they maintain an undifferentiated phenotype and retain expression of multiple stem cell markers (Kawauchi et al., 2012). We tested whether the loss of Miz1 binding affected the proliferation and/or cell death of tumorspheres derived from primary Myc/G3 MB, MycVD and Myc/ΔPOZ tumors. Myc/G3 MB primary tumor cells grew as round spheres while MycVD and Myc/ΔPOZ primary tumor cells formed more diffuse colonies (Figure 2A), and both could be passaged continuously (Figure 2B). In contrast to Myc-derived tumorspheres, the total number of cells in MycVD and Myc/ΔPOZ tumorspheres did not increase during passage, suggesting that only a small number of tumor cells had self-renewing capacity (Figure 2B). To avoid potential genetic drift, tumorsphere lines were analyzed at early passage (p3) for proliferation using Ki67 staining (Figure 2C) and BrdU pulse-labeling (Figure 2D), and at p4 for apoptosis by caspase-3 staining (Figure 2C) and fluorescence activated cell sorting (FACS) with Annexin V staining (Figure 2E). MycVD and Myc/ΔPOZ tumors formed tumorspheres that were less proliferative (Figures 2C and 2D), and highly apoptotic (Figures 2C and 2E) relative to Myc tumorspheres. The differences in cell proliferation and apoptosis from the MycVD and Myc/ΔPOZ tumors compared to the Myc/G3MBs were not due to variance in Myc levels (Figures S2A and S2B), nor to the incomplete deletion of the POZ domain by the Cre recombinase in Myc/ΔPOZ tumors (Figures S2C and S2D). Human G3 MBs with MYC amplification are often metastatic leading to leptomeningeal spread (Gajjar and Robinson, 2014; Roussel and Robinson, 2013). In accordance with this phenotype, Myc/G3 MB-derived tumorsphere cells had a greater migration (Figure S2E) and invasion (Figure S2F) index compared to MycVD- and Myc/ΔPOZ-driven tumors.
Figure 2. Myc/Miz1 interaction is required for proliferation of tumorspheres.
(A) Representative images of Myc, MycVD, and Myc/ΔPOZ tumorspheres, scale bar = 200μm. (B) Proliferation of tumorsphere cells passaged in vitro. (C) Immunofluorescence of RFP marked Myc, MycVD, and Myc/ΔPOZ tumorspheres with antibodies to cleaved caspase-3 (cell death), Ki67 (proliferation), and DAPI (nuclei), scale bar = 50 μm. (D) BrdU pulse and FACS analysis to measure tumorspheres proliferation. (E) Apoptosis measured by Annexin V staining and FACS analysis. p values (shown at the top) are calculated by an unpaired two-tailed t-test from three independent experiments. Data are represented as the mean ± SD. See also Figure S2.
Identification of Myc/Miz1 targets in G3 MB
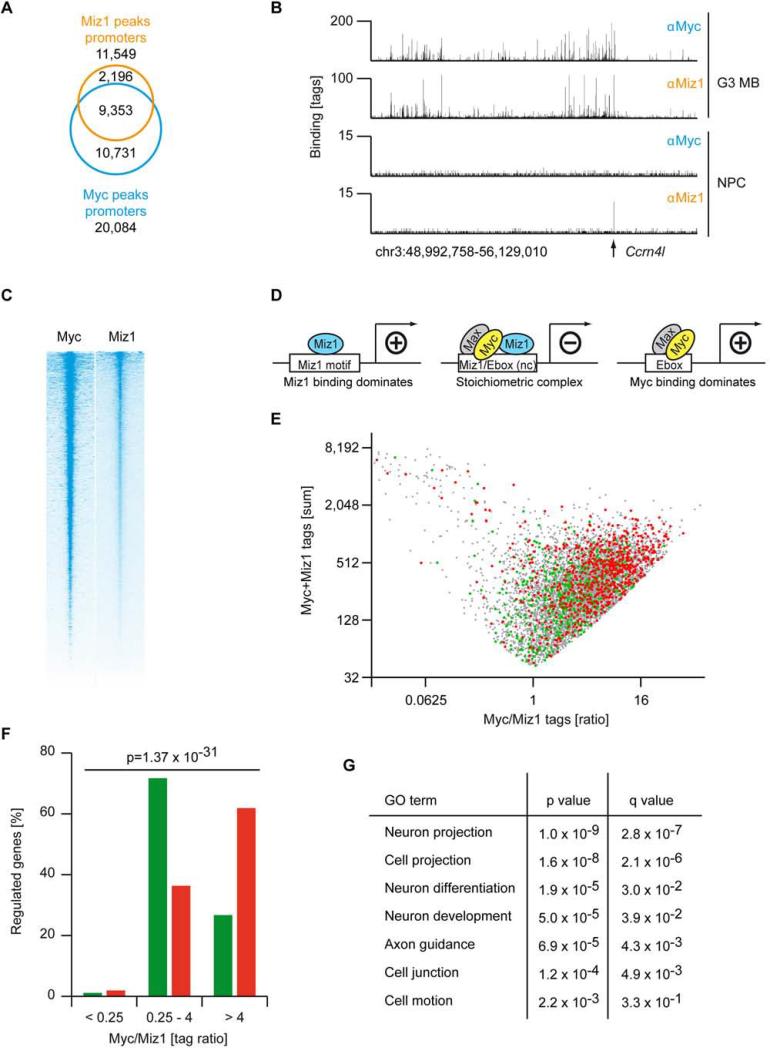
To analyze the molecular mechanism by which the Myc/Miz1 interaction regulates G3 MB development, we performed Chromatin Immunoprecipitation followed by Sequencing (ChIP-Seq) with antibodies to Myc and Miz1 to determine whether a Myc/Miz1-complex was present on chromatin in G3 MBs. We previously showed that Miz1 occupies approximately 140 promoters in neuronal progenitor cells (NPC), which carry the cognate Miz1 binding sequence (Wolf et al., 2013) (Figure S3A). In stark contrast, 11,549 Miz1 peaks are located in promoters in G3 MBs (Figure 3A). Myc co-occupied 9,353 binding sites with Miz1 in promoters (p<2.2×10−308, based on hypergeometric distribution) (Figures 3A, S3B, and S3C). A similarly broad binding of Miz1 to Myc-bound promoters is observed in several other human and mouse tumor types, since Myc recruits Miz1 to its binding sites (Walz et al., 2014). Consistent with these data, ranking of all promoters according to Miz1 occupancy showed that the overall intensity of Myc binding overlapped with Miz1 (Figures 3B and 3C) (Walz et al., 2014).
Figure 3. Promoter binding of Myc and Miz1 determines the transcriptional output.
(A) Venn diagram displaying the number of binding sites for Myc and Miz1 in promoter regions (+/− 5 kb) of RNA polymerase II transcribed genes in G3 MB. (B) Genome browser picture documenting binding of Miz1 and Myc to a large genomic region (chr3:48,99,758-56,129,010) in G3 MB and neuronal progenitor cells (NPCs) (Wolf et al., 2013). The arrow indicates the position of the Ccrn4l promoter, which contains a Miz1 binding motif. (C) Heat map documenting binding of Myc and Miz1 to genes transcribed by RNA-Pol-II in G3 MB. All 31,194 refseq genes are shown and Myc/Miz1-peaks are centred to the adjacent transcriptional start site (TSS, +/− 5 kb). Genes are sorted according to the occupancy by Miz1. (D) Schematic representation of DNA binding motifs and transcriptional function of Miz1 alone, Miz1 bound to the Myc/Max, complex and Myc bound to Max (“nc”: non-consensus). (E) The diagram shows the sum of Myc and Miz1 binding tags versus the ratio of Myc/Miz1 tags for promoters that are bound by Myc and Miz1 in G3 MB. Colour dots designate regulated genes after 12 hr of activation of Myc-ER with 4OHT in GNPs: grey dot, Myc/Miz1 bound; red dot, Myc activated; green dot, Myc repressed. Microarray analysis identified 475 down- (log2FC<−0.322, green dots) and 696 up-regulated (log2FC>0.322, red dots) genes upon activation of Myc. (F) Quantification of the distribution of repressed (green bars) and induced (red bars) genes in respect to the Myc/Miz1 ratio. p value (shown at the top) was calculated by a Chi-squared test. (G) GO terms (DAVID) of the analysis of the 475 genes that are repressed (log2FC<−0.322) 12 hr after the activation of Myc with 4OHT. See also Figure S3.
To identify genes repressed by Myc/Miz1 complexes in G3 MBs we analyzed microarray experiments from three engineered G3 MBs and three SHH MBs (labeled Ptch) that spontaneously arose in Ptch1+/−;Cdkn2c−/− mice (Figure 1D). This analysis yielded a total of 4,282 genes downregulated in G3 MB (Log2FC<−0.3) and 5,166 genes upregulated in G3 relative to SHH MBs. Myc and Miz1 were present at the promoters of 1,736 downregulated and 1,822 upregulated genes, suggesting that they are directly regulated by Myc or by Myc/Miz1 complexes in vivo. To test this hypothesis, we analyzed microarrays for an additional seven tumors expressing MycVD and for four more Myc/ΔPOZ tumors. Of the direct target genes repressed by Myc and Miz1, 713 genes were upregulated in both MycVD and Myc/ΔPOZ tumors, demonstrating that they are directly repressed by Myc/Miz1 complexes in G3 MB.
MycVD has a reduced affinity to Miz1, providing an explanation why target genes repressed by Myc/Miz1 are upregulated in MycVD-driven tumors (Figure S3D) (Herold et al., 2002). In contrast, the POZ domain of Miz1 is not part of the domain of Miz1 that interacts with Myc (Peukert et al., 1997). Consistent with these observations, Miz1ΔPOZ is not compromised in binding to Myc (Figure S3E), raising the question why Myc/Miz1 repressed genes are upregulated in Myc/ΔPOZ tumors. To explain this finding, we performed ChIP experiments and found that Miz1ΔPOZ bound as efficiently as wild-type Miz1 to promoters with cognate Miz1 binding sites (Figure S3F; see Vps27). In contrast, association of Miz1ΔPOZ to promoters that are bound together with Myc and that have E-boxes or non-consensus binding sites for both proteins was significantly compromised (Figure S3F). This finding is consistent with previous observations on the Cdkn1a promoter (Kosan et al., 2010) and argues that the POZ domain is required for formation of a stable chromatin-complex of Miz1 with Myc.
Both Myc and Miz1 are transcriptional activators that form a repressive complex upon binding to each other; hence the ratio of Myc to Miz1 bound to each promoter correlates with the direction of the transcriptional response to Myc in several tumor models (Walz et al., 2014) (Figure 3D). Consistently, the ratio of Myc/Miz1 tags at repressed target genes of Myc was around one and significantly higher than one at Myc-activated target genes (Figures 3E and 3F; p = 1.37×10−31, calculated by a Chi-squared test) suggesting that the ratio of Myc and Miz1 bound to promoters determines the transcriptional output of Myc in G3 MBs.
Consistent with this analysis, GSEA analysis showed that genes with a high Myc or Miz1 occupancy corresponded to canonical target genes of both proteins (Figures S3G-S3I). In contrast, genes with a tag ratio around 1 showed a highly significant overlap with sets of genes that are repressed in embryonic stem cells and with sets of target genes of the polycomb-repressive complex 2 (PRC2), supporting the notion that Myc/Miz1-mediated repression has a direct role in maintaining the “stemness” of G3 MB cells (Figure S3I).
Genes repressed by Myc/Miz1 complexes control ciliogenesis, differentiation, and survival
To better understand how Myc/Miz1-mediated repression promotes the development of G3 MBs, we functionally annotated the most strongly repressed genes. GO-term analyses showed a significant enrichment of genes encoding proteins of neuron projections, cell junctions, and cell motion (Figure 3G).
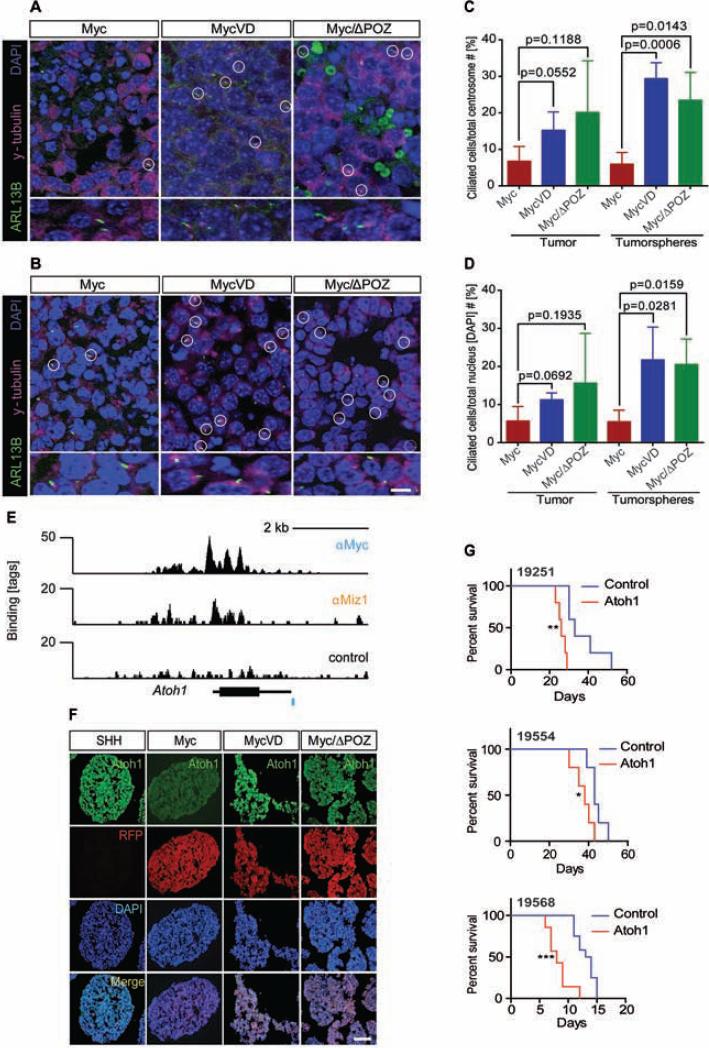
The predominant projections found in GNPs are primary cilia, which mediate signal transduction by several signaling pathways, including WNT and SHH (Goetz and Anderson, 2010). Primary cilia were previously identified in human MBs with activation of SHH or WNT signaling but not in G3 and G4 MBs (Han et al., 2009). Consistent with these findings, primary cilia were mostly absent in mouse G3 MBs tumor sections and tumorspheres (Figures 4A and 4B). In contrast, primary cilia were more frequently detected in MycVD and Myc/ΔPOZ tumor tissues (Figures 4A, and 4B) arguing that Myc/Miz1 complexes suppress ciliogenesis in G3 MBs. These results were quantified for ciliated cells over the total number of centrosomes and basal bodies in tumors and tumorspheres (Figure 4C) and ciliated cells over the total number of nuclei (Figure 4D).
Figure 4. MycVD and Myc/ΔPOZ tumors and tumorspheres express primary cilia which loss did not affect tumor onset or penetrance.
(A, B) Detection of primary cilia in tumor sections (A) and tumorspheres (B) from indicated tumors by immunofluorescence staining for Arl13b (green), to detect primary cilia, and γ-tubulin (purple), to identify basal bodies. DAPI (blue) was used to detect nuclei. Scale bar = 50 μm. (C, D) The numbers of the basal body (C) or nuclei (D) were used as a denominator to calculate the percentage of ciliated cells. p values were calculated by an unpaired two-tailed t-test. Data are represented as the mean ± SD. (E) ChIP-Seq analysis for Myc and Miz1 binding to the Atoh1 locus (black bar). A canonical E box-sequence is shown as a blue bar below the binding trace. (F) Immunofluorescence of RFP marked Myc, MycVD, and Myc/ΔPOZ tumorspheres with antibodies against Atoh1, scale bar = 50 μm. (G) Kaplan-Meier survival curves of mice transplanted with three individual G3 tumorsphere lines (# 19251, 19554, and 19568) infected with retroviruses empty vector control (blue) and encoding Atoh1 (red). Top panel, line #19251 median survival (ms) 33 days for control (n = 5) and 26 days for Atoh1 (n = 5). **, p = 0.0018 control versus Atoh1. Medium panel, line #19554, ms = 43 days for control (n = 5) and ms = 38 days for Atoh1 (n = 5). *, p = 0.0373 control versus Atoh1. Line #19568, ms = 13.5 days for control (n = 12) and ms = 8 days for Atoh1 (n = 7). ***, p = 0.0001 control versus Atoh1. See also Figure S4 and Table S1.
In addition, several of the genes that are most strongly repressed in Myc relative to MycVD and Myc/ΔPOZ tumors encode transcription factors implicated in neuronal differentiation and survival in the cerebellum (Table S1). These include, for example, Atoh1, a bHLH transcription factor that is expressed in proliferating but not post-mitotic cerebellar GNPs and in SHH MBs; Foxg1, which is required for survival of postmitotic cerebellar granule neurons (Dastidar et al., 2011); Neurog2, which regulates progenitor cell-cycle progression and Purkinje cell dendritogenesis in cerebellar development (Florio et al., 2012); and Otx1, which functions in establishing cerebellar cell identities (Foucher et al., 2006). ChIP-Seq analysis confirmed that both Myc and Miz1 directly bind to the promoter of Atoh1 in G3 MBs (Figure 4E). We confirmed by immunofluorescence that Atoh1 expression was suppressed in Myc MB compared to MycVD and Myc/ΔPOZ tumorspheres (Figure 4F).
We previously showed that Atoh1 overexpression did not transform GNPs purified from the cerebella of C57BL/6 mice but that it prevented their differentiation into granule neurons (Ayrault et al., 2010). We therefore tested whether repression of ciliogenesis or Atoh1 was sufficient to account for the role of Myc/Miz1-mediated repression in formation of G3 MBs. Notably, repression of cilia formation in MycVD tumors by knock-down of Ift88, an essential ciliogenic factor, did not affect proliferation of tumorspheres in vitro nor changed the tumor phenotype, the incidence, timing or penetrance of the MycVD tumors (Figures S4A-S4G). Conversely, enforced expression of Atoh1 in three independently derived mouse G3 MB tumorspheres accelerated rather than delayed tumor onset in each tumor line (Figure 4G) and did not converted these tumors to the SHH subtype, changed their pathology, or increased ciliogenesis (Figures S4H-S4K). Analysis of gene expression by RNA-Seq showed that ectopically expressed Atoh1 was functional in G3 MBs since it increased the expression of genes in the Notch and Hedgehog signaling pathways and the circadian clock (Figure S4L-S4M).
Collectively, these data argue that Myc/Miz1-mediated gene repression targets multiple cellular processes to promote tumorigenesis and to maintain an undifferentiated phenotype in G3 MBs.
Myc/Miz1-mediated repression discriminates G3 MB from other subgroups
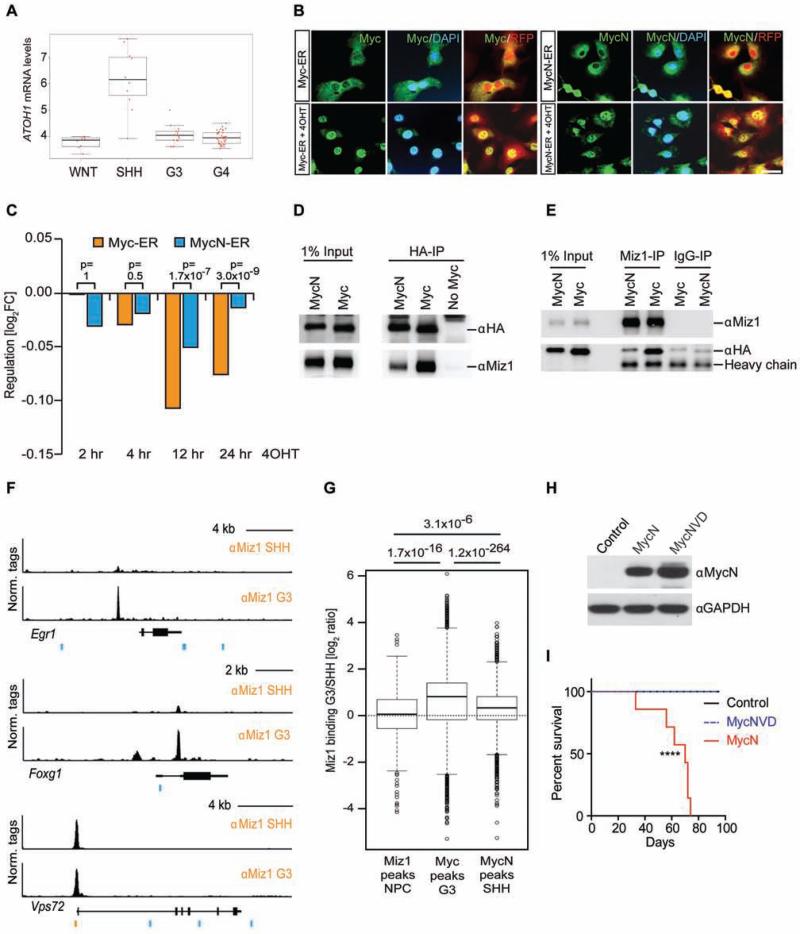
In mice and humans, a number of genes, including ATOH1, are highly expressed in SHH MBs but repressed in G3 MB (Figure 5A). We found that many of these genes were de-repressed in MycVD and Myc/ΔPOZ tumors. This suggested that Myc/Miz1-mediated repression might discriminate G3 MBs from SHH MBs that overexpress MycN rather than Myc. To test this hypothesis and to further confirm that Myc/Miz1 target genes are directly repressed by Myc, we enforced the expression of conditional Myc-ER or MycN-ER chimeras in GNPs purified from the cerebella of Trp53−/−;Cdkn2c−/− mice. In these cells Myc proteins translocated to the nucleus after 4-hydoxy-tamoxifen (4OHT) addition (Figure 5B). We observed a rapid drop in median expression of the genes that are directly repressed by Myc/Miz1 in G3MBs within the first 12 hr after activation of Myc-ER by adding 4OHT (Figure 5C). In contrast, we observed no consistent change in the expression of genes when MycN was activated instead of Myc in response to 4OHT (Figure 5C).
Figure 5. Myc/Miz1 mediated repression discriminate G3 MB from other subgroups.
(A) ATOH1 expression in WNT, SHH, G3, G4 MBs from St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project (PCGP) database. The black line represents the median value, the boxes reflect first and third quartile of the interquartile range, and jitter outliers represent the red dots. (B) Immunofluorescence of Myc or MycN (green) in GNPs cells from Trp53−/−;Cdkn2c−/− mice expressing a conditional Myc-ER or MycN-ER chimeric construct and RFP, before and after treatment with 4OHT. Co-expression of Myc and RFP (red and yellow). DAPI (blue) was used to detect nuclei, scale bar = 50 μm. (C) The median regulation of all genes (n = 713) that are bound by Myc and Miz1 in G3 MB and are repressed in G3 MB and re-expressed in MycVD and Myc/ΔPOZ tumors after 2 hr, 4 hr, 12 hr and 24 hr activation of Myc- and MycN-ER by 4OHT. p values (above each time point) depict if target genes are more repressed by Myc than by MycN (non-parametric 1-tailed 2-sample Wilcoxon signed rank test). (D, E) Co-IP of exogenous HA-tagged versions of Myc (MycN or Myc) and untagged Miz1 expressed in HEK293 cells by precipitation of Myc with HA antibody (D, αHA) or of Miz1 with a Miz1 antibody (E, αMiz1). (F) Binding of Miz1 at Vsp72, Foxg1, and Egr1 loci in G3 and SHH MB tumorspheres. Miz1 binding motifs (orange bars) and canonical Ebox-sequences (blue bars) are shown below the binding traces. (G) Box plot comparing Miz1 binding in Myc-driven G3 and SHH tumorspheres (log ratio) of Miz1 target genes in NPCs, Myc target genes in G3 tumors and MycN target genes in SHH tumorspheres. The black line indicates the median value, bottom and top of the boxes reflect first and third quartile, whiskers represent 1.5 interquartile range, and outliers are shown as dots (Tukey box plot). p values (shown at the top) are calculated by an unpaired two-tailed t-test. (H) Protein expression in GNPs from the cerebella of 7 days old Trp53−/−;Cdkn2c−/− mice infected or not with retroviruses encoding Myc or MycN and RFP. (I) Survival of mice transplanted with GNPs transduced with MSCV-IRES-GFP vector empty (control, black line, n = 5), encoding MycN (red line, n = 7) or MycNVD (dashed blue line, n=10). ****, p< 0.0001 control versus MycN. See also Figure S5.
Previous work showed that the interaction of MycN with Miz1 is weaker than that of Myc in two-hybrid assays (Peukert et al., 1997). Consistent with these observations, transiently expressed HA-tagged Myc efficiently co-precipitated with Miz1 whereas complex formation between HA-tagged MycN and Miz1 was much weaker (Figures 5D, 5E, and S5A). The interaction of MycN with Miz1 was also weaker than that of Myc when complex formation was assayed by GST-pull-down of in vitro translated proteins (Figure S5B). Taken together, the above results suggested that the differences between Myc and MycN in complex formation with Miz1 contributed to their propensity to form G3 and SHH MBs, respectively.
To further explore this possibility, we performed a series of ChIP-Seq experiments from mouse Myc/G3 and SHH tumorspheres that express MycN (Kawauchi et al., 2012). To compare different ChIP-Seq experiments, we used the overall occupancy by Miz1 at target sites with a cognate Miz1 binding sequence for normalization (see Vps72 trace in Figure 5F). Miz1 occupancy at E-box or non-consensus Myc binding sites was much weaker in tumorspheres from SHH MBs than G3 MBs (see Egr1 and Foxg1 trace in Figure 5F). Quantification of global binding showed that the overall Miz1 occupancy at sites bound by Myc in G3 MBs was much higher than occupancy of the same sites in SHH MB (Figures 5G, S5C). We were concerned that this might reflect the fact that at least some of the promoters that are bound by Myc in G3 MBs were not accessible in SHH MBs. As an important control, we therefore analyzed Miz1 occupancy at sites that are bound by MycN in SHH MB tumorspheres. Although not all of these are accessible in G3 MBs, median Miz1 occupancy at these promoters was higher in tumorspheres from Myc-driven G3 MBs than in SHH MBs (Figure 5G, S5C) arguing that the difference in Miz1 occupancy is due to differential recruitment by Myc and MycN.
To evaluate whether the weak Miz1 binding to MycN was relevant for the development of SHH MBs, we generated a MycNV394D mutant (referred as MycNVD thereafter). We confirmed that the V394D mutation further diminished the interaction of HA-tagged MycN with Miz1 (Figure S5D). GNPs purified from the cerebella of Trp53−/−;Cdkn2c−/− mice were infected with high titer retroviruses expressing MycNVD and GFP, sorted for GFP positive cells and injected into the cortices of CD1 nude recipient mice. Whereas MycN and MycNVD protein expression was comparable in GNPs before transplant (Figure 5H), none of the mice bearing GNPs expressing MycNVD developed tumors 99 days after transplantation (Figure 5I). This was in contrast to mice bearing GNPs expressing MycN that, as expected, developed tumors with complete penetrance between 30 and 75 days post-transplant (Figure 5I), arguing that the residual binding of MycN to Miz1 contributes to SHH MB development.
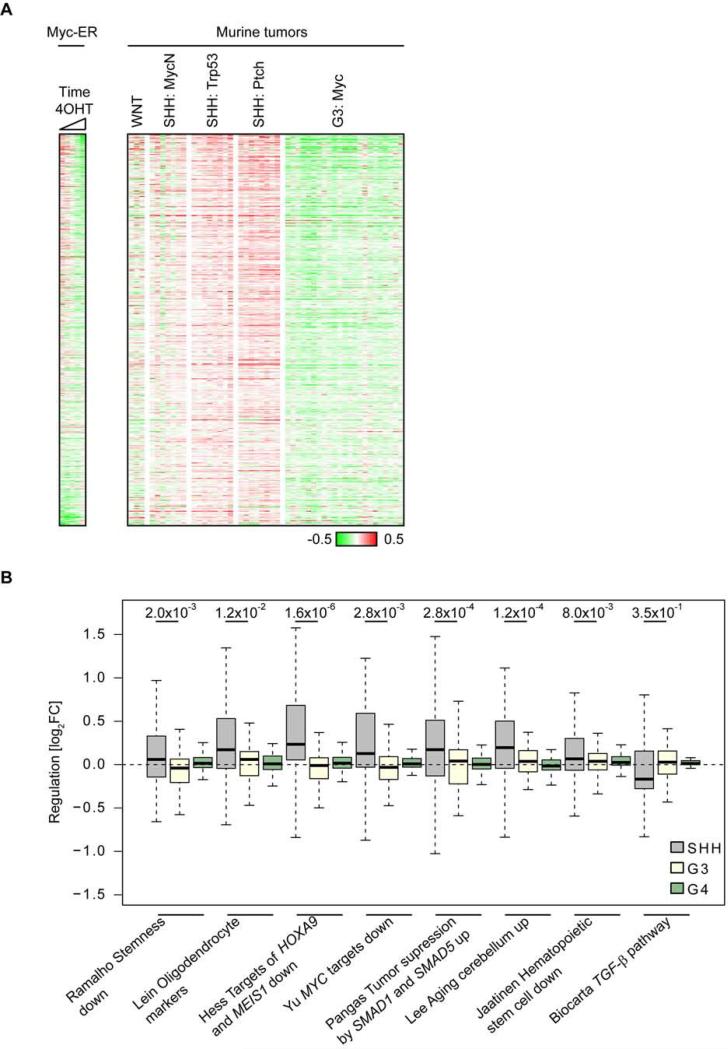
We explored publicly available gene expression profiles to test whether repression by Myc and Miz1 discriminates between different mouse and human MB subgroups. Consistent with the molecular analysis, genes that are repressed by Myc and Miz1 in response to Myc expression were consistently upregulated in GNPs and in three murine models of SHH MBs either engineered by enforced expression of MycN in GNPs from Trp53−/−;Cdkn2c−/− mice (MycN) (Kawauchi et al., 2012) or spontaneously arising from Trp53−/−;Cdkn2c−/− (Trp53) (Zindy et al., 2007) and Ptch1+/−;Cdkn2c−/− (Ptch) mice (Uziel et al., 2005) (Figure 6A). Furthermore, while the entire group sets of Myc/Miz1 repressed genes did not discriminate human G3 from SHH MBs (not shown), multiple individual gene sets of Myc/Miz1 repressed genes (see Figure 1D) discriminated human G3 from SHH MBs with high significance (Figure 6B). Expression of Myc/Miz1-repressed genes did not discriminate G3 from G4 MBs tumors. In addition, both human and mouse G3 MBs showed gene expression profiles indicating that multiple growth and proliferation related pathways are highly active in these tumors, reflecting their aggressive growth behavior (Figure S6). We concluded that Myc/Miz1-mediated gene repression is a defining feature of G3 MBs.
Figure 6. Comparison of global gene expression between murine MB subgroups.
(A) Gene expression profiles using publicly available data sets of the mouse WNT-subgroup (Ctnnb1+/lox(ex3);Blbp-Cre;Trp53−/−), of murine SHH MBs engineered by enforced expression of MycN in GNPs from Trp53−/−;Cdkn2c−/− mice (MycN) or spontaneously arisen from Trp53−/−;Cdkn2c−/− (Trp53), and Ptch1+/−;Cdkn2c−/− (Ptch), and of G3 MBs (Uziel et al., 2005). The heatmaps contain genes that are repressed by the Myc/Miz1 complex (bound by Myc and Miz1, repressed by Myc in tumors and re-expressed in MycVD- and Myc/ΔPOZ-genes: n=713) and are sorted according to fold expression after 2 hr, 4 hr, 12 hr and 24 hr induction of Myc-ER (left). (B) Expression data from different MB subgroups (GSE37382) were RMA normalized, median centered and the expression of each gene was averaged within the subgroups. The black line indicates the median value, bottom and top of the boxes reflect first and third quartile, whiskers represent 1.5 interquartile range, and outliers are not shown (Tukey box plot). p values were calculated using a paired two-tailed Wilcoxon signed-rank. See also Figure S6.
DISCUSSION
We here show that the interaction of Myc with Miz1 is critical for the development of G3 MBs and distinguishes G3 from other MB subgroups. Specifically, enforced expression of a Myc protein that has reduced binding to Miz1 (MycV394D) in GNPs from the cerebella of Trp53−/−;Cdkn2−/− mice or of a wild-type Myc in GNPs from Trp53 fl/fl;Miz1 ΔPOZ/POZ;Nestin-Cre mice induces tumors that develop later than Myc/G3 MBs and that display a pathology no longer characterized as MB and has no counterpart in humans.
Comparison of the transcriptional profiles of G3 MBs showed that, instead of their repression, target genes of the Myc/Miz1-complex are up-regulated in MycVD and Myc/ΔPOZ tumors that have reduced Myc/Miz1-complex activity. We previously observed that Myc induces G3 MBs while MycN generates SHH MBs after enforced expression in GNPs from the cerebella of Trp53−/−;Cdkn2c−/− mice (Kawauchi et al., 2012). Using co-precipitation assays and ChIP-Seq analysis, we now show that complexes of MycN with Miz1 are much less detectable than those formed between Myc and Miz1. Acute activation of Myc-ER in GNPs represses Myc/Miz1-target genes, whereas activation of MycN-ER does not. Furthermore, in both mouse and human, expression of Myc/Miz1-repressed genes set apart G3 MB from the other subgroups arguing that Myc/Miz1-mediated gene repression is a defining feature of G3 MBs. Consistent with this interpretation, genes repressed by Myc/Miz1 complexes include transcription factors known to play critical roles in GNPs development. For example, Atoh1, a bHLH transcription factor that is required for proliferation of GNPs and for SHH MB development (Roussel and Hatten, 2011; Zhao et al., 2008), was transcriptionally silenced in G3 MBs in a Miz1-dependent manner. This suggested that repression of Atoh1 expression by the Myc/Miz1 complex was likely required to convert SHH-dependent neuronal progenitors to G3 MB tumor cells that lack SHH dependence and are insensitive to Smoothened pathway inhibition (Kawauchi et al., 2012). We previously found that Atoh1 inhibits GNPs differentiation but did not induce tumorigenesis unless co-expressed with Gli1 (Ayrault et al., 2010). Genes repressed by Myc/Miz1 complexes are also required for ciliogenesis; indeed, previous work suggested that Miz1 participates in primary cilia function and SHH signaling (Lu et al., 2013). While mouse and human SHH or WNT tumors express primary cilia that are required for signaling and tumor formation, mouse and human Myc/G3 and G4 MBs do not (Han et al., 2009). MycVD and Myc/ΔPOZ tumors display neuroepithelial characteristics, express primary cilia, and fail to form either G3 or SHH tumors, suggesting that the loss of primary cilia is a specific characteristic of G3 MB.
Notably, neither enforced expression of Atoh1 in G3 MBs nor knock-down of Ift88, which is required for ciliogenesis, in MycVD tumors changed the tumors’ phenotype arguing that Myc/Miz1 represses additional factors and processes that are important for the genesis of G3 MBs. Myc/G3 tumors express many stem cell markers (Kawauchi et al., 2012), and in accordance, Myc/Miz1 complexes enhance the cellular self-renewal of neural progenitor cells (Kerosuo et al., 2008). In contrast, a predominant feature of cultured MycVD and Myc/ΔPOZ tumorspheres is an enhanced rate of apoptosis. Therefore, repression by Myc/Miz1 suppresses both apoptosis and differentiation in G3 MBs.
Myc activation not only promotes growth and proliferation but also induces metastasis. One of the characteristics of human G3 MBs with MYC amplification is their aggressiveness and ability to spread through the leptomeninges, making them difficult to treat (Gajjar and Robinson, 2014). We found that loss of Miz1 binding to Myc dramatically reduced migration and invasion of tumor cells, suggesting that inhibition of Miz1 by a small molecule inhibitor might suppress leptomeningeal spread of G3 MBs.
Myc and Miz1 form a ternary complex with Gfi-1 at the promoter of Cdkn2b, Cdkn1a and Cdkn1b (Liu et al., 2010). Remarkably, recent genomic analysis showed specific and mutually exclusive activation of Gfi-1 or Gfi-1B in G3 and G4 MBs by juxtaposition of their coding sequences to enhancer elements, including super-enhancers, stimulating their oncogenic activity (Northcott et al., 2014). Indeed, ectopic overexpression of Gfi-1 and Myc was sufficient to induce G3 MBs without the loss of Trp53, suggesting that Gfi-1 may collaborate with Myc and Miz1 to repress genes required for G3 MB development. Further analysis of repressed genes in this model is warranted to confirm this possibility.
We conclude that the interaction of Myc with Miz1 is required for G3 MB development and that active repression of genes by Myc/Miz1 complexes maintains the identity of G3 tumors. In contrast, MycN forms complexes with Miz1 less efficiently in the same cerebellar progenitor cells, and instead induces tumors of the SHH subgroup.
This differential ability of Myc and MycN to form complexes with Miz1 is central to distinguishing two members of the Myc gene family from one another, and reveals their distinct and as yet unappreciated biological functions.
EXPERIMENTAL PROCEDURES
A detailed description of the Experimental Procedures utilized in this work can be found in the Supplementary Experimental Procedures.
Animal Husbandry
Breeding and genotyping of mice were done as reported previously (Uziel et al., 2005). Mice were genotyped by genomic PCRs using specific primers (Supplementary Experimental Procedures). Percoll enriched GNPs from cerebella of postnatal (P) days P6-P7 pups were obtained from Trp53−/−;Cdkn2c−/− and Trp53fl/fl;Miz1ΔPOZ/POZ;Nestin-Cre pups, as previously described (Kawauchi et al., 2012; Wolf et al., 2013). Spontaneous SHH MBs occurred in Trp53−/−;Cdkn2c−/− and Ptch1+/−;Trp53−/− mice, described previously (Uziel et al., 2005; Zindy et al., 2007). CD-1 nu/nu mice (Charles River Laboratories, Wilmington, MA) were used as a recipient for cranial transplantation. All animal experiments were approved by and conducted in accordance with St. Jude Children's Research Hospital Animal Care and Use Committee guidelines, as required by the United States Animal Welfare Act and the National Institutes of Health's policy to ensure proper care and use of laboratory animals for research.
Cell Culture, Retrovirus Production and Infections
Purification of GNPs and other progenitor populations, retrovirus production, infections, and orthotopic transplants were performed as previously described (Ayrault et al., 2010). Plasmids used for retrovirus production were generated using the mouse stem cell virus (MSCV) backbone expressing the red fluorescent protein DsRed (RFP) or green fluorescent protein (GFP) downstream of the internal ribosomal entry site (IRES), pMSCV-IRES-RFP. Appropriate cDNAs were inserted downstream of the long terminal repeat (LTR) to generate MSCV-Myc-IRES-RFP, MSCV-MycV394D-IRES-RFP, MSCV-MycN-IRES-RFP, MSCV-MycNV394D-IRES-RFP, MSCV-Myc-ER-IRES-RFP, MSCV-MycN-ER-IRES-RFP, or MSCV-Atoh1-IRES-GFP. To knockdown Ift88, a sequence targeting TAGAAATTGATGAAGATGATA was cloned into a miR-30-based short hairpin cassette downstream of MSCV promoter. A non-targeting sequence, CCGGCTGAAGAGCCTGATCAA, was used as a control. Non-replicative retroviruses stocks were generated in 293T cells as described previously (Ayrault et al., 2010; Kawauchi et al., 2012). Infection efficiency was analyzed using fluorescence activated cell sorting (FACS) for RFP expression. In all cases, viral infection efficiency of GNPs ranged from 35 to 50%. GNPs were infected for 2 consecutive days, harvested and suspended with Matrigel (BD Bioscience, San Jose) before transplant into the cerebral cortices of CD-1 nu/nu recipient mice, as described previously (Ayrault et al., 2010; Kawauchi et al., 2012).
Tumor Harvest and Tumorsphere Assays
After transplant of 2 × 106 virus-infected progenitor cells per mouse in the cortices of CD1 nu/nu mice, animals were examined daily for symptoms of sickness (doming of the head, ataxia, and reduced activity). When mice became moribund, tumors were extracted and tumor cells purified with Percoll gradient, followed by extraction of RNAs and proteins, or grown in vitro as tumorspheres in neural stem cell culture conditions, as previously described (Kawauchi et al., 2012; Taylor et al., 2005). Briefly, cells from G3 MB/Myc, MycVD and Myc/ΔPOZ tumors were purified and plated on an ultra-low attachment dish. Human recombinant basic FGF and EGF (Peprotech, Rocky Hill, NJ, USA) were added to the culture medium every 3 days.
Histopathology, Immunohistochemistry, and Immunoblotting
For histopathology, samples were formalin-fixed, paraffin-embedded, and sectioned at 5 μm thickness. For each sample, a section was stained using a standard hematoxylin and eosin (H&E) protocol. For IHC of cultured spheres, samples were sectioned at 10 μm thickness after fixation with 2% PFA in PBS for 2 hours on ice, and cryo-protected with 30% sucrose in PBS, followed by staining without heat antigen retrieval. Primary antibodies are listed in Table S2. Fluorescently coupled secondary antibodies, including Alexa anti-rabbit 488, anti-mouse 488, and anti-rabbit 647 (Invitrogen, Grand Island, NY, USA) were used. Immunohistochemistry to detect cilia was performed on Myc, MycVD and Myc/ΔPOZ tumor sections and tumorspheres with antibodies to ARL13B and γ-tubulin (See Table S2) and with DAPI. Representative images of each sample/stain combination were captured and analyzed using Axiovision software (Carl Zeiss Microscopy, Thornwood, NY, USA). Immunoblotting was performed on tumor cells purified from mouse G3 MB (Myc), MycVD and Myc/ΔPOZ tumor, lysed in RIPA buffer and proteins separated on SDS/PAGE gels detected with primary antibodies against Myc, β-actin, and GAPDH (see Table S2), as previously described (Ayrault et al., 2010; Kawauchi et al., 2012).
Affymetrix Microarray Analysis
RNA from G3 MB/Myc, MycVD and Myc/ΔPOZ tumor cells was subjected to hybridization using Affymetrix Mouse Genechips HT430PM.
RNA-Sequencing Analysis
Three G3 MB tumorsphere lines (#19251, 19554, and 19568) were infected with retroviruses encoding Atoh1 followed by cranial implants into the cortices of recipient animals. Tumor cells were dissociated, purified by Percoll gradient and total RNA was extracted with TRIzol reagent (Life Technologies). Libraries for RNA-Seq samples were constructed following manufactory's instructions using the TruSeq Stranded Total RNA Kit (Illumina) and sequenced on an Illumina HiSeq 2500.
Co-Immunoprecipitations (Co-IPs)
Co-IPs were performed in HEK293 cells transfected with CMV driven plasmids encoding Miz1, HA-Myc and HA-MycN. Cells were harvested 48 h after transfection with polyethylenimine and lysed in lysis buffer (20 mM Hepes pH 7.9, 200 mM NaCl, 0.2% NP40, 0.5 mM EDTA, 10% glycerol). Myc was precipitated with αHA- (Y-11, Santa Cruz) and Miz1 was precipitated with antibody to Miz1, αMiz1- (10E2) coupled to magnetic beads (Life Technologies) for 6 h. After washing with lysis buffer, proteins were separated by PAGE, transferred on PVDV-membrane and immunostained with αHA- and αMiz1-antibodies.
ChIP and Parallel Sequencing
ChIPs (Chromatin IPs) from fluorescence sorted tumor cells or tumor cells expanded in culture (tumorspheres) were performed as described previously (Walz et al., 2014). Briefly, cells were fixated with 1% formaldehyde, incubated with glycine (50 mM final) and swelled. Afterwards, nuclei were resuspended in sonication buffer (10 mM Tris/HCl pH 7.5, 150 mM NaCl, 1% NP40, 1% DOC, 0.1% SDS, 1 mM EDTA) and DNA was fragmented to nucleosomal size using a Branson sonifier. Antibodies for Miz1 (10E2), Myc (N262, Santa Cruz) and MycN (B8.4.B, Santa Cruz) were coupled to magnetic beads and incubated with the chromatin. Precipitated material was eluted (input Chromatin was used as control), the crosslink was reverted and DNA was purified by chloroform/phenol extraction. Sequencing libraries were prepared with the ChIP-Seq Library Prep Master Mix Set (New England BioLabs) and adaptor-ligated DNA was size-selected (200 bp +/−25 bp) by agarose gel electrophoresis and purified with Qiagen gel extraction kit. DNA-fragments were amplified by PCR with indexed amplifications primers (New England BioLabs) and subjected to Illumina GAIIx sequencing according to the manufacturer's instructions.
ChIP-Sequencing Analysis
After base calling high quality PF-clusters were de-multiplexed into a single fastq-file for each individual sample and aligned to a precompiled murine reference genome (mm9) with BOWTIE v.0.12.7 (Langmead, 2010), primary tumor cells: “-- best - m 1 -v 0” options, tumorspheres: standard options). Mapped reads were used for subsequent peaks calling, visualization of binding profiles and heat maps. Peak calling of ChIP-Seq datasets (control: input samples) was performed with MACS v.1.4.2 (the -keep-dup parameter was adjusted depending on the ChIP enrichment at the highest peaks). For the data sets from tumor cells only peaks with more than 20 tags and a FDR < 1% were considered. Miz1 ChIP-Seq from G3- and SHH-tumorspheres were normalized by taking into account the average tag number in Miz1-peaks, present in NPCs (containing a Miz1 binding motif) to account for technical ChIP-to-ChIP-variance.
In vitro transcription and translation assays (IVT)
Experimental details for the in vitro interaction assays between Miz1 and Myc proteins are described in the Supplemental Experimental Procedures.
Supplementary Material
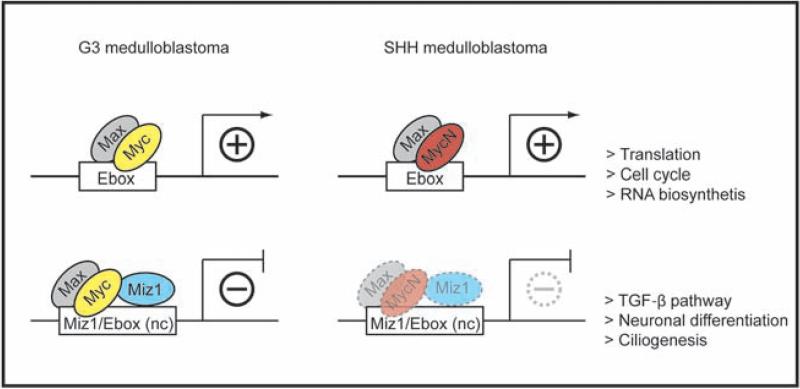
Figure 7. Schematic model of the Myc/Max/Miz1 transcriptional network in G3 and SHH MBs.
Both Myc/Max and MycN/Max complexes bind to E-boxes to activate transcription in G3 and SHH MBs, respectively. The Myc/Max/Miz1 complex also binds to non-canonical (“nc”) Myc/Max and Miz1 binding sites to repress transcription whereas the reduced interaction of MycN/Max with Miz1 attenuates gene repression. The lighter shades of the MycN/Max/Miz1 represent the weaker but significant interaction with Miz1.
HIGHLIGHTS.
Myc/Miz1 interaction is required for Group 3 medulloblastoma.
Myc and MycN differ in their interaction with Miz1.
Myc/Miz1 complexes suppress ciliogenesis in Group 3 medulloblastoma.
Myc/Miz1-repressed genes discriminate between SHH and Group 3 medulloblastomas.
SIGNIFICANCE.
Myc proteins are generally considered to be functionally interchangeable, and yet human medulloblastomas driven by Myc or MycN differ in their gene expression profiles, biological behavior and prognosis. Here, we show that target gene transactivation by Myc and MycN is similar in distinct mouse models of medulloblastoma, whereas both proteins differ in their ability to repress transcription via interactions with Miz1. In highly aggressive Myc-dependent G3 medulloblastomas, Myc/Miz1 complexes repress genes that are involved in neuronal differentiation, ciliogenesis, and the TGF-β pathway, maintaining a stem cell-like gene expression profile. Our data suggest that inhibition of Myc/Miz1-dependent repression may hold therapeutic promise specifically for G3 medulloblastomas.
ACKNOWLEDGMENTS
Authors gratefully acknowledge Dr. Charles J. Sherr for his critical review of the manuscript and all members of the Roussel/Sherr laboratory for helpful discussions and comments during the course of these experiments. We are indebted to the Flow Cytometry and Cell Sorting Shared Resource facility; Dr. Frederique Zindy and Sarah Robinson for managing the mouse colony; Melissa Johnson and Shantel Brown for cranial implants; Drs. Yannan Ouyang and Jennifer Peters for confocal imaging assistance. We thank Drs. Derek Persons and Satish Nandakumar for providing us the shRNA-Ift88 plasmid. This work was funded in part by the National Institutes of Health grants CA-096832 and CA-21765 to M.F.R., by grants from the Deutsche Forschungsgemeinschaft (222/12-1) to M.E., the German Excellence Initiative via the Graduate School of Life Sciences (University of Würzburg) to E.W., Cancer Center Development Funds CA021765 and the Sontag Foundation Distinguished Scientist Award to Y-G.H., the Sununu Fellowship to B.H.V., the Anderson Fellowship to D.K., and the American Lebanese Syrian Charities of St. Jude Children's Research Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
B.T.V., E.W., M.E., and M.F.R. designed and oversaw the project. B.T.V., E.W., D.K., and A.G. performed experiments and collected data. D.F., S.W., B.L.M., D.K., and B.T.V. analyzed Affymetrix microarray data. B.T.V., E.W., S.W., M.E., and M.F.R. analyzed and interpreted the data. B.T.V., Y.H.Y., and Y.G.H. analyzed primary cilia data. J.E.R. performed pathologic review. B.T.V., D.K., and B.L.M. harvested tumors and cultured tumorspheres. M.E., B.T.V., E.W., and M.F.R. wrote the manuscript.
REFERENCES
- Adhikary S, Peukert K, Karsunky H, Beuger V, Lutz W, Elsasser HP, Moroy T, Eilers M. Miz1 is required for early embryonic development during gastrulation. Mol. Cell Biol. 2003;23:7648–7657. doi: 10.1128/MCB.23.21.7648-7657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrault O, Zhao H, Zindy F, Qu C, Sherr CJ, Roussel MF. Atoh1 inhibits neuronal differentiation and collaborates with Gli1 to generate medulloblastoma-initiating cells. Cancer Res. 2010;70:5618–5627. doi: 10.1158/0008-5472.CAN-09-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacci-Sorrell M, McFerrin L, Eisenman RN. An overview of MYC and its interactome. Cold Spring Harb Perspect. Med. 2014;4:a014357. doi: 10.1101/cshperspect.a014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastidar SG, Landrieu PM, D'Mello SR. FoxG1 promotes the survival of postmitotic neurons. J. Neurosci. 2011;31:402–413. doi: 10.1523/JNEUROSCI.2897-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio M, Leto K, Muzio L, Tinterri A, Badaloni A, Croci L, Zordan P, Barili V, Albieri I, Guillemot F, et al. Neurogenin 2 regulates progenitor cell-cycle progression and Purkinje cell dendritogenesis in cerebellar development. Development. 2012;139:2308–2320. doi: 10.1242/dev.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher I, Mione M, Simeone A, Acampora D, Bally-Cuif L, Houart C. Differentiation of cerebellar cell identities in absence of Fgf signalling in zebrafish Otx morphants. Development. 2006;133:1891–1900. doi: 10.1242/dev.02352. [DOI] [PubMed] [Google Scholar]
- Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nat. Rev. Clin. Oncol. 2014;11:714–722. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat. Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S, Wanzel M, Beuger V, Frohme C, Beul D, Hillukkala T, Syvaoja J, Saluz HP, Haenel F, Eilers M. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol. Cell. 2002;10:509–521. doi: 10.1016/s1097-2765(02)00633-0. [DOI] [PubMed] [Google Scholar]
- Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C, Finkelstein D, Qu C, Pounds S, Ellison DW, et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell. 2012;21:168–180. doi: 10.1016/j.ccr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Kerosuo L, Piltti K, Fox H, Angers-Loustau A, Hayry V, Eilers M, Sariola H, Wartiovaara K. Myc increases self-renewal in neural progenitor cells through Miz-1. J. Cell Sci. 2008;121:3941–3950. doi: 10.1242/jcs.024802. [DOI] [PubMed] [Google Scholar]
- Kosan C, Saba I, Godmann M, Herold S, Herkert B, Eilers M, Moroy T. Transcription factor miz-1 is required to regulate interleukin-7 receptor signaling at early commitment stages of B cell differentiation. Immunity. 2010;33:917–928. doi: 10.1016/j.immuni.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Langmead B. Aligning short sequencing reads with Bowtie. Curr Protoc Bioinformatics. 2010 doi: 10.1002/0471250953.bi1107s32. Chapter 11, Unit 11 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Basu S, Qiu Y, Tang F, Dong F. A role of Miz-1 in Gfi-1-mediated transcriptional repression of CDKN1A. Oncogene. 2010;29:2843–2852. doi: 10.1038/onc.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Chen M, Ren XR, Wang J, Lyerly HK, Barak L, Chen W. Regulation of hedgehog signaling by Myc-interacting zinc finger protein 1, Miz1. PLoS One. 2013;8:e63353. doi: 10.1371/journal.pone.0063353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn BA, de Alboran IM, O'Hagan RC, Bronson R, Davidson L, DePinho RA, Alt FW. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000;14:1390–1399. [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Lee C, Zichner T, Stutz AM, Erkek S, Kawauchi D, Shih DJ, Hovestadt V, Zapatka M, Sturm D, et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature. 2014;511:428–434. doi: 10.1038/nature13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, Stutz AM, Korshunov A, Reimand J, Schumacher SE, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ, Witt H, Korshunov A, Read TA, Sun JL, et al. An animal model of MYC-driven medulloblastoma. Cancer Cell. 2012;21:155–167. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peukert K, Staller P, Schneider A, Carmichael G, Hanel F, Eilers M. An alternative pathway for gene regulation by Myc. EMBO J. 1997;16:5672–5686. doi: 10.1093/emboj/16.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel MF, Hatten ME. Cerebellum development and medulloblastoma. Curr. Top. Dev. Biol. 2011;94:235–282. doi: 10.1016/B978-0-12-380916-2.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel MF, Robinson GW. Role of MYC in Medulloblastoma. Cold Spring Harb. Perspect. Biol. 2013:5. doi: 10.1101/cshperspect.a014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat. Cell Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Moroy T, Bartek J, Massague J, Hanel F, Eilers M. Repression of p15INK4b expression by Myc through association with Miz-1. Nat. Cell Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Uziel T, Zindy F, Xie S, Lee Y, Forget A, Magdaleno S, Rehg JE, Calabrese C, Solecki D, Eberhart CG, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev. 2005;19:2656–2667. doi: 10.1101/gad.1368605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riggelen J, Muller J, Otto T, Beuger V, Yetil A, Choi PS, Kosan C, Moroy T, Felsher DW, Eilers M. The interaction between Myc and Miz1 is required to antagonize TGFbeta-dependent autocrine signaling during lymphoma formation and maintenance. Genes Dev. 2010;24:1281–1294. doi: 10.1101/gad.585710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz S, Lorenzin F, Morton J, Wiese KE, von Eyss B, Herold S, Rycak L, Dumay-Odelot H, Karim S, Bartkuhn M, et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature. 2014;511:483–487. doi: 10.1038/nature13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E, Gebhardt A, Kawauchi D, Walz S, von Eyss B, Wagner N, Renninger C, Krohne G, Asan E, Roussel MF, Eilers M. Miz1 is required to maintain autophagic flux. Nat. Commun. 2013;4:2535. doi: 10.1038/ncomms3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E, Lin CY, Eilers M, Levens DL. Taming of the beast: shaping Myc-dependent amplification. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ayrault O, Zindy F, Kim JH, Roussel MF. Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev. 2008;22:722–727. doi: 10.1101/gad.1636408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Knoepfler PS, Xie S, Sherr CJ, Eisenman RN, Roussel MF. NMyc and the cyclin-dependent kinase inhibitors p18Ink4c and p27Kip1 coordinately regulate cerebellar development. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11579–11583. doi: 10.1073/pnas.0604727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Uziel T, Ayrault O, Calabrese C, Valentine M, Rehg JE, Gilbertson RJ, Sherr CJ, Roussel MF. Genetic alterations in mouse medulloblastomas and generation of tumors de novo from primary cerebellar granule neuron precursors. Cancer Res. 2007;67:2676–2684. doi: 10.1158/0008-5472.CAN-06-3418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.