Abstract
The kidneys play a key role in the homeostasis of body water and electrolyte balance. Aquaporin-2 (AQP2) is the vasopressin-regulated water-channel protein expressed at the connecting tubule and collecting duct, and plays a key role in urine concentration and body-water homeostasis through short-term and long-term regulation of collecting duct water permeability. The signaling transduction pathways resulting in the AQP2 trafficking to the apical plasma membrane of the collecting duct principal cells, including AQP2 phosphorylation, RhoA phosphorylation, actin depolymerization, and calcium mobilization, and the changes of AQP2 abundance in water-balance disorders have been extensively studied. Dysregulation of AQP2 has been shown to be importantly associated with a number of clinical conditions characterized by body-water balance disturbances, including hereditary nephrogenic diabetes insipidus (NDI), lithium-induced NDI, electrolytes disturbance, acute and chronic renal failure, ureteral obstruction, nephrotic syndrome, congestive heart failure, and hepatic cirrhosis. Recent studies exploiting omics technology further demonstrated the comprehensive vasopressin signaling pathways in the collecting ducts. Taken together, these studies elucidate the underlying molecular mechanisms of body-water homeostasis and provide the basis for the treatment of body-water balance disorders.
Keywords: Aquaporins, Arginine vasopressin, Phosphorylation, Ubiquitination, Water–electrolyte balance
Introduction
The kidneys play a key role in the homeostasis of body water and electrolyte balance. Water is reabsorbed as urine passes through the renal tubules. Absorption in the renal tubule depends on the driving force for water reabsorption and osmotic equilibration of water across the tubular epithelium. Accordingly, transcellular water transport across the renal tubular epithelial cells is essential to the homeostasis of body-water balance. Approximately, 180 L/day of glomerular filtrate is produced in an adult human, and the majority of glomerular filtrate is constitutively reabsorbed by the proximal tubules and descending thin limbs of Henle′s loop, where aquaporin-1 (AQP1) is abundantly present at both the apical and basolateral membranes of the epithelia. The ascending thin limbs and thick limbs are relatively impermeable to water and they deliver the tubular fluid into the distal convoluted tubules, connecting tubule segments, and collecting ducts. In particular, the collecting ducts are importantly involved in the regulation of body-water balance, because vasopressin-regulated water reabsorption occurs in this segment. Basal epithelial water permeability in the collecting duct principal cells is low, but water permeability can increase to very high levels, when principal cells are stimulated by arginine vasopressin (AVP, also known as antidiuretic hormone).
Although water can slowly diffuse through lipid bilayers and all biomembranes have some degree of water permeability, water channels have to exist in the cells having high water permeability, such as red blood cells and renal tubular epithelial cells. The discovery of the first water-channel protein (AQP1) provided an answer to the important biophysical question of how water specifically and rapidly crosses biomembranes [1], [2], [3]. Moreover, complementary DNAs for many of the important transporters localized at the renal tubules were cloned and sequenced [4]. Thirteen mammalian AQPs are now known [5], [6], [7], and they can be classified into three major subtypes, which are mainly determined by their transport capabilities: (1) the classical AQPs (AQP1, AQP2, AQP4, and AQP5), which are water-selective channels transporting only water; (2) aquaglyceroporins (AQP3, AQP7, AQP9, and AQP10), which are permeated by small uncharged molecules in addition to water; and (3) unorthodox AQPs (AQP6, AQP8, AQP11, and AQP12), whose function is currently being elucidated. Of the known AQPs, eight AQPs (AQP1, AQP2, AQP3, AQP4, AQP6, AQP7, AQP8, and AQP11) are expressed in the mammalian kidney. In the present review, we will focus on the regulation of AQP2, which is the vasopressin-regulated water-channel protein expressed at the connecting tubule and collecting duct, and plays a key role in urine concentration and body-water homeostasis.
Expression of AQP2 in the kidney collecting duct
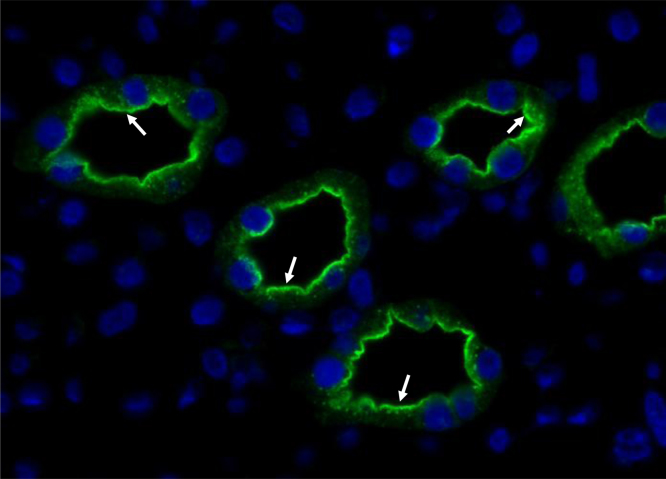
AQP2 is abundantly expressed at the apical plasma membrane and subapical vesicles in the principal cells of the kidney collecting duct (Fig. 1) and less abundantly expressed in the connecting tubules [8]. In addition, some AQP2 immunolabeling has also been found to be associated with the basolateral plasma membrane [9], [10]. AQP2 is the target protein for vasopressin-regulated water permeability in the collecting ducts [11], [12], [13], [14]. This finding was established by the studies revealing a direct correlation between AQP2 expression at the apical plasma membrane and collecting duct water permeability in isolated tubule studies in response to vasopressin stimulation [12] and studies demonstrating that humans with mutations in the AQP2 gene [15], [16] or rats with profound nephrogenic diabetes insipidus (NDI) [17], [18], [19] exhibited massive polyuria and impaired urinary concentrating capacity. Moreover, a severe urinary concentrating defect and postnatal death were directly observed in AQP2 gene-deficient mice [20]. This indicates that AQP2 plays an essential role in renal tubular water reabsorption in both the connecting tubule and the collecting ducts. Consistent with this, dysregulation of AQP2 is importantly associated with a number of clinical conditions exhibiting body-water balance disturbance, including hereditary NDI, lithium-induced NDI, electrolytes disturbance, acute and chronic renal failure, ureteral obstruction, nephrotic syndrome, congestive heart failure, and hepatic cirrhosis [16], [21], [22], [23], [24].
Figure 1.
Immunofluorescence microscopy of AQP2 in the inner medullary collecting duct of rat kidney. The AQP2 is localized at the apical plasma membrane and intracellular vesicles in the inner medullary collecting duct cells, indicated by arrows. AQP2, aquaporin-2.
Regulation of renal AQP2 by vasopressin
The signaling transduction pathways resulting in the AQP2 trafficking to the apical plasma membrane of the collecting duct principal cells and the changes to AQP2 abundance during times of water-balance disorders have been extensively studied (Table 1). AQP2 plays a key role in both short-term regulation and long-term adaptation of collecting duct water permeability [12], [14], [25], [26], [27], [28], [29]. Short-term regulation is the process by which vasopressin rapidly increases water permeability of the collecting duct principal cells by stimulating vasopressin V2 receptor (V2R) in the basolateral plasma membrane and translocation of AQP2 from intracellular vesicles to the apical plasma membrane. This response was measured within 5–30 minutes after increasing the peritubular vasopressin concentration [30], [31]. Long-term adaptation of collecting duct water permeability is seen when circulating vasopressin levels are increased over a period of hours to days, resulting in an increase of the AQP2 abundance per cell in the collecting ducts [25], [27], [28]. This process allows urine concentration and is essential for water-balance homeostasis. In addition, recent studies demonstrated that ubiquitination and subsequent proteasomal and/or lysosomal degradation of AQP2 could play a critical role in the regulation of AQP2 abundance [32], [33], [34]. The degradation pathways, therefore, balance the abundance of AQP2.
Table 1.
Intracellular signaling pathways for AQP2 trafficking or endocytosis
| Trafficking |
| cAMP/PKA signaling pathway [27], [40] |
| Intracellular calcium (Ca2+) mobilization (calcium–calmodulin-mediated myosin activation) [78] |
| PI3K-dependent activation of Akt [87] |
| AS160 Phosphorylation [73] |
| RhoA-dependent cytoskeletal dynamics [88] |
| Endocytosis |
| Clathrin-mediated endocytosis [89] |
| Ubiquitination of AQP2 C-terminus at K270 [33], [34] |
| PGE2, dopamine [52], [67] |
AQP2, aquaporin-2; cAMP, cyclic adenosine monophosphate; PGE2, prostaglandin E2; PKA, protein kinase A.
Vasopressin-induced AQP2 trafficking
An increase in blood osmolality and/or a decrease in blood volume trigger the neurohypophyseal release of vasopressin [35]. Isolated perfused renal collecting ducts demonstrated that AVP induces a rapid increase in the osmotic water permeability of the epithelium. Kinetic studies in isolated perfused inner medullary collecting ducts (IMCDs) revealed an increase in osmotic water permeability after only 40 seconds of incubation at 37 °C, and half of the maximal water permeability was reached within 10 minutes [31]. The increase in water permeability of the collecting duct epithelium is a result of translocation of AQP2 from subapical vesicles to the apical plasma membrane [12], [25]. In the absence of AVP, most of the AQP2 resides in intracellular vesicles thought to be recycling endosomes [36]. This was demonstrated by the findings of colocalization of AQP2 and Rab11 protein, a marker of apical recycling endosomes [36]. By contrast, immunoelectron microscopy revealed that AVP stimulation resulted in a fivefold increase in the appearance of AQP2 immunogold particles in the apical plasma membrane accompanied by a markedly decreased immunogold labeling of intracellular AQP2 [12]. This redistribution was associated with an increase in osmotic water permeability of similar magnitude [12]. These findings were reproduced in vivo by administrating vasopressin in rats, which also caused translocation of AQP2 to the apical plasma membrane of collecting duct principal cells [37] or vice versa upon withdrawing vasopressin stimulation [34].
The vasopressin signaling network between vasopressin stimulation and AQP2 trafficking to the apical plasma membrane has been identified [38]. Insertion of AQP2 at the apical plasma membrane is induced by vasopressin binding to the V2R expressed at the basolateral plasma membrane of the collecting duct principal cells. This activates G proteins, which stimulate adenylyl cyclase, resulting in increased intracellular cyclic adenosine monophosphate (cAMP) concentration and activation of protein kinase A (PKA). AQP2 contains a PKA phosphorylation consensus site at serine 256, and phosphorylation of the serine 256 in three of four AQP2 monomers in an AQP2 heterotetramer is involved in the regulated translocation of AQP2 to the apical plasma membrane [39], [40]. A number of proteins have been demonstrated to be involved in the cAMP-dependent AQP2 trafficking, such as PKA-anchoring proteins (AKAPs), phosphodiesterases (PDEs), cytoskeletal components (F-actin and microtubules), small guanosine triphosphatases (GTPases) of the Rho family, motor proteins transporting AQP2-bearing vesicles, vesicle-targeting receptors (soluble N-ethylmaleimide-sensitive factor attachment protein receptor or SNAREs), and 70-kDa heat-shock protein [41], [42].
AQP2 phosphorylation in vasopressin-stimulated AQP2 translocation to the plasma membrane
V2R-mediated cAMP/PKA signaling has been demonstrated to be one of the principal pathways to induce AQP2 trafficking and expression [22], [40]. Stimulation of V2R by AVP at the basolateral plasma membrane of the collecting duct principal cells triggers activation of Gsα-mediated adenylyl cyclase activity [43]. Sequentially, increased intracellular cAMP concentration and PKA activation result in recruitment of PKA to AQP2-containing vesicles by AKAPs [44]. In fact, AKAP18 delta has been shown to colocalize with AQP2 in intracellular vesicles [45]. In addition, rolipram-mediated inhibition of the cAMP-specific PDE4D increases AKAP-tethered PKA activity in AQP2-bearing vesicles and enhances AQP2 trafficking [46]. This finding suggested that a compartmentalized cAMP-dependent signal transduction pathway consisting of anchored PDE4D, AKAP18 delta, and PKA plays a role in AQP2 trafficking [47].
AQP2 contains a consensus site for PKA phosphorylation (RRQS) in the cytoplasmic COOH terminus at seine 256 (S256), which has been shown to be critical for vasopressin-induced cell-surface accumulation of AQP2 [48], [49]. This has been demonstrated by mutational analysis. The AQP2-S256A mutant cannot be phosphorylated by PKA and it does not traffic to the plasma membrane in response to cAMP-elevating agents [40], [50]. By contrast, the AQP2-S256D mutant, mimicking phosphorylation, resides in the plasma membrane, independent of cAMP level [50]. Interestingly, immunoelectron microscopy revealed that phosphorylated AQP2 (at serine 256) is localized in both the plasma membrane and intracellular vesicles [51], suggesting that even in low-circulating vasopressin states it is constitutively phosphorylated, and/or the phosphorylation of AQP2 at serine 256 per se is not sufficient to translocate AQP2 to the plasma membrane or to maintain AQP2 at the plasma membrane. In Madin-Darby Canine Kidney (MDCK) cells expressing AQP2-S256D, its internalization was induced by treatment of the PKA inhibitor H89 [52]. This finding suggested that PKA-dependent phosphorylation of other regulatory proteins could also be involved in the regulation of AQP2 trafficking or maintaining AQP2 in the plasma membrane. Moreover, dopamine and prostaglandin E2 (PGE2) cause internalization of the AQP2-S256D mutant [52].
Recently, phosphoproteomics studies have revealed that in addition to S256, AQP2 is further phosphorylated on residues S261, S264, and S269 [53] in response to AVP stimulation. Immunoelectron microscopy demonstrated that these phosphorylated forms of AQP2 are localized to different intracellular compartments [54], [55]. The precise role that these additional phosphorylation sites play remains undefined [55]. Although phosphorylation of AQP2 at S256 is important in AQP2 trafficking, it remains unclear as to how the phosphorylated AQP2 actually causes intracellular trafficking. One possibility is that phosphorylation of AQP2 directly influences an interaction between AQP2-containing vesicles and the cell cytoskeleton, microtubules, or accessory crosslinking proteins. Indeed, S256 is important for a direct interaction of AQP2 with 70-kDa heat-shock protein and, ultimately, the AQP2 trafficking [41]. Moreover, a recent study revealed that forskolin-induced AQP2 phosphorylation (S256) was not significantly induced in the mpkCCD cells with small interfering RNA (siRNA)-directed knockdown of 70-kDa heat-shock protein [42]. Alternatively, phosphorylation might attenuate AQP2 endocytosis, leading to an accumulation at the cell surface [56].
Retrieval of AQP2 from the plasma membrane and possible role of ubiquitination in AQP2 degradation
In contrast to the relatively well-established pathways involved in vasopressin-regulated AQP2 trafficking and de novo synthesis of AQP2, retrieval of AQP2 from the plasma membrane and intracellular degradation of the proteins including AQP2 are poorly understood. During endocytosis of AQP2, it accumulates in clathrin-coated pits prior to being internalized through a clathrin-mediated process [57], [58], [59]. Internalization of AQP2 is likely to be independent on its phosphorylation state. For example, both PGE2 and dopamine can promote removal of AQP2 from the cell membrane despite the phosphorylation state of AQP2 [52], [60]. Furthermore, PKC activation mediates AQP2 endocytosis independent of phosphorylation state [50]. Once internalized, AQP2 is retrieved to EEA1-positive early endosomes through a phosphatidylinositol-3-kinase (PI3K)-dependent mechanism prior to being transferred to Rab11-positive recycling vesicles [61]. The actin filament is involved in this process, as the disruption of actin filaments results in the accumulation of AQP2 in the EEA1-positive early endosomes [62]. Following AVP restimulation, AQP2 may be recycled to the apical plasma membrane, a process that is thought to involve the protein Rab11 [62], [63]. Interestingly, despite the disruption of microtubules, AQP2 in Rab11-positive vesicles respond to AVP stimulation, resulting in AQP2 trafficking [36], [62]. The Rab family of proteins are known to play an important role in intracellular vesicle trafficking. In the IMCD cells of rat kidney, proteomic analysis of AQP2-expressing vesicles previously revealed the expression of a number of Rab proteins including Rab2, Rab10, and Rab14 [63]. Moreover, transcriptome analysis of rat kidney IMCD revealed a number of transcripts corresponding to Rab proteins including Rab2, Rab8A, Rab8B, Rab10, and Rab14 [64]. In addition, immunogold electron microscopy showed that Rab5, Rab7, and Rab11 are present in AQP2-immunoisolated vesicles and an immunoblot analysis also showed that Rab4, Rab5, Rab7, and Rab11 are present in the AQP2-bearing vesicles [63]. Among them, Rab11, a marker of apical recycling endosomes, is known to be associated with the AQP2-storage compartment [65].
Ubiquitination is likely to be important for AQP2 endocytosis [33]. AQP2 is polyubiquitinated at the plasma membrane on a single residue (K270), resulting in internalization of AQP2, transport to multivesicular bodies (MVBs), and subsequent proteasomal degradation. Consistent with this, either MG132 (a specific proteasome inhibitor) or chloroquine (a blocker of the lysosomal pathway of protein degradation) treatment in primary cultured IMCD cells significantly reduced AQP2 degradation [34], indicating that ubiquitination and subsequent proteasomal and/or lysosomal degradation of AQP2 plays a critical role in the regulation of AQP2 abundance. A proportion of AQP2 that is internalized to MVBs can be excreted into the urine as exosomes [66]. A recent study demonstrated a profile of genes and proteins of E3 ubiquitin-protein ligases (E3s) in rat kidney, which could be involved in the intracellular degradation of proteins associated with vasopressin-induced urine concentration [34]. Both in vivo and in vitro results suggest that the selected three E3s, BRE1B, NEDD4, and CUL5, could play a potential role in urine concentration. For example, (1) immunoblots revealed an increase in NEDD4 and CUL5 during dDAVP withdrawal after long-term stimulation or an increase in NEDD4 and BRE1B in rats with lithium-induced NDI; and (2) siRNA-mediated gene silencing of NEDD4 or CUL5 significantly decreased the rate of AQP2 degradation in mpkCCDc14 cells [34].
Other signal transduction pathways involved in vasopressin regulation of AQP2 trafficking
Other signal transduction pathways including prostaglandins, angiotensin II, aldosterone, PI3K/Akt pathways, cytoskeleton, intracellular Ca2+ concentration, and vesicle-targeting receptors have been described in previous studies and reviews [22], [67], [68], [69]. Prostaglandins are associated with retrieval of AQP2 from the plasma membrane, but this appears to be independent of AQP2 phosphorylation by PKA [67]. Angiotensin II has a crosstalk to the vasopressin-induced signaling transduction pathways for AQP2 trafficking/expression [70], [71], [72]. Phosphorylation of other cytoplasmic or vesicular regulatory proteins may also be involved. These issues remain to be investigated directly. The PI3K/Akt pathways are activated in response to AVP stimulation and are also importantly involved in the regulation of AQP2 trafficking through Rab GTPase activity of AS160 [36], [73]. The cytoskeleton has been known to be involved in the AQP2 trafficking in kidney collecting duct [74]. In particular, a microtubular network has been implicated in this process, because chemical disruption of microtubules inhibits the increase in permeability in both the toad bladder and the mammalian collecting duct [75], [76]. The intracellular Ca2+ concentration has been shown to increase upon stimulation of isolated perfused rat IMCDs with vasopressin or dDAVP [77]. These observations have been followed by a number of studies concerning the role of the Ca2+ concentration in the vasopressin-induced increase in water permeability [78]. The mechanism by which AQP2 vesicles are targeted to the apical plasma membrane and the mechanism by which cAMP controls docking and fusion of vesicles has been investigated [79], [80]. Vesicle-targeting receptors (often referred to as SNAREs) are believed to induce specific interaction of vesicles with membrane sites. Vesicle-targeting receptors chiefly associated with translocating vesicles are known as VAMPs (vesicle-associated membrane proteins, also referred to as synaptobrevins) and synaptotagmins. Two other families of membrane proteins are believed to serve as receptors in target membranes, namely the syntaxins and SNAP-25 homologs. Several of these SNAREs have been found in the renal collecting duct [63], [81], [82], [83], [84], [85], [86]. Although numerous vesicle docking and fusion proteins are associated with AQP2, their importance remains undefined.
Conflict of interest
The authors have declared that no competing interests exist.
Acknowledgments
This study was supported by a National Research Foundation Grant (Grant No. 2010-0019393) funded by the Ministry of Education, Science and Technology (MEST), Korea, and the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Korea (Grant No. A111345).
References
- 1.Preston G.M., Carroll T.P., Guggino W.B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 2.Preston G.M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith B.L., Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991;266:6407–6415. [PubMed] [Google Scholar]
- 4.Knepper M.A., Masilamani S. Targeted proteomics in the kidney using ensembles of antibodies. Acta Physiol Scand. 2001;173:11–21. doi: 10.1046/j.1365-201X.2001.00880.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujiyoshi Y., Mitsuoka K. de Groot BL, Philippsen A, Grubmüller H, Agre P, Engel A: Structure and function of water channels. Curr Opin Struct Biol. 2002;12:509–515. doi: 10.1016/s0959-440x(02)00355-x. [DOI] [PubMed] [Google Scholar]
- 6.Rojek A., Praetorius J., Frøkiaer J., Nielsen S., Fenton R.A. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70:301–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen S. Renal aquaporins: an overview. BJU Int. 2002;90(Suppl 3):1–6. doi: 10.1046/j.1464-410x.90.s3.1.x. [DOI] [PubMed] [Google Scholar]
- 8.Coleman R.A., Wu D.C., Liu J., Wade J.B. Expression of aquaporins in the renal connecting tubule. Am J Physiol Renal Physiol. 2000;279:F874–F883. doi: 10.1152/ajprenal.2000.279.5.F874. [DOI] [PubMed] [Google Scholar]
- 9.van Balkom B.W., van Raak M., Breton S., Pastor-Soler N., Bouley R., van der Sluijs P., Brown D., Deen P.M. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem. 2003;278:1101–1107. doi: 10.1074/jbc.M207339200. [DOI] [PubMed] [Google Scholar]
- 10.Seigneux S., Nielsen J., Olesen E.T., Dimke H., Kwon T.H., Frøkiaer J., Nielsen S. Long-term aldosterone treatment induces decreased apical but increased basolateral expression of AQP2 in CCD of rat kidney. Am J Physiol Renal Physiol. 2007;293:F87–F99. doi: 10.1152/ajprenal.00431.2006. (de) [DOI] [PubMed] [Google Scholar]
- 11.Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen S., Chou C.L., Marples D., Christensen E.I., Kishore B.K., Knepper M.A. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabolić I., Katsura T., Verbavatz J.M., Brown D. The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol. 1995;143:165–175. doi: 10.1007/BF00233445. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T., Sasaki S., Fushimi K., Ishibashi K., Yaoita E., Kawasaki K., Marumo F., Kihara I. Vasopressin increases AQP-CD water channel in apical membrane of collecting duct cells in Brattleboro rats. Am J Physiol. 1995;268:C1546–C1551. doi: 10.1152/ajpcell.1995.268.6.C1546. [DOI] [PubMed] [Google Scholar]
- 15.Deen P.M., Verdijk M.A., Knoers N.V., Wieringa B., Monnens L.A., van Os C.H., van Oost B.A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 16.Loonen A.J., Knoers N.V., van Os C.H., Deen P.M. Aquaporin 2 mutations in nephrogenic diabetes insipidus. Semin Nephrol. 2008;28:252–265. doi: 10.1016/j.semnephrol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Christensen B.M., Kim Y.H., Kwon T.H., Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291:F39–F48. doi: 10.1152/ajprenal.00383.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kwon T.H., Laursen U.H., Marples D., Maunsbach A.B., Knepper M.A., Frokiaer J., Nielsen S. Altered expression of renal AQPs and Na+ transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol. 2000;279:F552–F564. doi: 10.1152/ajprenal.2000.279.3.F552. [DOI] [PubMed] [Google Scholar]
- 19.Marples D., Christensen S., Christensen E.I., Ottosen P.D., Nielsen S. Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest. 1995;95:1838–1845. doi: 10.1172/JCI117863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojek A., Füchtbauer E.M., Kwon T.H., Frøkiaer J., Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci USA. 2006;103:6037–6042. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon T.H., Hager H., Nejsum L.N., Andersen M.L., Frøkiaer J., Nielsen S. Physiology and pathophysiology of renal aquaporins. Semin Nephrol. 2001;21:231–238. doi: 10.1053/snep.2001.21647. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen S., Frøkiaer J., Marples D., Kwon T.H., Agre P., Knepper M.A. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 23.Schrier R.W. Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin Nephrol. 2008;28:289–296. doi: 10.1016/j.semnephrol.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen S., Kwon T.H., Frøkiaer J., Agre P. Regulation and dysregulation of aquaporins in water balance disorders. J Intern Med. 2007;261:53–64. doi: 10.1111/j.1365-2796.2006.01760.x. [DOI] [PubMed] [Google Scholar]
- 25.DiGiovanni S.R., Nielsen S., Christensen E.I., Knepper M.A. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ecelbarger C.A., Terris J., Frindt G., Echevarria M., Marples D., Nielsen S., Knepper M.A. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 27.Lankford S.P., Chou C.L., Terada Y., Wall S.M., Wade J.B., Knepper M.A. Regulation of collecting duct water permeability independent of cAMP-mediated AVP response. Am J Physiol. 1991;261:F554–F566. doi: 10.1152/ajprenal.1991.261.3.F554. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S., DiGiovanni S.R., Christensen E.I., Knepper M.A., Harris H.W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terris J., Ecelbarger C.A., Nielsen S., Knepper M.A. Long-term regulation of four renal aquaporins in rats. Am J Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- 30.Kuwahara M., Verkman A.S. Pre-steady-state analysis of the turn-on and turn-off of water permeability in the kidney collecting tubule. J Membr Biol. 1989;110:57–65. doi: 10.1007/BF01870993. [DOI] [PubMed] [Google Scholar]
- 31.Wall S.M., Han J.S., Chou C.L., Knepper M.A. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol. 1992;262:F989–F998. doi: 10.1152/ajprenal.1992.262.6.F989. [DOI] [PubMed] [Google Scholar]
- 32.Tamma G., Robben J.H., Trimpert C., Boone M., Deen P.M. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am J Physiol Cell Physiol. 2011;300:C636–C646. doi: 10.1152/ajpcell.00433.2009. [DOI] [PubMed] [Google Scholar]
- 33.Kamsteeg E.J., Hendriks G., Boone M., Konings I.B., Oorschot V., van der Sluijs P., Klumperman J., Deen P.M. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci USA. 2006;103:18344–18349. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y.J., Lee J.E., Choi H.J., Lim J.S., Jung H.J., Baek M.C., Frøkiær J., Nielsen S., Kwon T.H. E3 ubiquitin-protein ligases in rat kidney collecting duct: response to vasopressin stimulation and withdrawal. Am J Physiol Renal Physiol. 2011;301:F883–F896. doi: 10.1152/ajprenal.00117.2011. [DOI] [PubMed] [Google Scholar]
- 35.Dunn F.L., Brennan T.J., Nelson A.E., Robertson G.L. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973;52:3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vossenkämper A., Nedvetsky P.I., Wiesner B., Furkert J., Rosenthal W., Klussmann E. Microtubules are needed for the perinuclear positioning of aquaporin-2 after its endocytic retrieval in renal principal cells. Am J Physiol Cell Physiol. 2007;293:C1129–C1138. doi: 10.1152/ajpcell.00628.2006. [DOI] [PubMed] [Google Scholar]
- 37.Marples D., Knepper M.A., Christensen E.I., Nielsen S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol. 1995;269:C655–C664. doi: 10.1152/ajpcell.1995.269.3.C655. [DOI] [PubMed] [Google Scholar]
- 38.Knepper M.A. Systems biology in physiology: the vasopressin signaling network in kidney. Am J Physiol Cell Physiol. 2012;303:C1115–C1124. doi: 10.1152/ajpcell.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moeller H.B., MacAulay N., Knepper M.A., Fenton R.A. Role of multiple phosphorylation sites in the COOH-terminal tail of aquaporin-2 for water transport: evidence against channel gating. Am J Physiol Renal Physiol. 2009;296:F649–F657. doi: 10.1152/ajprenal.90682.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsura T., Gustafson C.E., Ausiello D.A., Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol. 1997;272:F817–F822. [PubMed] [Google Scholar]
- 41.Lu H.A., Sun T.X., Matsuzaki T., Yi X.H., Eswara J., Bouley R., McKee M., Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem. 2007;282:28721–28732. doi: 10.1074/jbc.M611101200. [DOI] [PubMed] [Google Scholar]
- 42.Park E.J., Lim J.S., Jung H.J., Kim E., Han K.H., Kwon T.H. The role of 70-kDa heat shock protein in dDAVP-induced AQP2 trafficking in kidney collecting duct cells. Am J Physiol Renal Physiol. 2013;304:F958–F971. doi: 10.1152/ajprenal.00469.2012. [DOI] [PubMed] [Google Scholar]
- 43.Rieg T., Tang T., Murray F., Schroth J., Insel P.A., Fenton R.A., Hammond H.K., Vallon V. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol. 2010;21:2059–2068. doi: 10.1681/ASN.2010040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klussmann E., Maric K., Wiesner B., Beyermann M., Rosenthal W. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem. 1999;274:4934–4938. doi: 10.1074/jbc.274.8.4934. [DOI] [PubMed] [Google Scholar]
- 45.Henn V., Edemir B., Stefan E., Wiesner B., Lorenz D., Theilig F., Schmitt R., Vossebein L., Tamma G., Beyermann M., Krause E., Herberg F.W., Valenti G., Bachmann S., Rosenthal W., Klussmann E. Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J Biol Chem. 2004;279:26654–26665. doi: 10.1074/jbc.M312835200. [DOI] [PubMed] [Google Scholar]
- 46.Stefan E., Wiesner B., Baillie G.S., Mollajew R., Henn V., Lorenz D., Furkert J., Santamaria K., Nedvetsky P., Hundsrucker C., Beyermann M., Krause E., Pohl P., Gall I., MacIntyre A.N., Bachmann S., Houslay M.D., Rosenthal W., Klussmann E. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol. 2007;18:199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- 47.Fenton R.A., Moeller H.B. Recent discoveries in vasopressin-regulated aquaporin-2 trafficking. Prog Brain Res. 2008;170:571–579. doi: 10.1016/S0079-6123(08)00444-5. [DOI] [PubMed] [Google Scholar]
- 48.Fushimi K., Sasaki S., Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 49.Katsura T., Ausiello D.A., Brown D. Direct demonstration of aquaporin-2 water channel recycling in stably transfected LLC-PK1 epithelial cells. Am J Physiol. 1996;270:F548–F553. doi: 10.1152/ajprenal.1996.270.3.F548. [DOI] [PubMed] [Google Scholar]
- 50.van Balkom B.W., Savelkoul P.J., Markovich D., Hofman E., Nielsen S., van der Sluijs P., Deen P.M. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem. 2002;277:41473–41479. doi: 10.1074/jbc.M207525200. [DOI] [PubMed] [Google Scholar]
- 51.Christensen B.M., Zelenina M., Aperia A., Nielsen S. Localization and regulation of PKA-phosphorylated AQP2 in response to V2-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol. 2000;278:F29–F42. doi: 10.1152/ajprenal.2000.278.1.F29. [DOI] [PubMed] [Google Scholar]
- 52.Nejsum L.N., Zelenina M., Aperia A., Frøkiaer J., Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol. 2005;288:F930–F938. doi: 10.1152/ajprenal.00291.2004. [DOI] [PubMed] [Google Scholar]
- 53.Hoffert J.D., Pisitkun T., Wang G., Shen R.F., Knepper M.A. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA. 2006;103:7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffert J.D., Nielsen J., Yu M.J., Pisitkun T., Schleicher S.M., Nielsen S., Knepper M.A. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol. 2007;292:F691–F700. doi: 10.1152/ajprenal.00284.2006. [DOI] [PubMed] [Google Scholar]
- 55.Fenton R.A., Moeller H.B., Hoffert J.D., Yu M.J., Nielsen S., Knepper M.A. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA. 2008;105:3134–3139. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moeller H.B., Praetorius J., Rützler M.R., Fenton R.A. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA. 2010;107:424–429. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouley R. Hawthorn G, Russo LM, Lin HY, Ausiello DA, Brown D: Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in “endocytosis-resistant” membrane domains after vasopressin treatment. Biol Cell. 2006;98:215–232. doi: 10.1042/BC20040054. [DOI] [PubMed] [Google Scholar]
- 58.Lu H., Sun T.X., Bouley R., Blackburn K., McLaughlin M., Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol. 2004;286:F233–F243. doi: 10.1152/ajprenal.00179.2003. [DOI] [PubMed] [Google Scholar]
- 59.Russo L.M., McKee M., Brown D. Methyl-beta-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol. 2006;291:F246–F253. doi: 10.1152/ajprenal.00437.2005. [DOI] [PubMed] [Google Scholar]
- 60.Tamma G., Wiesner B., Furkert J., Hahm D., Oksche A., Schaefer M., Valenti G., Rosenthal W., Klussmann E. The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J Cell Sci. 2003;116:3285–3294. doi: 10.1242/jcs.00640. [DOI] [PubMed] [Google Scholar]
- 61.Takata K., Tajika Y., Matsuzaki T., Aoki T., Suzuki T., Abduxukur A., Hagiwara H. Molecular mechanisms and drug development in aquaporin water channel diseases: water channel aquaporin-2 of kidney collecting duct cells. J Pharmacol Sci. 2004;96:255–259. doi: 10.1254/jphs.fmj04004x3. [DOI] [PubMed] [Google Scholar]
- 62.Tajika Y., Matsuzaki T., Suzuki T., Ablimit A., Aoki T., Hagiwara H., Kuwahara M., Sasaki S., Takata K. Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem Cell Biol. 2005;124:1–12. doi: 10.1007/s00418-005-0010-3. [DOI] [PubMed] [Google Scholar]
- 63.Barile M., Pisitkun T., Yu M.J., Chou C.L., Verbalis M.J., Shen R.F., Knepper M.A. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics. 2005;4:1095–1106. doi: 10.1074/mcp.M500049-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uawithya P., Pisitkun T., Ruttenberg B.E., Knepper M.A. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics. 2008;32:229–253. doi: 10.1152/physiolgenomics.00201.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nedvetsky P.I., Stefan E., Frische S., Santamaria K., Wiesner B., Valenti G., Hammer J.A., 3rd, Nielsen S., Goldenring J.R., Rosenthal W., Klussmann E. A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic. 2007;8:110–123. doi: 10.1111/j.1600-0854.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 66.Pisitkun T., Shen R.F., Knepper M.A. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zelenina M., Christensen B.M., Palmér J., Nairn A.C., Nielsen S., Aperia A. Prostaglandin E2 interaction with AVP: effects on AQP2 phosphorylation and distribution. Am J Physiol Renal Physiol. 2000;278:F388–F394. doi: 10.1152/ajprenal.2000.278.3.F388. [DOI] [PubMed] [Google Scholar]
- 68.Moeller H.B., Fenton R.A. Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch. 2012;464:133–144. doi: 10.1007/s00424-012-1129-4. [DOI] [PubMed] [Google Scholar]
- 69.Kwon T.H., Nielsen J., Møller H.B., Fenton R.A., Nielsen S., Frøkiaer J. Aquaporins in the kidney. Handb Exp Pharmacol. 2009:95–132. doi: 10.1007/978-3-540-79885-9_5. [DOI] [PubMed] [Google Scholar]
- 70.Kwon T.H., Nielsen J., Knepper M.A., Frøkiaer J., Nielsen S. Angiotensin II AT1 receptor blockade decreases vasopressin-induced water reabsorption and AQP2 levels in NaCl-restricted rats. Am J Physiol Renal Physiol. 2005;288:F673–F684. doi: 10.1152/ajprenal.00304.2004. [DOI] [PubMed] [Google Scholar]
- 71.Lee Y.J., Song I.K., Jang K.J., Nielsen J., Frøkiaer J., Nielsen S., Kwon T.H. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol. 2007;292:F340–F350. doi: 10.1152/ajprenal.00090.2006. [DOI] [PubMed] [Google Scholar]
- 72.Wang W., Li C., Summer S., Falk S., Schrier R.W. Interaction between vasopressin and angiotensin II in vivo and in vitro: effect on aquaporins and urine concentration. Am J Physiol Renal Physiol. 2010;299:F577–F584. doi: 10.1152/ajprenal.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim H.Y., Choi H.J., Lim J.S., Park E.J., Jung H.J., Lee Y.J., Kim S.Y., Kwon T.H. Emerging role of Akt substrate protein AS160 in the regulation of AQP2 translocation. Am J Physiol Renal Physiol. 2011;301:F151–F161. doi: 10.1152/ajprenal.00519.2010. [DOI] [PubMed] [Google Scholar]
- 74.Jang K.J., Cho H.S., Kang do H., Bae W.G., Kwon T.H., Suh K.Y. Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integr Biol (Camb) 2011;3:134–141. doi: 10.1039/c0ib00018c. [DOI] [PubMed] [Google Scholar]
- 75.Phillips M.E., Taylor A. Effect of nocodazole on the water permeability response to vasopressin in rabbit collecting tubules perfused in vitro. J Physiol. 1989;411:529–544. doi: 10.1113/jphysiol.1989.sp017588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phillips M.E., Taylor A. Effect of colcemid on the water permeability response to vasopressin in isolated perfused rabbit collecting tubules. J Physiol. 1992;456:591–608. doi: 10.1113/jphysiol.1992.sp019355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Star R.A., Nonoguchi H., Balaban R., Knepper M.A. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest. 1988;81:1879–1888. doi: 10.1172/JCI113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chou C.L., Yip K.P., Michea L., Kador K., Ferraris J.D., Wade J.B., Knepper M.A. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem. 2000;275:36839–36846. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- 79.Bajjalieh S.M., Scheller R.H. The biochemistry of neurotransmitter secretion. J Biol Chem. 1995;270:1971–1974. doi: 10.1074/jbc.270.5.1971. [DOI] [PubMed] [Google Scholar]
- 80.Söllner T., Bennett M.K., Whiteheart S.W., Scheller R.H., Rothman J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 81.Franki N., Macaluso F., Gao Y., Hays R.M. Vesicle fusion proteins in rat inner medullary collecting duct and amphibian bladder. Am J Physiol. 1995;268:C792–C797. doi: 10.1152/ajpcell.1995.268.3.C792. [DOI] [PubMed] [Google Scholar]
- 82.Inoue T., Nielsen S., Mandon B., Terris J., Kishore B.K., Knepper M.A. SNAP-23 in rat kidney: colocalization with aquaporin-2 in collecting duct vesicles. Am J Physiol. 1998;275:F752–F760. doi: 10.1152/ajprenal.1998.275.5.F752. [DOI] [PubMed] [Google Scholar]
- 83.Kishore B.K., Wade J.B., Schorr K., Inoue T., Mandon B., Knepper M.A. Expression of synaptotagmin VIII in rat kidney. Am J Physiol. 1998;275:F131–F142. doi: 10.1152/ajprenal.1998.275.1.F131. [DOI] [PubMed] [Google Scholar]
- 84.Liebenhoff U., Rosenthal W. Identification of Rab3-, Rab5a- and synaptobrevin II-like proteins in a preparation of rat kidney vesicles containing the vasopressin-regulated water channel. FEBS Lett. 1995;365:209–213. doi: 10.1016/0014-5793(95)00476-p. [DOI] [PubMed] [Google Scholar]
- 85.Mandon B., Nielsen S., Kishore B.K., Knepper M.A. Expression of syntaxins in rat kidney. Am J Physiol. 1997;273:F718–F730. doi: 10.1152/ajprenal.1997.273.5.F718. [DOI] [PubMed] [Google Scholar]
- 86.Nielsen S., Marples D., Birn H., Mohtashami M., Dalby N.O., Trimble M., Knepper M. Expression of VAMP-2-like protein in kidney collecting duct intracellular vesicles. Colocalization with Aquaporin-2 water channels. J Clin Invest. 1995;96:1834–1844. doi: 10.1172/JCI118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung H.J., Kwon T.H. Membrane trafficking of collecting duct water channel protein AQP2 regulated by Akt/AS160. Electrolyte Blood Press. 2010;8:59–65. doi: 10.5049/EBP.2010.8.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tamma G., Klussmann E., Procino G., Svelto M., Rosenthal W., Valenti G. cAMP-induced AQP2 translocation is associated with RhoA inhibition through RhoA phosphorylation and interaction with RhoGDI. J Cell Sci. 2003;116:1519–1525. doi: 10.1242/jcs.00355. [DOI] [PubMed] [Google Scholar]
- 89.Sun T.X., Van Hoek A., Huang Y., Bouley R., McLaughlin M., Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol. 2002;282:F998–F1011. doi: 10.1152/ajprenal.00257.2001. [DOI] [PubMed] [Google Scholar]