Abstract
Background
Periodontitis and chronic kidney disease (CKD) are important health issues; however, the association between periodontitis and CKD markers, especially in Korean adults, remains elusive.
Methods
Data on 15,729 Korean adults were obtained from the Korean National Health and Nutritional Examination Surveys IV and V. The CKD markers included a decreased estimated glomerular filtration rate (eGFR;<60 mL/min/1.73 m2), proteinuria, and hematuria. Odds ratios (ORs) and 95% confidence intervals were measured using stepwise multivariate logistic regression analyses for CKD markers based on the presence of periodontitis.
Results
Patients with periodontitis had greater unadjusted ORs for CKD markers compared to those without periodontitis, as follows: decreased eGFR, 4.07 (3.11–5.33); proteinuria, 2.12 (1.48–3.05); and hematuria, 1.25 (1.13–1.39, all P<0.001). Periodontitis was a significant predictor of decreased eGFR independent of all covariates [1.39 (1.03–1.89), P=0.034]. However, the effect of periodontitis on decreased eGFR seemed to be affected by hypertension and diabetes mellitus. Periodontitis was not an independent predictor of proteinuria; the significance disappeared after adjusting for hypertension and diabetes mellitus. Periodontitis was significantly correlated with hematuria, leading to similar ORs regardless of the adjustment for covariates [1.29 (1.15–1.46), P<0.001].
Conclusion
This study confirms the correlation between periodontitis and CKD markers, including decreased eGFR, proteinuria, and hematuria in Korean adults.
Keywords: Glomerular filtration rate, Hematuria, Periodontal diseases, Periodontitis, Proteinuria
Introduction
Early detection of chronic kidney disease (CKD) is a major global health concern, because CKD is associated with high morbidity and mortality [1], [2]. CKD is defined as either kidney damage or a decreased glomerular filtration rate (GFR), according to the Kidney Disease Outcomes Quality Initiative guidelines [3]. Proteinuria and hematuria are the most commonly used and principal markers of kidney damage [3], [4] although radiologic and pathologic abnormalities are also used. Despite the succinct definition of CKD and the implementation of strategies to control this disease, its prevalence has rapidly increased [5]. This phenomenon is attributable to the multifactorial and interactive nature of the etiologies of CKD. Consequently, efforts to identify potentially modifiable factors associated with CKD are required to reduce the large burden of this disease.
Periodontitis is a chronic inflammatory disease that leads to the destruction of periodontal tissue. This process is characterized by a pocket formation around the teeth and/or gum recession [6]. Recently, periodontitis received great attention for its association with several diseases including cardiovascular disease, diabetes, osteoporosis, respiratory disease, and rheumatologic diseases [7].
Previously, several factors have been identified as associated with CKD [8]. Among them, hypertension and diabetes mellitus are the leading causes [9]. A role for periodontitis in CKD has also been suggested [10], [11], [12], particularly because both diseases are based on the inflammatory milieu [13]. However, the data are limited in that most studies have focused only on moderately decreased GFR (<60 mL/min/1.73 m2) and not on proteinuria or hematuria, which are confirmed markers of CKD [10], [11], [12]. Decreased GFR is not always accompanied by proteinuria or hematuria. Furthermore, in the early stages of CKD, GFR may not decrease although other markers, such as urinary abnormalities, may be present [3]. Moreover, previous studies have not included the general Asian population [7], [10], [11], [12], [14]. CKD is a worldwide disease, with Asians displaying a high prevalence compared to other ethnicities [15]. In the present study, we aimed to examine the correlations between periodontitis and both decreased GFR and urinary abnormalities, including proteinuria and hematuria. In addition to this objective, we attempted to confirm the correlation between periodontitis and CKD markers in a representative Korean adult population using a dataset from the Korean National Health and Nutritional Examination Survey (KNHANES).
Methods
Study population
Data were obtained from the 2nd and 3rd years (2008–2009) of the KNHANES IV study and the 1st year (2010) of the KNHANES V study, which were conducted by the Korea Centers for Disease Control and Prevention. KNHANES used a rolling sampling design that involved a complex, stratified, multistage, probability-cluster survey of a representative sample of the non-institutionalized civilian population in South Korea. The survey consisted of health interviews, examinations including laboratory tests, and a nutritional survey. Totals of 12,528 participants, 12,722 participants, and 10,938 participants were sampled in 2008, 2009, and 2010, respectively. In total, 9,744 individuals (77.8% in 2008), 10,533 individuals (82.8% in 2009), and 8,958 individuals (81.9% in 2010) participated in the survey each year. The cross-sectional analysis presented here was restricted to 15,808 participants aged ≥20 years for whom periodontal and kidney results including serum creatinine and urinalysis were available. Furthermore, we excluded participants who were pregnant (n=72) or were menstruating (n=7) at the time of the examination. A total of 15,729 participants were analyzed in the present study. All participants signed informed consent forms. The institutional review board at the Korea Centers for Disease Control and Prevention approved the survey of the study population (Nos. 2008-04EXP-01-C, 2009-01CON-03-2C, and 2010-02CON-21-C).
Study variables
Demographic variables including age, sex, residential region, and education level were collected during the health interview. Age as reported at the time of the health interview was categorized into 20–59 years and ≥60 years. The residential region was categorized as urban or rural. Urban areas included Seoul and the surrounding metropolis in addition to six metropolitan cities. The remaining areas were categorized as rural. Education level was categorized as less than high school, high school, and college or higher. Weight (kg) and height (cm) were measured in participants wearing only a gown without shoes. Body mass index was calculated as [weight/height2 (kg/m2)]. Obesity was defined as body mass index ≥25.0 kg/m2 [16]. Smoking status was subdivided into nonsmoker, past smoker, or current smoker. Regular exercise was defined as either regular moderate exercise for ≥30 minutes/session more than five times/week or intense exercise for ≥20 minutes/session more than three times/week, or both. Moderate exercise was defined as any activity that leaves the participant somewhat breathless, such as badminton, table tennis, slow swimming, or volleyball. Intense exercise was defined as any activity that causes the participant to be out of breath, such as climbing, basketball, football, squash, or running. Participants reported whether they had been diagnosed with hypertension, diabetes mellitus, hypercholesterolemia, or cardiovascular disease including coronary artery disease or cerebrovascular disease, by a medical doctor. Blood pressure was measured while participants were in a sitting position following a 5-minute rest period. Then, blood pressure was measured on three occasions with a mercury sphygmomanometer on the right arm and averaged across three measured values. The presence of hypertension was defined as a systolic blood pressure≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or a diagnosis of hypertension.
Blood samples were collected during the fasting state for the health examination surveys. After collection, the samples were promptly refrigerated and transported to the designated central laboratory (NeoDin Medical Institute, Seoul, Korea). Serum creatinine, glucose, and cholesterol levels were measured with a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). The estimated GFR (eGFR) was calculated using the modification of diet in a renal disease equation [17] as follows:
Decreased eGFR was defined as an eGFR<60 mL/min/1.73 m2. The presence of diabetes mellitus was defined as fasting blood glucose>126 mg/dL or a diagnosis of diabetes mellitus. Participants with blood total cholesterol ≥240 mg/dL or diagnosis of hypercholesterolemia were identified as having hypercholesterolemia. Random urine samples were obtained using the clean-catch technique in a sterile container; participants were requested to submit early morning samples when possible. The results of the dipstick tests were scored semi-quantitatively from negative to +4. Proteinuria and hematuria were defined as ≥+1.
The periodontal examination was performed by trained dentists. The World Health Organization (WHO) community periodontal index (CPI) was used to assess periodontitis. Periodontitis was defined as a CPI≥code 3, which indicates a pathological pocket of 4–5 mm in at least one site (code 4,≥6 mm). The index tooth numbers were 11, 16, 17, 26, 27, 31, 36, 37, 46, and 47. A CPI probe that met the WHO guideline was used [18]. The mouth was divided into sextants. A probing force of approximately 20 g was used.
Statistical analysis
All of the analyses and calculations were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). In all of the analyses, the complex sampling and survey sample weights of the KNHANES were used. The data were presented as the weighted means or proportions (standard error). Proportions were compared using the Pearson's Chi-square test. A logistic regression analysis was used to examine the odds ratios (ORs) and 95% confidence intervals for the CKD markers, including decreased eGFR, proteinuria, and hematuria. In addition to the unadjusted analysis, stepwise multivariate logistic regression models of periodontitis were tested as follows: Model 1, adjusted for age and sex; Model 2, Model 1 plus region, education, smoking, obesity, and exercise; Model 3, Model 2 plus hypertension; Model 4, Model 2 plus diabetes mellitus; Model 5, Model 2 plus hypercholesterolemia; Model 6, Model 2 plus cardiovascular disease; and Model 7, adjusted for all of the covariates. These models were used because hypertension, diabetes mellitus, hypercholesterolemia, and cardiovascular disease were the strongest predictors of CKD in the univariate analysis. A P-value of <0.05 was considered significant in the logistic regression model. The model fit of logistic regression analysis was measured using pseudo R2, whereby larger values indicated a higher predictability of the model. These values were compared between the models both with and without consideration of periodontitis as an independent variable.
Results
Baseline characteristics
The weighted mean age of the participants was 43.4±0.2 years. A total of 32.7% of the participants exhibited periodontitis. The weighted mean eGFR was 95.5±0.3 mL/min/1.73 m2. The weighted proportions of the participants with decreased eGFR, proteinuria, and hematuria were 1.6%, 1.2%, and 14.8%, respectively. Other demographic findings are presented in Table 1.
Table 1.
Baseline characteristics of the study participants
| Total (n=15,729) | |
|---|---|
| Age | 43.4±0.2 |
| <60 years old | 85.6±0.4 |
| >60 years old | 14.4±0.4 |
| Sex | |
| Male | 53.7±0.4 |
| Female | 46.3±0.4 |
| Residential region | |
| Urban | 71.0±1.0 |
| Rural | 29.0±1.0 |
| Education level | |
| Less than high school | 26.5±0.6 |
| High school | 40.6±0.6 |
| College or more | 32.9±0.7 |
| Body mass index | 23.7±0.1 |
| No obesity | 67.9±0.5 |
| Obesity | 32.1±0.5 |
| Smoking status | |
| None | 50.5±0.4 |
| Past smoker | 6.7±0.3 |
| Current smoker | 42.8±0.4 |
| Exercise | |
| No | 74.1±0.5 |
| Moderate | 13.6±0.4 |
| Intensive | 17.8±0.4 |
| Regular | 25.9±0.5 |
| Hypertension | |
| No | 77.3±0.4 |
| Yes | 22.7±0.4 |
| Diabetes mellitus | |
| No | 92.4±0.3 |
| Yes | 7.6±0.3 |
| Hypercholesterolemia | |
| No | 86.9±0.3 |
| Yes | 13.1±0.3 |
| Cardiovascular disease | |
| No | 97.7±0.1 |
| Yes | 2.3±0.1 |
| Periodontitis | |
| No | 67.3±0.7 |
| Yes | 32.7±0.7 |
| Decreased eGFR | 95.5±0.3 |
| No | 98.4±0.1 |
| Yes | 1.6±0.1 |
| Proteinuria | |
| No | 98.8±0.1 |
| Yes | 1.2±0.1 |
| Hematuria | |
| No | 85.2±0.4 |
| Yes | 14.8±0.4 |
Data are expressed as weighted means±standard error for continuous variables and weighted percentages±standard error for categorical variables.
eGFR, estimated glomerular filtration rate.
Association between periodontitis and markers of CKD
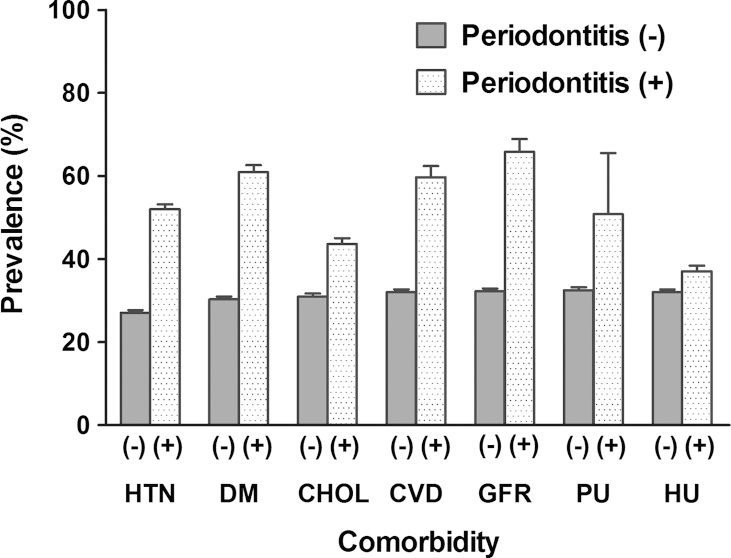
Fig. 1 shows the weighted prevalence of periodontitis according to individual comorbidities. The prevalence of periodontitis was different across all of the comorbidities (all P<0.001). Logistic regression analyses were conducted to evaluate the association between periodontitis and CKD markers. Individuals with periodontitis had greater ORs for CKD markers compared with participants without periodontitis as follows: decreased eGFR, 4.07 (3.11–5.33); proteinuria, 2.12 (1.48–3.05); and hematuria, 1.25 (1.13–1.39, all P<0.001). The unadjusted ORs of other covariates are shown in Table S1 in the supplementary material online. We adjusted all of the covariates for the associations between independent variables and CKD markers (Table 2). We found that age, education level, exercise, hypertension, diabetes mellitus, and a history of cardiovascular disease were significant predictors of decreased eGFR. For proteinuria, hypertension and diabetes mellitus were significant predictors. For hematuria, age, sex, education level, diabetes mellitus, and hypercholesterolemia remained significant in the multivariate analyses. Subsequently, we conducted stepwise multivariate analyses to assess whether the correlations between periodontitis and CKD markers remained consistent after adjusting the covariates (Table 3). Periodontitis was a significant predictor of decreased eGFR independent of all covariates. When ORs and P values were considered, hypertension and diabetes mellitus seemed to exert a relatively strong effect on the correlation between periodontitis and decreased eGFR, compared to hypercholesterolemia or a history of cardiovascular disease. For proteinuria, periodontitis was not an independent predictor. Among the covariates, hypertension and diabetes mellitus had stronger effects on the correlation with proteinuria than hypercholesterolemia or a history of cardiovascular disease. For hematuria, periodontitis was significantly correlated and had a similar OR, regardless of the adjusted covariates. We compared the predictive strengths of the CKD markers between the logistic models with and without periodontitis as an independent variable (Table 4). The predictive strengths were measured using a pseudo R2 calculated by three different methods. All levels of the pseudo R2 in the model that considered periodontitis were greater than the levels in the model that did not account for periodontitis.
Figure 1.
Weighted prevalence of periodontitis according to the comorbidity. Bar represents standard error. CHOL, hypercholesterolemia; CVD, cardiovascular disease; DM, diabetes mellitus; GFR, decreased estimated glomerular filtration rate; HTN, hypertension; HU, hematuria; PU, proteinuria.
Table 2.
Multivariate logistic analysis⁎ between covariates and markers of chronic kidney disease
| Decreased eGFR | Proteinuria | Hematuria | |
|---|---|---|---|
| Age≥60 y (vs.<60 y) | 4.50 (2.99–6.76)§ | 0.80 (0.54–1.17) | 1.16 (1.00–1.34)† |
| Male (vs. female) | 0.98 (0.62–1.55) | 1.64 (0.91–2.97) | 0.31 (0.26–0.36)§ |
| Urban (vs. rural) | 1.36 (0.97–1.92) | 1.27 (0.86–1.86) | 0.94 (0.84–1.06) |
| Educational level | |||
| Less than high school | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| High school | 0.93 (0.65–1.34) | 1.05 (0.67–1.63) | 0.81 (0.71–0.93)‡ |
| College or more | 0.44 (0.26–0.75)‡ | 0.87 (0.53–1.44) | 0.71 (0.60–0.83)§ |
| Obesity (vs. none) | 1.30 (0.96–1.76) | 1.16 (0.80–1.69) | 0.96 (0.86–1.08) |
| Smoking status | |||
| None | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Past smoker | 1.51 (0.81–2.81) | 0.70 (0.31–1.58) | 0.90 (0.68–1.19) |
| Current smoker | 1.29 (0.83–2.03) | 0.90 (0.53–1.54) | 1.15 (0.99–1.33) |
| Exercise (vs. none) | 0.66 (0.46–0.93)§ | 1.00 (0.67–1.49) | 0.99 (0.87–1.12) |
| Hypertension (vs. none) | 3.94 (2.69–5.78)§ | 3.23 (2.20–4.73)§ | 1.10 (0.96–1.25) |
| Diabetes mellitus (vs. none) | 2.83 (2.09–3.82)§ | 3.93 (2.64–5.87)§ | 0.59 (0.45–0.77)§ |
| Hypercholesterolemia (vs. none) | 1.34 (0.94–1.90) | 1.54 (0.97–2.43) | 0.85 (0.74–0.98)† |
| Cardiovascular disease (vs. none) | 2.52 (1.66–3.84)§ | 0.93 (0.46–1.90) | 1.27 (0.96–1.70) |
Data are expressed as odds ratios (95% confidence intervals).
eGFR, estimated glomerular filtration rate.
Adjusted for all covariates including age, sex, region, education, obesity, smoking, exercise, hypertension, diabetes mellitus, hypercholesterolemia, cardiovascular disease, and periodontitis.
P<0.05.
P<0.01.
P<0.001.
Table 3.
Multivariate logistic regression models for chronic kidney disease by periodontitis
| Model⁎ | Decreased eGFR |
Proteinuria |
Hematuria |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| 1 | 1.90 (1.39–2.59) | <0.001 | 1.86 (1.26–2.74) | 0.002 | 1.34 (1.20–1.51) | < 0.001 |
| 2 | 1.71 (1.25–2.34) | 0.001 | 1.75 (1.17–2.63) | 0.007 | 1.27 (1.12–1.43) | < 0.001 |
| 3 | 1.50 (1.11–2.03) | 0.009 | 1.50 (1.01–2.22) | 0.044 | 1.26 (1.12–1.43) | < 0.001 |
| 4 | 1.52 (1.10–2.08) | 0.011 | 1.44 ((0.96–2.17) | 0.081 | 1.30 (1.15–1.47) | < 0.001 |
| 5 | 1.71 (1.26–2.34) | 0.001 | 1.70 (1.13–2.55) | 0.010 | 1.27 (1.13–1.44) | < 0.001 |
| 6 | 1.67 (1.23–2.29) | 0.001 | 1.75 (1.16–2.62) | 0.007 | 1.27 (1.12–1.43) | < 0.001 |
| 7 | 1.39 (1.03–1.89) | 0.034 | 1.29 (0.87–1.91) | 0.208 | 1.29 (1.15–1.46) | < 0.001 |
CI, confidence interval; eGFR estimated glomerular filtration rate; OR odds ratio.
Model 1, adjusted for age and sex; Model 2, model 1 plus region, education, obesity, smoking, and exercise; Model 3, model 2 plus hypertension; Model 4, model 2 plus diabetes mellitus; Model 5, model 2 plus hypercholesterolemia; Model 6, model 2 plus cardiovascular disease; Model 7, adjusted for all covariates.
Table 4.
Predictive strengths of the logistic regression model for chronic kidney disease
| Method | Decreased eGFR |
Proteinuria |
Hematuria |
|||
|---|---|---|---|---|---|---|
| Periodontitis (−) | Periodontitis (+) | Periodontitis (−) | Periodontitis (+) | Periodontitis (−) | Periodontitis (+) | |
| Cox and Snell | 0.0393 | 0.0396 | 0.0120 | 0.0121 | 0.0446 | 0.0460 |
| Nagelkerke | 0.2659 | 0.2679 | 0.1014 | 0.1026 | 0.0784 | 0.0809 |
| McFadden | 0.2507 | 0.2526 | 0.0958 | 0.0970 | 0.0542 | 0.0560 |
Data are expressed as pseudo R2.
eGFR, estimated glomerular filtration rate.
Discussion
Both periodontitis and CKD are important health issues, but their potential relationship is limited because previous studies have only considered decreased eGFR and have not included Koreans. In this population-based study using data from a nationally representative survey, we first evaluated the correlation between periodontitis and CKD markers, including proteinuria and hematuria in addition to decreased eGFR. Multivariate analyses showed independent correlations of periodontitis with decreased eGFR and hematuria; however, its correlation with proteinuria was not significant after adjusting for hypertension or diabetes mellitus. Furthermore, the model for predicting the presence of CKD markers was enhanced when periodontitis was included, which indicates that periodontitis can be considered when exploring the factors related to CKD.
The present study has important implications. CKD and end-stage renal disease, the final stage of CKD, are the strongest causes of cardiovascular morbidity and mortality [1], [2]. Therefore, efforts to identify potentially modifiable factors associated with CKD are required to reduce the large burden of CKD. Previously, studies have suggested periodontitis to be a modifiable risk factor of moderately decreased eGFR (< 60 mL/min/1.73 m2) [8], [9], [10]. However, moderately decreased eGFR appears in late-stage CKD (≥Stage 3) [3]. It is plausible that earlier identification of CKD will lead to a greater reduction in the burden of CKD and end-stage renal disease. Several blood and urinary markers have been proposed or are under development as early markers of kidney damage [19]. Among them, urinary abnormalities, such as proteinuria and hematuria, can be easily measured in both inpatient and outpatient settings. Both parameters can also predict morbidity and mortality, as can decreased eGFR [20], [21]. Taken together, the present study has important implications by demonstrating an association between periodontitis and markers of CKD, including proteinuria and hematuria. Furthermore, this study is the first to confirm such associations in the general Asian population. Although a potential association between periodontitis and CKD in Asians has been suggested, all previous studies have focused on correlations in population subgroups, such as in elderly participants [22], [23].
Periodontitis is derived from the interaction between specific bacterial species and components of the host immune response. Subsequently, periodontitis leads to systemic inflammation, which has been verified in previous studies showing increased systemic inflammatory markers in participants with periodontitis [24]. In this regard, a correlation between periodontitis and CKD is plausible because systemic inflammation is a well-known traditional risk factor of CKD [25] although further experimental studies are warranted. However, whether each CKD marker is separately related with periodontitis is not yet known. For decreased eGFR, both direct and indirect effects of periodontitis were identified [13]. In this study, hypertension and diabetes mellitus were possible mediators between periodontitis and decreased eGFR. The present study showed that periodontitis was an independent predictor of decreased eGFR although the correlation power seemed to be affected by hypertension and diabetes mellitus because the ORs and the significance of periodontitis decreased after adjusting for these conditions. Viewed from this result, it can be suggested that periodontitis has an indirect effect on decreased eGFR via hypertension and diabetes mellitus, in addition to its direct effect, all of which support previous study results [13]. This explanation is plausible because periodontitis is known to increase the risk of hypertension and diabetes mellitus [7], [26]. According to the previous studies including a meta-analysis [7], [27], periodontitis is associated with cardiovascular disease. With this respect, the correlation between periodontitis and CKD may be attributable to cardiovascular disease.
The correlation of periodontitis with proteinuria was significant in the univariate analysis but not in the multivariate analyses, especially after adjusting hypertension and diabetes mellitus. Unlike the present results, two previous studies showed a significant correlation between periodontitis and proteinuria [28], [29]. However, participants in those studies were hypertensive [28] or diabetic [29], and not representative of the general population. It can be suggested that, if participants are under the conditions of hypertension or diabetes mellitus, periodontitis is an additional predictor of proteinuria. However, the effect of periodontitis in the general population may be weak in causing proteinuria compared with hypertension or diabetes mellitus. This explanation is plausible because hypertension and diabetes mellitus are the two most common causes of proteinuria [9], [30], [31].
Unlike decreased eGFR and proteinuria, the correlation between periodontitis and hematuria was strong, regardless of other covariates such as hypertension and diabetes mellitus. Hematuria is an important marker of kidney damage and a common sign of glomerulonephritis [21], [32], whereas it is not common in patients with hypertensive nephrosclerosis or diabetic nephropathy, who often have decreased eGFR and proteinuria [33]. Because glomerulonephritis is characterized by inflammation of the glomerulus, it can be suggested that periodontitis is directly linked to hematuria of glomerulonephritis via an inflammatory process [34], and not indirectly via hypertension or diabetes mellitus. Taken together, hematuria may be more reliable than eGFR or proteinuria as a marker of direct kidney damage, especially in light of glomerulonephritis being linked to periodontitis.
The present study used a dataset from the KNHANES, a study that was conducted in a representative sample of the general Korean population under strict quality control criteria. Although the present study has important strengths, it also has some limitations. First, reverse causation cannot be excluded as an explanation for our results because of the cross-sectional study design. Additional interventional studies are needed to address this issue although some preliminary data have shown improved kidney function after periodontal treatment [35]. Second, CKD markers including decreased eGFR, proteinuria, and hematuria mostly originate from kidney damage, but these markers may be present after damage to other organs such as heart and liver, albeit with low frequency. Third, we conducted this study only in Korean adults; this aspect is both a strength and a weakness. Care must be taken when applying our study results to other ethnicities. Fourth, the CPI method has been shown to overestimate or underestimate the incidence of periodontitis. However, such a bias would have supported the null hypothesis. Fifth, the subgroup analysis in the participants with decreased eGFR could not be conducted because of the small sample size (n=349). The next objective is to conduct a study with a sufficient sample size that targets participants with decreased eGFR or other comorbidities.
In conclusion, this study was the first to confirm a correlation between periodontitis and CKD markers, including decreased eGFR, proteinuria, and hematuria in Korean adults. The correlation trends were different according to each CKD marker; these results may be attributable to the different interaction between periodontitis and CKD. The present findings should be considered in future clinical studies that address periodontitis and CKD. In addition, further interventional and experimental studies are warranted to validate the present findings and to determine whether periodontal treatment can ameliorate the risk of CKD.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by a grant from the Korean Healthcare Technology Research and Development Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084001).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.krcp.2013.09.001.
Appendix A. Supplementary materials
Supplementary material
References
- 1.Weiner D.E., Tighiouart H., Amin M.G., Stark P.C., MacLeod B., Griffith J.L., Salem D.N., Levey A.S., Sarnak M.J. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 4.Keane W.F., Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J., Selvin E., Stevens L.A., Manzi J., Kusek J.W., Eggers P., Van Lente F., Levey A.S. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.Armitage G.C. Development of a classification system for periodontal disease and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Otomo-Corgel J., Pucher J.J., Rethman M.P., Reynolds M.A. State of the science: chronic periodontitis and systemic health. J Evid Based Dent Pract. 2012;12:20–28. doi: 10.1016/S1532-3382(12)70006-4. [DOI] [PubMed] [Google Scholar]
- 8.Tangri N., Stevens L.A., Griffith J., Tighiouart H., Djurdjev O., Naimark D., Levin A., Levey A.S. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 9.Levey A.S., Astor B.C., Stevens L.A., Coresh J. Chronic kidney disease, diabetes, and hypertension: what's in a name? Kidney Int. 2010;78:19–22. doi: 10.1038/ki.2010.115. [DOI] [PubMed] [Google Scholar]
- 10.Kshirsagar A.V., Moss K.L., Elter J.R., Beck J.D., Offenbacher S., Falk R.J. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study. Am J Kidney Dis. 2005;45:650–657. doi: 10.1053/j.ajkd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Fisher M.A., Taylor G.W., Shelton B.J., Jamerson K.A., Rahman M., Ojo A.O., Sehgal A.R. Periodontal disease and other nontraditional risk factors for CKD. Am J Kidney Dis. 2008;51:45–52. doi: 10.1053/j.ajkd.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M.A., Taylor G.W. A prediction model for chronic kidney disease includes periodontal disease. J Periodontol. 2009;80:16–23. doi: 10.1902/jop.2009.080226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher M.A., Taylor G.W., West B.T., McCarthy E.T. Bidirectional relationship between chronic kidney and periodontal disease: a study using structural equation modeling. Kidney Int. 2011;79:347–355. doi: 10.1038/ki.2010.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grubbs V., Plantinga L.C., Crews D.C., Bibbins-Domingo K., Saran R., Heung M., Patel P.R., Burrows N.R., Ernst K.L., Powe N.R. Centers for Disease Control and Prevention CKD Surveillance Team: Vulnerable populations and the association between periodontal and chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:711–717. doi: 10.2215/CJN.08270910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q.L., Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation: Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 17.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) Oral Health Surveys: Basic Methods. 4th edition. World Health Organization; Geneva: 1997. pp. 6–39. [Google Scholar]
- 19.Ferguson M.A., Waikar S.S. Established and emerging markers of kidney function. Clin Chem. 2012;58:680–689. doi: 10.1373/clinchem.2011.167494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmelgarn B.R., Manns B.J., Lloyd A., James M.T., Klarenbach S., Quinn R.R., Wiebe N., Tonelli M. Alberta Kidney Disease Network: Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 21.Vivante A., Afek A., Frenkel-Nir Y., Tzur D., Farfel A., Golan E., Chaiter Y., Shohat T., Skorecki K., Calderon-Margalit R. Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA. 2011;306:729–736. doi: 10.1001/jama.2011.1141. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara A., Deguchi T., Hanada N., Miyazaki H. Renal function and periodontal disease in elderly Japanese. J Periodontol. 2007;78:1241–1248. doi: 10.1902/jop.2007.070025. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki M., Taylor G.W., Nesse W., Vissink A., Yoshihara A., Miyazaki H. Periodontal disease and decreased kidney function in Japanese elderly. Am J Kidney Dis. 2012;59:202–209. doi: 10.1053/j.ajkd.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Slade G.D., Ghezzi E.M., Heiss G., Beck J.D., Riche E., Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163:1172–1179. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 25.Fried L., Solomon C., Shlipak M., Seliger S., Stehman-Breen C., Bleyer A.J., Chaves P., Furberg C., Kuller L., Newman A. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 26.Tsioufis C., Kasiakogias A., Thomopoulos C., Stefanadis C. Periodontitis and blood pressure: the concept of dental hypertension. Atherosclerosis. 2011;219:1–9. doi: 10.1016/j.atherosclerosis.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Bahekar A.A., Singh S., Saha S., Molnar J., Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 28.Tsioufis C., Thomopoulos C., Soldatos N., Kasiakogias A., Andrikou I., Kordalis A., Toutouzas K., Giamarelos G., Tousoulis D., Kallikazaros I., Stefanadis C. Periodontal disease severity and urinary albumin excretion in middle-aged hypertensive patients. Am J Cardiol. 2011;107:52–58. doi: 10.1016/j.amjcard.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 29.Shultis W.A., Weil E.J., Looker H.C., Curtis J.M., Shlossman M., Genco R.J., Knowler W.C., Nelson R.G. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–311. doi: 10.2337/dc06-1184. [DOI] [PubMed] [Google Scholar]
- 30.Mogensen C.E. How to protect the kidney in diabetic patients: with special reference to IDDM. Diabetes. 1997;46(Suppl 2):S104–S111. doi: 10.2337/diab.46.2.s104. [DOI] [PubMed] [Google Scholar]
- 31.Tozawa M., Iseki K., Iseki C., Oshiro S., Ikemiya Y., Takishita S. Influence of smoking and obesity on the development of proteinuria. Kidney Int. 2002;62:956–962. doi: 10.1046/j.1523-1755.2002.00506.x. [DOI] [PubMed] [Google Scholar]
- 32.D'Amico G. Vol. 64. 1987. The commonest glomerulonephritis in the world: IgA nephropathy; pp. 709–727. (Q J Med). [PubMed] [Google Scholar]
- 33.O'Neill W.M., Jr, Wallin J.D., Walker P.D. Hematuria and red cell casts in typical diabetic nephropathy. Am J Med. 1983;74:389–395. doi: 10.1016/0002-9343(83)90956-7. [DOI] [PubMed] [Google Scholar]
- 34.Ardalan M.R., Ghabili K., Pourabbas R., Shoja M.M. A causative link between periodontal disease and glomerulonephritis: a preliminary study. Ther Clin Risk Manag. 2011;7:93–98. doi: 10.2147/TCRM.S14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graziani F., Cei S., La Ferla F., Vano M., Gabriele M., Tonetti M. Effects of non-surgical periodontal therapy on the glomerular filtration rate of the kidney: an exploratory trial. J Clin Periodontol. 2010;37:638–643. doi: 10.1111/j.1600-051X.2010.01578.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material