Abstract
Background
The aim of this study was to evaluate the clinical characteristics of nondiabetic nephropathy in type 2 diabetes mellitus patients and to find a clinical significance of renal biopsy and immunosuppressive treatment in such a patient.
Methods
Renal biopsy results, clinical parameters, and renal outcomes were analyzed in 75 diabetic patients who underwent kidney biopsy at Chungnam National University Hospital from January 1994 to December 2010.
Results
The three most common reasons for renal biopsy were nephrotic range proteinuria (44%), proteinuria without diabetic retinopathy (20%), and unexplained decline in renal function (20.0%). Ten patients (13.3%) had only diabetic nephropathy (Group I); 11 patients (14.7%) had diabetic nephropathy with superimposed nondiabetic nephropathy (Group II); and 54 patients (72%) had only nondiabetic nephropathy (Group III). Membranous nephropathy (23.1%), IgA nephropathy (21.5%), and acute tubulointerstitial nephritis (15.4%) were the three most common nondiabetic nephropathies. Group III had shorter duration of diabetes and lesser diabetic retinopathy than Groups I and II (P=0.008). Group II had the lowest baseline estimated glomerular filtration rate (P=0.002), with the greatest proportion of renal deterioration during follow-up (median 38.0 months, P<0.0001). The patients who were treated with intensive method showed better renal outcomes (odds ratio 4.931; P=0.01). Absence of diabetic retinopathy was associated with favorable renal outcome in intensive treatment group (odds ratio 0.114; P=0.032).
Conclusion
Renal biopsy should be recommended for type 2 diabetic patients with atypical nephropathy because a considerable number of these patients may have nondiabetic nephropathies. And intensive treatment including corticosteroid or immunosuppressants could be recommended for type 2 diabetic patients with nondiabetic nephropathy, especially if the patients do not have diabetic retinopathy.
Keywords: Biopsy, Diabetic nephropathy, Immunosuppressive agents, Type 2 diabetes mellitus
Introduction
The prevalence of type 2 diabetes mellitus is rapidly rising worldwide [1]. The total number of people with diabetes globally is projected to increase from 171 million in 2000 to 366 million in 2030 [2]. The burden of diabetes and its complications may double in the span of 30 years. Diabetic nephropathy (DN) is the leading cause of renal replacement therapy and affects approximately 40% of type 1 and type 2 diabetic patients [3]. DN is also one of the most common causes of renal replacement therapy disease in Korea (45.4%) [4].
However, not all nephropathies in diabetic patients are due to DN. The prevalence of nondiabetic nephropathy (NN) is presumed to exist in between one-sixth and two-thirds of diabetic patients with overt proteinuria [5], [6], [7], [8]. In Korea, Kim et al. [9] reported 74 cases of renal biopsy performed in diabetic patients, and nearly half of them had NN.
It is important to distinguish between NN and DN because some NNs are reversible, whereas DN rarely improves. However, it is difficult to differentiate between these two diseases based on clinical findings alone. Renal biopsy is recommended if diabetic patients present with an atypical course of nephropathy. However, this procedure is invasive and might lead to some complications.
Although several studies have been performed to evaluate the prevalence and predictors of NN in diabetic patients, there are no definitive indications for renal biopsy. In previous studies, predictors of NN included evidence of acute renal failure, microscopic hematuria, short duration of diabetes, absence of retinopathy, or nephritic proteinuria [9], [10], [11].
We evaluated the prevalence and clinical characteristics of NN in type 2 diabetes mellitus patients who underwent renal biopsy. We analyzed the significance of renal biopsy and immunosuppressive treatment in NN with type 2 diabetes mellitus patients.
Methods
Data were collected retrospectively from medical records at Chungnam National University Hospital. All patients were diagnosed with type 2 diabetes mellitus prior to renal biopsy or during the admission period. Our dataset included renal biopsies from January 1994 to December 2010. Exclusion criteria were as follows: (1) patients with kidney transplants who had undergone renal biopsy for diagnosis of rejection; (2) patients who had undergone renal biopsy for distinguishing subtype of lupus nephritis; and (3) patients who had undergone renal biopsy of renal tumors. Ninety-four diabetic patients who underwent renal biopsy were screened, and 19 patients were excluded.
The reasons for renal biopsy were as follows: (1) nephrotic range proteinuria (>3 g/day) with atypical course of nephropathy; (2) proteinuria without diabetic retinopathy [urine total protein (TP)>500 mg/24 hours or spot urine TP/creatinine (Cr)>500 mg/g]; (3) unexplained abrupt decline in renal function [decrease to>50% of estimated glomerular filtration rate (eGFR) or increase to>200% of serum creatinine within 1 month]; (4) unexplained hematuria (>3/high power field); and (5) proteinuria with short duration of diabetes (24 hour urine TP>500 mg or spot urine TP/Cr>500 mg/g within 5 years after diabetes diagnosis).
All biopsy specimens were obtained via percutaneous needle biopsy and analyzed via light microscopy, electron microscopy, and immunofluorescence. All diagnoses were confirmed by the same experienced renal pathologist. DN was diagnosed by the presence of mesangial expansion and diffuse intercapillary glomerulosclerosis with or without the nodular Kimmelstiel–Wilson formation, basement membrane thickening, fibrin caps, or capsular drops. Nondiabetic renal diseases were categorized following orthodox pathological criteria.
Based on the biopsy findings, the patients were classified into three groups: Group I (isolated DN); Group II [NN with underlying DN (DN+NN)]; and Group III (isolated NN).
Patient characteristics analyzed included: age at biopsy, sex, duration of diabetes (years), presence of diabetic retinopathy, HbA1c (%), 24-hour urine total protein (mg/day), blood urea nitrogen (BUN, mg/dL), serum creatinine (mg/dL), albumin (g/L), triglyceride (mg/dL), total cholesterol (mg/dL), C-reactive protein (CRP, mg/dL) as a marker of inflammation, complement (C3, C4, mg/dL), and immunoglobulin (Ig) G/A/M (mg/dL). Estimated GFR (mL/min/1.73 m2) was calculated by Modification of Diet in Renal Disease (MDRD) equation. Baseline characteristics and renal outcomes after the follow-up period (decline of eGFR>50% from baseline eGFR) were analyzed. Baseline was defined as the latest data prior to renal biopsy.
Patients with NN (Groups II and III; n=65) were then reclassified into two groups based on treatment modalities (i.e., immunosuppressant or glucocorticoid therapy vs. conservative measures).
Treatment modalities and renal outcomes in patients with NN were analyzed. Renal outcomes were evaluated by changes of eGFR and proteinuria. Treatment response group was defined as proteinuria<3.5 g/day and least 50% reduction from initial value and stable renal function from baseline (change in creatinine<25%), or improvement of eGFR>50% from baseline over a 6-month period of observation.
Statistical analysis
Collected data were processed using Microsoft Office Excel 2010, IBM SPSS Statistics version 20, and PASW(Predictive Analytics SoftWare) Statistics 18. Quantitative data are expressed as mean±standard deviation. Negative or positive values were expressed as count/total sample counts. Comparisons between groups were assessed using an unpaired t test, analysis of variance, Chi-squared test, nonparametric test. Relationships between baseline characteristics and renal outcomes were assessed using binary univariate and multivariate regression analysis. A P value<0.05 was considered statistically significant.
Results
Reasons for renal biopsy
Seventy-five renal biopsies among 75 diabetic patients were performed during the study period. Ten patients were categorized into Group I (isolated DN), 11 patients into Group II (DN+NN), and 54 patients into Group III (isolated NN).
The reasons for renal biopsy are shown in Table 1. Among them, nephrotic range proteinuria with atypical course of diabetic nephropathy was the most common (44.0%). Proteinuria without diabetic retinopathy and unexplained decline in renal function were both ranked second (20.0%, each). The other reasons for biopsy included unexplained microscopic hematuria (13.3%) and proteinuria with short duration of diabetes (2.7%; Table 1).
Table 1.
| Reason for renal biopsy | Group I (n=10) | Group II (n=11) | Group III (n=54) | P‡ |
|---|---|---|---|---|
| Nephrotic range proteinuria with atypical course (n=33) | 5 (15.2) | 8 (24.2) | 20 (60.6) | 0.003 |
| Proteinuria without diabetic retinopathy (n=15) | 2 (13.3) | 1 (6.7) | 12 (80.0) | 0.001 |
| Unexplained decline of renal function (n=15) | 1 (6.7) | 1 (6.7) | 13 (86.7) | <0.001 |
| Unexplained hematuria (n=10) | 1 (10.0) | 1 (10.0) | 8 (80.0) | 0.007 |
| Proteinuria with short duration of diabetes (n=2) | 1 (50.0) | 0 (0) | 1 (50.0) | NS |
Data are presented as n (%).
DN, diabetic nephropathy; NN, nondiabetic nephropathy; NS, nonsignificant value.
Differences in prevalence among the three groups were analyzed separately with nonparametric tests according to reason for biopsy.
Group I: DN, Group II: DN+NN, Group III: NN.
P=0.514 by Fisher′s exact test.
Patients with nephrotic range proteinuria with an atypical course, proteinuria without diabetic retinopathy, unexplained decline in renal function, or unexplained microscopic hematuria had significantly higher incidences of NN (Group III) compared to the DN or combined groups (Groups I and II).
Baseline characteristics
Duration of diabetes (P=0.008) and prevalence of diabetic retinopathy (P=0.008) were significantly lower in Group III compared to Groups I and II. The patients in Group II had the lowest eGFR, whereas those in Group III had the highest level of eGFR (P=0.022). No other laboratory parameters showed significant differences (P>0.05, Table 2).
Table 2.
Baseline characteristics of type 2 diabetic patients who underwent renal biopsy
| Parameter | Group I (n=10) | Group II (n=11) | Group III (n=54) | P |
|---|---|---|---|---|
| Age (y) | 65.0±13.1⁎ | 53.2±14.9 | 54.6±14.1⁎ | NS |
| M:F ratio | 9:1 | 10:1⁎ | 1.35:1⁎ | 0.024 |
| DM duration (y) | 8.3±7.5 | 9.6±7.9 | 4.2±4.8 | 0.008 |
| Presence of DR | 5/9 (55.6) | 7/10 (70.0)⁎ | 11/46 (23.9)⁎ | 0.008 |
| Laboratory data HbA1c (%) | 7.11±1.60 | 7.17±1.30 | 6.87±1.28 | NS |
| 24 h UP (mg/d) | 5274.2±5749.5 | 4564.5±2354.6 | 4077.3±5372.6 | NS |
| eGFR (mL/min/1.73m2) | 51.0±31.0 | 33.4±21.1⁎ | 61.5±31.9⁎ | 0.022 |
| BUN (mg/dL) | 26.3±15.1⁎ | 42.4±16.0⁎ | 26.8±19.2⁎ | 0.037 |
| Cr (mg/dL) | 2.1±1.4 | 3.2±2.4 | 2.0±2.0 | NS |
| Albumin (g/L) | 3.2±1.1 | 2.9±0.7 | 3.2±1.0 | NS |
| TG (mg/dL) | 256.9±126.7⁎ | 145.7±55.9⁎ | 252.6±237.9 | NS |
| Total cholesterol (mg/dL) | 224.7±95.0 | 197.2±49.5 | 234.3±137.9 | NS |
| CRP (mg/dL) | 0.2±0.1 | 1.4±3.1 | 1.4±3.3 | NS |
| C3 (mg/dL) | 87.0±19.4 | 103.4±22.6 | 101.5±30.6 | NS |
| C4 (mg/dL) | 36.6±9.0 | 36.9±9.4 | 30.7±17.2 | NS |
| IgG (mg/dL) | 967.7±375.9 | 1179.0±535.4 | 1072.4±537.8 | NS |
| IgA (mg/dL) | 273.7±120.5 | 347.8±194.6 | 304.5±145.3 | NS |
| IgM (mg/dL) | 92.0±65.0 | 126.6±98.0 | 139.4±77.4 | NS |
Data are presented as mean±SD or positive count/total count (%).
BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; DM, diabetes mellitus; DR, diabetic retinopathy; Ig, immunoglobulin; NS, nonsignificant value; UP, urine total protein.
Statistically significant findings in analysis between two groups: between Groups I and II – serum BUN (P=0.029), triglyceride (P=0.025); between Groups I and III – age at biopsy (P=0.039); and between Groups II and III – sex (P=0.036), presence of diabetic retinopathy (P=0.016), baseline eGFR (P=0.002), serum BUN (P=0.012).
Results of renal biopsy
Table 3 presents the pathologic diagnoses and reasons for renal biopsy in NNs (isolated DN is excluded from this table). The three most common NNs were membranous nephropathy (23.1%), IgA nephropathy (21.5%), and acute tubulointerstitial nephritis (15.4%). Nephrotic range proteinuria was the most common reason for renal biopsy in cases of membranous nephropathy and IgA nephropathy. Unexplained decline of renal function was the most common reason for renal biopsy in acute tubulointerstitial nephritis.
Table 3.
Results of renal biopsy in nondiabetic nephropathy⁎
| Pathology | Total (n=65) | A | B | C | D | E |
|---|---|---|---|---|---|---|
| MN | 15 (23.1) | 8 (53.3) | 5 (33.3) | 0 (0) | 1 (6.7) | 1 (6.7) |
| IgA nephropathy | 14 (21.5) | 6 (42.9) | 3 (21.4) | 3 (21.4) | 2 (14.3) | 0 (0) |
| ATIN | 10 (15.4) | 4 (40.0) | 1 (10.0) | 4 (40.0) | 1 (10.0) | 0 (0) |
| FSGS | 6 (9.2) | 3 (50.0) | 1 (16.7) | 2 (33.3) | 0 (0) | 0 (0) |
| MCD | 5 (7.7) | 1 (20.0) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 0 (0) |
| HSP nephritis | 4 (6.2) | 2 (50.0) | 0 (0) | 1 (25.0) | 1 (25.0) | 0 (0) |
| DPGN | 3 (4.6) | 1 (33.3) | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) |
| MesPGN | 2 (3.1) | 1 (50.0) | 0 (0) | 0 (0) | 1 (50.0) | 0 (0) |
| ANCA-GN | 2 (3.1) | 0 (0) | 0 (0) | 2 (100.0) | 0 (0) | 0 (0) |
| HT nephropathy | 2 (3.1) | 0 (0) | 0 (0) | 1 (50.0) | 1 (50.0) | 0 (0) |
| MPGN | 1 (1.5) | 1 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IgM nephropathy | 1 (1.5) | 1 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Data are presented as n (%).
ATIN, acute tubulointerstitial nephritis; DPGN, diffuse proliferative glomerulonephritis; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; HSP, Henoch–Schönlein purpura; HT, hypertensive; MCD, minimal change disorder; MesPGN, mesangial proliferative glomerulonephritis; MN, membranous nephropathy; MPGN, membranous proliferative glomerulonephritis.
A, Nephrotic range proteinuria (>3 g/day) with atypical course; B, Proteinuria without diabetic retinopathy [urine total protein (TP)>500 mg/day or spot urine TP/Cr>500 mg/g]; C, Unexplained decline of renal function; D, Unexplained hematuria (>3 red blood cells/high-power field); E, Proteinuria with short duration of diabetes (24 h urine TP>500 mg or spot urine TP/creatinine >500 mg/g within 5 years of diabetes).
Renal outcomes and clinical characteristics in NN
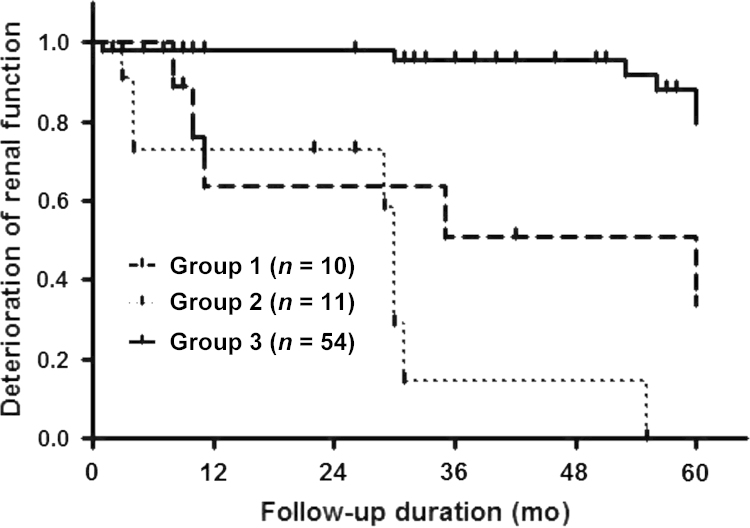
The overall median follow-up duration was 38 months, and those in Group I, Group II, and Group III were 23 months, 26 months, and 50.5 months, respectively. Group III had the best long-term renal survival rate, and Group II had the worst long-term renal survival rates. Renal deterioration was defined as decline the of eGFR>50% from the baseline (Fig. 1).
Figure 1.
Renal outcomes of patients during follow-up (median 38.0 months). Group 3 showed the best renal survival rate among the three groups. Group II showed the largest proportion of renal deterioration during follow-up [P<0.0001 by Log Rank (Mantel–Cox)]. Key: Group 1, diabetic nephropathy; Group 2, underlying diabetic nephropathy+superimposed nondiabetic nephropathy; Group 3, nondiabetic nephropathy.
Compared to the nonimproved renal outcome group, improved renal outcome group showed lower prevalence of DN (P=0.001), more intensive treatment (P=0.010, Table 4, Table 5).
Table 4.
Univariate (binomial) logistic regression analysis (NDRD and renal outcome)⁎
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Age (y) | 1.028 | 0.990–1.066 | NS |
| DM duration (y) | 0.904 | 0.816–1.001 | NS |
| Presence of DR | 0.360 | 0.112–1.155 | NS |
| Presence of DN | 0.053 | 0.006–0.447 | 0.007 |
| Aggressive treatment | 5.077 | 1.696–15.195 | 0.004 |
| 24 h UP (mg/d) | 1.000 | 1.000–1.000 | NS |
| HbA1c (%) | 0.967 | 0.646–1.447 | NS |
| eGFR (mL/min/1.73 m2) | 0.100 | 0.997–1.031 | NS |
| Albumin (g/L) | 0.844 | 0.501–1.422 | NS |
| TG (mg/dL) | 1.003 | 0.999–1.007 | NS |
| CRP (mg/dL) | 1.083 | 0.887–1.322 | NS |
CI, confidence interval; Cr, creatinine; CRP, C-reactive protein; DN, diabetic nephropathy; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; NDRD, nondiabetic renal diseases; NS, not significant; TG, triglyceride; UP, urine total protein.
Treatment response: proteinuria <3.5 g/day and least 50% reduction from initial value, and stable renal function from baseline (change in Cr<25%), or improvement of eGFR>50% from baseline over a 6-month period of observation.
Table 5.
Multivariate logistic regression analysis results (NDRD and renal outcome)⁎
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Presence of DN | 0.055 | 0.006–0.502 | 0.010 |
| Aggressive treatment | 4.931 | 1.464–16.611 | 0.010 |
CI, confidence intervals; DN, diabetic nephropathy; NDRD, nondiabetic renal diseases.
Treatment response: proteinuria <3.5 g/day and least 50% reduction from initial value, and stable renal function from baseline (change in creatinine <25%), or improvement of estimated glomerular filtration rate >50% from baseline over a 6-month period of observation.
Treatment modalities and renal outcomes in NNs
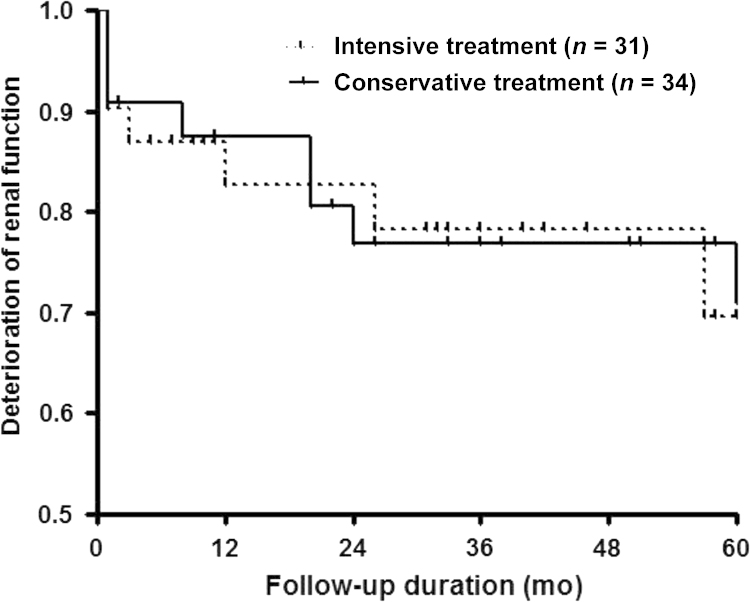
During the maximal follow-up (up to 5 years), 31 patients were treated with intensive methods (i.e., immunosuppressants, glucocorticoids, or combination therapy), and 22 patients (70.9%) achieved improvement in renal function and proteinuria. Thirty-four patients were treated with conservative methods, and 13 patients (38.2%) achieved renal improvement. Intensive treatment methods showed more favorable renal outcome in univariate regression analysis. Long-term survival did not show statistical significant differences between the two groups (P=0.9315, Fig. 2).
Figure 2.
Renal outcomes of patients according to treatment modality (median 40.0 months). The patients who treated with intensive methods showed slightly better renal survival rate than the patients who treated with conservative methods [P=0.9315 by Log Rank (Mantel–Cox)].
Three patients in the isolated nondiabetic renal disorder group (Group III) showed remarkable improvement in eGFR at the 6-month follow-up period, even with a baseline eGFR>15 mL/min/1.73m2. Two patients were diagnosed with focal segmental glomerulosclerosis, and another was diagnosed with IgA nephropathy. One of the focal segmental glomerulosclerosis patients received a combination therapy of corticosteroid, rituximab, and mycophenolic acid, whereas the other patient received corticosteroid only. The patient with IgA nephropathy was treated with cyclophosphamide only. Group II patients (DN+NN) did not show such a significant improvement in eGFR.
Renal outcomes of intensive treatment group
We evaluated several factors to find a prognostic factor in NN in diabetes patients with intensive treatment. In the intensive treatment group, clinical parameters, and renal outcomes were analyzed using binomial logistic regression (dependent variable: treatment response group). Absence of diabetic retinopathy was associated with favorable renal outcome in intensive treatment group (Table 6).
Table 6.
Univariate (binomial) logistic regression analysis (NDRD and renal outcome in intensive treatment group)⁎
| Odd ratio | 95% CI | P | |
|---|---|---|---|
| Age (y) | 1.074 | 0.994–1.161 | NS |
| DM duration (y) | 0.940 | 0.798–1.109 | NS |
| Presence of DR | 0.114 | 0.016–0.828 | 0.032 |
| Presence of DN | 0.000 | 0.000 | NS |
| 24 h UP (mg/d) | 1.000 | 1.000–1.000 | NS |
| HbA1c (%) | 1.376 | 0.606–3.123 | NS |
| eGFR (mL/min/1.73 m2) | 1.010 | 0.983–1.038 | NS |
| Albumin (g/L) | 1.701 | 0.531–5.450 | NS |
| TG (mg/dL) | 1.005 | 0.997–1.013 | NS |
| CRP (mg/dL) | 1.036 | 0.801–1.340 | NS |
CI, confidence intervals; Cr, creatinine; CRP, C-reactive protein; DN, diabetic nephropathy; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; NDRD, nondiabetic renal diseases; NS, not significant; TG, triglyceride; UP, urine total protein.
Treatment response: proteinuria <3.5 g/day and least 50% reduction from initial value, and stable renal function from baseline (change in creatinine<25%), or improvement of eGFR>50% from baseline over a 6-month period of observation.
Discussion
It is important to distinguish NN from DN because some cases of NN are reversible, whereas DN is rarely improves.
Several clinical scenarios supportive of NN include rapidly decreasing kidney function with or without increasing proteinuria (particularly if nephritic), active urinary sediment, evidence of other systemic disease, absence of diabetic retinopathy, and proteinuria in a type 1 diabetic patient with<5 years of disease duration [12], [13], [14].
Although several studies have been performed to evaluate clinical predictors of NN in diabetic patients, there is no definitive indication for renal biopsy in diabetic patients with proteinuria or declining renal function.
In this study, patients with isolated NN had a shorter duration of diabetes and less diabetic retinopathy, consistent with findings in preceding studies. The isolated NN group also showed higher baseline eGFR compared to other groups, which was not demonstrated in previous studies [8], [9], [15], [16].
Chong et al. [10] suggested that the presence of diabetic retinopathy and longer duration of diabetes may be predictors of DN, whereas acute renal failure and microscopic hematuria may be predictors of NN. Kim et al. [9] and Soni et al. [11] suggested that a shorter duration of diabetes, absence of retinopathy, and absence of nephritic proteinuria may be predictors of NN. Monga et al. [17] analyzed superimposed glomerulopathies on diabetic glomerulosclerosis (DGS) and suggested that duration of diabetes favors superimposition of glomerulonephritis on DGS.
However, some studies did not show such correlations. Hayashi et al. [18] analyzed renal biopsy specimens of Japanese DM patients, and found that duration of DM was not associated with all morphologic parameters. A study by Lin et al. [19] demonstrated that diabetic retinopathy was not exclusively seen in NN among type 2 DM patients. Mak et al. [20] suggested that DN and NN were associated with similar durations of disease, as well as incidences of retinopathy and neuropathy.
There were no statistically significant differences between our three study groups regarding age, HbA1c, creatinine level, or 24-hour urine total protein. Our population characteristics were similar to the study of NN in type II DM patients by Zajjari et al. [21]. Mak et al. [20] also found that patients with both isolated DN and NN had no difference in serum creatinine or glycosylated hemoglobin levels. Ghani et al. [22] did not find significant differences between DGS and NN groups according to age or serum creatinine.
In this study, the prevalence of biopsy-proven NN in diabetic patients was 86.7%, which was higher than previously reported findings. The variety in reported prevalence of NN is mainly due to a varying selection criteria for renal biopsy [5], [6], [7], [8], [23]. Therefore, it may be helpful to collect more valuable prognostic data.
Furthermore, renal outcomes in NN patients were analyzed according to the treatment method. This emphasizes the importance of confirming the diagnosis of NN including via renal biopsy in diabetic patients, as well as the application of intensive treatments such as immunosuppressants or glucocorticoids. Wong et al. [8] suggested that the presence of DGS is a risk factor for end-stage renal disease compared to NN. Kim et al. [9] demonstrated that half of diabetic patients who were treated with immunosuppressants achieved complete remission of NN.
In this study, we observed impressively better renal outcomes in patients who treated with intensive treatment including corticosteroid or immunosuppressants. Compared to the nonimproved renal outcome group, improved renal outcome group showed lower prevalence of diabetic nephropathy and more intensive treatment. This suggests that intensive treatment including corticosteroid or immunosuppressants should be recommended for type 2 diabetic patients with NN, even though the patients has poor initial renal function confirmed at the diagnosis.
Absence of diabetic retinopathy was associated with favorable renal outcome in the intensive treatment group. It is possible that absence of diabetic retinopathy is a prognostic factor for a good effect of intensive treatment.
There are several limitations to this study. First, the retrospective study design allows for findings to be subject to many other variables not analyzed in this study. Next, the number of male patients was much higher than that of females, making our results less generalizable. Further evaluation of predictors and prognostic factor of NN such as larger multicenter prospective trials or meta-analyses of existing studies might be helpful.
In conclusion, renal biopsy should be recommended for type 2 diabetic patients with atypical nephropathy because a considerable number of diabetic patients may have NNs that are reversible. Intensive treatment including corticosteroid or immunosuppressants could be recommended for type 2 diabetic patients with NN, especially if the patients do not have diabetic retinopathy.
Conflicts of interest
None.
References
- 1.Zhou J., Chen X., Xie Y., Li J., Yamanaka N., Tong X. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23:1940–1945. doi: 10.1093/ndt/gfm897. [DOI] [PubMed] [Google Scholar]
- 2.Wild S., Roglic G., Green A., Sicree R., King H.: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053, 2004 [DOI] [PubMed]
- 3.Gross J.L. de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T: Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 4.Jin D.C. Current status of dialysis therapy in Korea. Korean J Intern Med. 2011;26:123–131. doi: 10.3904/kjim.2011.26.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambara V., Mecca G., Remuzzi G., Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3:1458–1466. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 6.Ruggenenti P., Gambara V., Perna A., Bertani T., Remuzzi G. The nephropathy of non-insulin-dependent diabetes: predictors of outcome relative to diverse patterns of renal injury. J Am Soc Nephrol. 1998;9:2336–2343. doi: 10.1681/ASN.V9122336. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y., Ueno M., Hayashi H., Nishi S., Satou H., Karasawa R., Inn H., Suzuki S., Maruyama Y., Arakawa M. A light microscopic study of glomerulosclerosis in Japanese patients with noninsulin-dependent diabetes mellitus: the relationship between clinical and histological features. Clin Nephrol. 1994;42:155–162. [PubMed] [Google Scholar]
- 8.Wong T.Y., Choi P.C., Szeto C.C., To K.F., Tang N.L., Chan A.W., Li P.K., Lai F.M. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care. 2002;25:900–905. doi: 10.2337/diacare.25.5.900. [DOI] [PubMed] [Google Scholar]
- 9.Kim B.S., Chang Y.K., Song H.C., Lee S.Y., Jang S.N., Kim H.W., Shin Y.S., Choi Y.J., Jin D.C., Kim Y.S. The clinical differentiations between diabetic nephropathy and non-diabetic renal disease in type 2 diabetic patients. Korean J Nephrol. 2009;28:588–594. [Google Scholar]
- 10.Chong Y.B., Keng T.C., Tan L.P., Ng K.P., Kong W.Y., Wong C.M., Cheah P.L., Looi L.M., Tan S.Y. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail. 2012;34:323–328. doi: 10.3109/0886022X.2011.647302. [DOI] [PubMed] [Google Scholar]
- 11.Soni S.S., Gowrishankar S., Kishan A.G., Raman A. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11:533–537. doi: 10.1111/j.1440-1797.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 12.Bi H., Chen N., Ling G., Yuan S., Huang G., Liu R. Nondiabetic renal disease in type 2 diabetic patients: a review of our experience in 220 cases. Ren Fail. 2011;33:26–30. doi: 10.3109/0886022X.2010.536292. [DOI] [PubMed] [Google Scholar]
- 13.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Parving H.H., Hommel E., Mathiesen E., Skott P., Edsberg B., Bahnsen M., Lauritzen M., Hougaard P., Lauritzen E. Vol. 296. 1988. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes; pp. 156–160. (Br Med J (Clin Res Ed)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E.Y., Chung C.H., Choi S.O. Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J. 1999;40:321–326. doi: 10.3349/ymj.1999.40.4.321. [DOI] [PubMed] [Google Scholar]
- 16.Tone A., Shikata K., Matsuda M., Usui H., Okada S., Ogawa D., Wada J., Makino H. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69:237–242. doi: 10.1016/j.diabres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Monga G., Mazzucco G., di Belgiojoso G.B., Confalonieri R., Sacchi G., Bertani T. Pattern of double glomerulopathies: a clinicopathologic study of superimposed glomerulonephritis on diabetic glomerulosclerosis. Mod Pathol. 1989;2:407–414. [PubMed] [Google Scholar]
- 18.Hayashi H., Karasawa R., Inn H., Saitou T., Ueno M., Nishi S., Suzuki Y., Ogino S., Maruyama Y., Kouda Y., Arakawa M. An electron microscopic study of glomeruli in Japanese patients with non-insulin dependent diabetes mellitus. Kidney Int. 1992;41:749–757. doi: 10.1038/ki.1992.117. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y.L., Peng S.J., Ferng S.H., Tzen C.Y., Yang C.S. Clinical indicators which necessitate renal biopsy in type 2 diabetes mellitus patients with renal disease. Int J Clin Pract. 2009;63:1167–1176. doi: 10.1111/j.1742-1241.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 20.Mak S.K., Gwi E., Chan K.W., Wong P.N., Lo K.Y., Lee K.F., Wong A.K. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12:2588–2591. doi: 10.1093/ndt/12.12.2588. [DOI] [PubMed] [Google Scholar]
- 21.Zajjari Y., Benyahia M., Montasser Ibrahim D., Kassouati J., Maoujoud O., El Guendouz F., Oualim Z. Non-diabetic renal disease in type II diabetes mellitus patients in Mohammed V Military Hospital, Rabat, Morocco. East Mediterr Health J. 2012;18:620–623. [Article in French] [PubMed] [Google Scholar]
- 22.Ghani A.A., Al Waheeb S., Al Sahow A., Hussain N. Renal biopsy in patients with type 2 diabetes mellitus: indications and nature of the lesions. Ann Saudi Med. 2009;29:450–453. doi: 10.4103/0256-4947.57167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen S., Mogensen C.E. How often is NIDDM complicated with non-diabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia. 1996;39:1638–1645. doi: 10.1007/s001250050628. [DOI] [PubMed] [Google Scholar]