Abstract
Osteomyelitis caused by nontuberculous mycobacteria (NTM) can have severe consequences and a poor prognosis. Physicians therefore need to be alert to this condition, especially in immunocompromised patients. Although the pathogenesis of NTM osteomyelitis is still unclear, studies in immunodeficient individuals have revealed close relationships between NTM osteomyelitis and defects associated with the interleukin-12–interferon-γ–tumor necrosis factor-α axis, as well as human immunodeficiency virus infection, various immunosuppressive conditions, and diabetes mellitus. Culture and species identification from tissue biopsies or surgical debridement tissue play crucial roles in diagnosing NTM osteomyelitis. Suitable imaging examinations are also important. Adequate surgical debridement and the choice of appropriate, combined antibiotics for long-term anti-mycobacterial chemotherapy, based on in vitro drug susceptibility tests, are the main therapies for these bone infections. Bacillus Calmette–Guerin vaccination might have limited prophylactic value. The use of multiple drugs and long duration of treatment mean that the therapeutic process needs to be monitored closely to detect potential side effects. Adequate duration of anti-mycobacterial chemotherapy together with regular monitoring with blood and imaging tests are key factors determining the recovery outcome in patients with NTM osteomyelitis.
Keywords: Osteomyelitis, diabetes mellitus, nontuberculous mycobacteria
Introduction
Nontuberculous mycobacteria (NTM) comprise more than 150 species of mycobacteria (http://www.bacterio.net/mycobacterium.html), excluding Mycobacterium tuberculosis complex and Mycobacterium leprae [1]. NTM are widely distributed in the environment and can be cultured from samples including soil and lake water [2]. NTM have recently gained increased attention because of their ability to cause infections in immunocompromised patients, such as those with human immunodeficiency virus (HIV) infection, cystic fibrosis, or organ transplants, whereas NTM rarely infects immunocompetent individuals [2]. Although the skin and skeletal muscle can become infected following injection or trauma [3,4], the respiratory system is the main site of infection. There have been numerous case reports concerning either immunocompromised or immunocompetent patients with NTM osteomyelitis, but few reviews. Here, we review the status of NTM osteomyelitis and report on a patient with diabetes mellitus (DM) who developed osteomyelitis caused by multi-antibiotic-resistant M. intracellulare after surgery.
Species distribution and pathogenesis
NTM can be isolated from the natural environment and from hospital equipment, such as xylocaine for injection, hemodialysis water, and laparoscopic instruments. NTM show weaker virulence than M. tuberculosis (MTB) and M. leprae [4–6]. NTM are gram-positive and acid-fast-positive bacteria. They might be misdiagnosed as MTB, but are naturally resistant to antituberculosis antibiotics because of the complex structure of their cell wall. NTM were first identified as pulmonary pathogens in the 1950s by Runyon [7], and increasing numbers of NTM have been recognized as emerging pathogens, especially in immunocompromised individuals.
NTM infections may involve multiple organs, including the bones, and the diversity of pathogen species involved increases the difficulty of treating these infections. NTM osteomyelitis was rarely reported before the 1980s, but its prevalence seems to have increased recently, reflected by case reports and large-scale clinical research. Many slow-growing NTM species have demonstrated the ability to cause NTM osteomyelitis, including M. avium-intracellulare complex (MAC) [3,8–48], M. ulcerans [49–51], M. marinum [52–57], M. kansasii [9,33,36,58–63], M. xenopi [64–66], M. gordonae [67], M. haemophilium [33,68,69], M. scrofulaceum [45,70], M. szulgai [71–74], M. longobardum [75], and M. flavescens [76]. Among the rapidly growing mycobacteria, osteomyelitis can be caused by M. abscessus [3,77–81], M. fortuitum [82–94], M. chelonae [5,25,83,95–104], M. smegmatis [105], M. peregrinum [82], and M. thermoresistibile [106]. Some authors have argued that the otomastoiditis attributed to M. fortuitum or M. chelonae was actually caused by M. abscessus [107], though this disagreement is actually a taxonomic issue. Different strains of the same species or subspecies of NTM have shown different degrees of virulence, with complex mechanisms of resistance against the host immune system [108–110]; strains may include some genotypes with a wide host spectrum, while others are avirulent [111]. Further research is needed to establish the genetic factors related to mycobacteria virulence [111].
Multiple bone loci can be infected by NTM through direct inoculation during surgery or via insertion of a contaminated prosthesis, for example, osteomyelitis in the sternum caused by cardiac surgery [91], otomastoiditis by myringotomy tube insertion [17,25,82,96,107,112], and osteomyelitis of the mandible by dental treatment [77]. In addition to nosocomial infections, NTM osteomyelitis can be caused by trauma, fracture or animal bites during activities such as gardening, farming, fish-keeping, playing in sand pits, and swimming [5,6,51,95]. These activities might allow environmental NTM to colonize and infect individuals through a wound, as an exogenous pathogen. If the infection extends beyond the skin or soft tissue, NTM might cause transient bacteremia and colonize other organs via the bloodstream or lymphatic system, including the reticuloendothelial system in bones, where it results in osteomyelitis [113]. Studies in animal models have shown that direct injection of mycobacterial components resulted in osteomyelitis with chronic inflammation [110]. However, in patients with no history of penetrating trauma, the ‘locus minoris resistentiae’ (place of least resistance) theory might offer a possible explanation. NTM can colonize or invade the mucosal surface of the respiratory and gastrointestinal tracts in immunocompetent patients. Macrophages in the blood might then come into contact with the mycobacteria, such as M. intracellulare, which could then enter the macrophages via endocytosis. Some currently undetermined structures in the macrophages might protect the mycobacteria, allowing them to survive, while complex mechanisms may inhibit phagosome–lysosome fusion [109,114–116]. It is possible that, in the absence of penetrating trauma, abnormal levels of nitric oxide may cause bone formation [117–120] and might thus be involved in the pathophysiological progression of NTM release from macrophages mobilized to the injury site, resulting in local osteomyelitis, as in some cases of vertebral osteomyelitis [3,121]. However, the dissemination of NTM to cause osteomyelitis is not always a direct progression, which might explain the low prevalence of NTM osteomyelitis.
The pathogenesis of NTM infection is not fully understood and an ideal NTM osteomyelitis animal model is currently lacking. However, it has been suggested that the immune system plays a crucial role in NTM infection, especially multifocal NTM osteomyelitis. Multifocal NTM osteomyelitis and disseminated NTM infection have been found in different members of the same family [22,122,123]. Cases of multifocal NTM osteomyelitis have occurred in the absence of adjacent organ infection, suggesting that an impaired host immune system or inherited immune deficiency contributed to the development of infection. Studies on pathogenesis have revealed key roles for interferon (IFN)-γ, interleukin (IL)-12, and tumor necrosis factor (TNF)-α in the human immune defense against mycobacterial infection [116,124–131]. Some inherited gene defects related to the IL-12–IFN-γ–TNF-α axis have been shown to be directly linked and associated with disseminated NTM infection and multifocal NTM osteomyelitis. These include mutations in human IFN-γ receptors 1 and 2, IFN regulatory factor, signal transducer and activator of transcription 1, IL-12 receptor β1, and IL-12 p40 subunit genes [22,108,131–134]. Mutations in the genes encoding cell surface receptors impaired the functions of and cascade regulation between these cytokines [131]. Interestingly, isolated NTM osteomyelitis has been found in patients with autosomal dominant partial IFN-γ receptor 1 deficiency, but not those with recessive complete IFN-γ receptor 1 deficiency [134], and the mechanism is unclear. IFN-γ antibodies, which are a major cause of NTM dissemination, have already been identified, although the genetic mechanisms, predisposing factors, and contributions of these antibodies remain unknown [126,133,135].
Diagnosis
It is difficult to diagnose and differentiate NTM osteomyelitis from other diseases such as Langerhans cell histiocytosis or osteomyelitis caused by MTB and other bacteria. These difficulties arise because of a lack of specific examination methods, and the fact that the clinical presentation is variable. Furthermore, the wide cross-reactivity between mycobacteria means that the tuberculosis skin test has limited value for the diagnosis of NTM osteomyelitis [136]. However, the IFN-γ release assay can help to distinguish between some cases of NTM osteomyelitis and MTB infection, but not other bacteria, because the RD-1 region differs between most NTM and MTB [137,138]. Given the limitations of current examination methods, an accurate diagnosis of NTM osteomyelitis depends largely on imaging examinations combined with histopathologic, cytological, and microbiological examinations via surgical debridement or biopsy.
Useful imaging examinations include X-ray plain film, ultrasonography, computed tomography (CT) scan, bone nuclear imaging, and magnetic resonance imaging (MRI). X-ray plain films have the advantages of low cost and being easy to perform, even in basic hospitals, and are able to provide comprehensive imaging of the anatomy and pathologic conditions. Ultrasonography is also an easy technique for detecting involved periosteum and soft tissues, and for helping to guide surgical drainage and biopsy. CT has limited use in NTM osteomyelitis because it is less sensitive than bone nuclear imaging and MRI, although it can help to guide biopsy procedures [139]. Bone nuclear imaging can reveal multifocal infected bone lesions; this is an advantage in the case of NTM osteomyelitis, which often involves multiple bone loci. MRI can provide detailed and accurate information on both the infected bone marrow and involved periosteum and soft tissues, and it has the highest sensitivity and specificity of all the current imaging techniques; however, the cost of MRI is high [140].
Extrapulmonary NTM infection normally shows as tenosynovitis, synovitis, skin lesions, subcutaneous granuloma, or osteomyelitis, and pathological examinations without acid-fast staining (AFB) performed routinely after surgical debridement or biopsy may not provide an accurate diagnosis of NTM infection. Some samples may have no obvious inflammatory signs, whereas others might show different signs of non-specific inflammation, such as neutrophilic abscesses or spindle cell proliferation. Furthermore, some samples might have granulomas with or without necrosis, whereas others might show typical caseous epithelioid granulomas [22,54,100,141,142]. Especially if the inflammatory lesion shows a tuberculotic ‘cold abscess’ [143], these indeterminate characteristics and pathology might easily lead to an incorrect diagnosis and the inappropriate use of antituberculous chemotherapy. NTM-infected tissue samples do not always show AFB-positive bacteria and moreover, these bacteria are difficult to differentiate from MTB. Some case reports found that the bacteria in M. kansasii osteomyelitis had specific microscopic features [60,144], but it is currently not possible to differentiate the bacterial species in NTM osteomyelitis by pathological examination.
Microbiological investigation after surgical debridement or biopsy play a crucial role in the diagnosis of NTM osteomyelitis. These examinations include the traditional AFB smear test and mycobacterial culture, combined with molecular identification methods such as polymerase chain reaction (PCR) or biochip testing.
Because of their low prevalence, NTM are unlikely to be considered as the first causative pathogens in an osteomyelitis patient, leading to a delay in diagnosis and chemotherapy, which might be associated with a poor prognosis. However, NTM osteomyelitis should be suspected in patients who are negative for normal bacterial culture and tests and resistant to antibacterial therapy with routine antibiotics.
The AFB smear test is routinely used for suspected MTB or NTM infection, although Nocardia, Rhodococcus, and some other bacteria are also AFB-positive. However, the main disadvantage of the AFB smear test is its low sensitivity for detecting NTM infection [145], and the AFB test may need to be repeated several times to demonstrate a positive result if NTM infection is suspected. Mycobacterial culture of infected tissue or pus should be performed at the same time as surgical debridement or biopsy, because blood culture might be negative in most immunocompetent patients [79]. If a positive isolate is acquired, subsequent identification tests and in vitro drug susceptibility tests can then be performed easily, even if the culture result demonstrates polymicrobial infection in open fracture patients [83]. However, NTM might also be a laboratory contaminant [22], and physicians should therefore interpret positive culture results carefully to avoid misdiagnosis. Increasing numbers of NTM species have been identified recently, and mycobacterial culture combined with PCR or other molecular tests may provide a more accurate and rapid diagnosis than conventional biochemical tests [83,112,146], given the urgency of mycobacterial identification as the key to the successful management of NTM osteomyelitis.
Therapy
Therapy for NTM infections remains difficult, and the wide range of NTM species means that there are currently no effective single anti-NTM antibiotics. Therapy for NTM osteomyelitis includes surgical debridement, abscess drainage, and long-term chemotherapy, with surgery playing a more important role in some cases.
Surgical debridement and drainage of abscesses are crucial for local NTM osteomyelitis, because the mycobacterial burden in the bone marrow is high and the dead bone might provide a storage site for mycobacteria [147]. Because the circulation does not reach dead bone, antibiotics would be ineffective, and thorough curettage, and even amputation, may be needed [56]. Surgical debridement requires the removal of all infected tissue, and multiple sequential debridements or persistent drainage may be required [79]. However, new anti-mycobacterial therapies are being developed for NTM osteomyelitis, and Kato et al. recently reported no infection recurrence in a patient with vertebral osteomyelitis treated by debridement and surgery using antibacterial iodine-supported instrumentation [78].
It has been suggested that surgical debridement is adequate treatment for NTM osteomyelitis [148], but appropriate, individualized anti-mycobacterial chemotherapy performed after debridement or abscess drainage may be beneficial [6].
Chemotherapy may limit the spread of mycobacteria and eliminate bacteria not removed during surgery, especially in cases with multifocal lesions. There are currently no guidelines for NTM osteomyelitis therapy. However, the American Thoracic Society recommends performing drug susceptibility tests before using antibiotics, using combination therapy to avoid inducing antibiotic resistance [149]. Because antibiotic resistance differs among NTM species, in vitro drug susceptibility tests should be performed as soon as possible after species identification to help physicians select the appropriate combination of antibiotics [79].
Osteomyelitis often involves deep tissue, and the decreased local concentration of antibiotics and slow growth of NTM [147] mean that anti-mycobacterial chemotherapy needs to be continued for at least 4–6 weeks after the clinical signs or cultures turn negative [6,65,79,83,100,150,151]. Chemotherapy may even need to be continued for years in immunocompromised patients, because the immune condition could affect the prognosis [152]. No guidelines or gold standards for the optimal duration of anti-mycobacterial chemotherapy have yet been developed, and associated clinical trials are lacking. Although the combination with surgical debridement might reduce the potential side effects of long-term use of combined antibiotics, the antibiotics should still be chosen carefully and monitored closely to avoid adverse drug events, such as loss of vision, rash, and hepatic or renal toxicity [153,154].
Long-term follow-up of NTM osteomyelitis patients after discharge is important for patient recovery. In addition to the use of oral antibiotics, imaging examinations and blood tests such as complete blood count (CBC), erythrocyte sedimentation rate (ESR), and liver and kidney function test should also be used to monitor the progress of the infection [155]. A significant improvement in bone imaging results could suggest the effectiveness of therapy, although bone nuclear imaging is not a useful technique for follow-up studies given that the results would take too long to become normalized [155]. In the case of slow-growing NTM like MAC, anti-mycobacterial chemotherapy should be continued for at least 1 year, whereas cases with fast-growing NTM like M. fortuitum and M. abscessus should receive chemotherapy for at least 6 months [149].
Prophylaxis
There are currently few prophylactic options for NTM osteomyelitis, especially in patients with IFN-receptor deficiency [22]. Bacillus Calmette–Guerin (BCG) vaccination was suggested to protect patients from osteomyelitis caused by M. ulcerans [113,146,156], but the value of BCG vaccination as a prophylaxis against other species is undetermined. Furthermore, BCG vaccination should be contraindicated in immunocompromised patients because of the potential to cause M. bovis infection [157]. Macrolides and rifabutin have been suggested as prophylactic agents for MAC infection in HIV patients with decreasing CD4+ T-cell counts [21,158–160]. However, there is currently no recommended drug for NTM osteomyelitis prophylaxis.
Immunocompromised patients
HIV-infected patients
NTM osteomyelitis is a rare complication in HIV patients, even though M. intracellulare and M. avium are major opportunistic pathogens responsible for disseminated infection among patients with acquired immune deficiency syndrome (AIDS) [161,162], and osteomyelitis in HIV patients caused by M. kansasii, M. haemophilum, M. fortuitum, M. terrae, and M. xenopi has also been reported [33,36,59,61,64,90,144,163]. NTM osteomyelitis often develops late in AIDS patients, when it involves multiple sites of the bones or joints related to cutaneous lesions, and is co-infected with other opportunistic fungi or bacteria. The spine is the most commonly involved location, with both arteries and veins providing hematogenous sources of infection [15]. The widespread use of highly active antiretroviral therapy (HAART) in patients with HIV infection seems to be associated with an increased prevalence of local NTM infections such as NTM osteomyelitis [18,21]. However, although the prevalence of NTM osteomyelitis in HIV-infected patients is higher than in healthy individuals, it is still lower than in injecting drug users [33,164]. Although CD4+ T cells play an important role in controlling NTM infection together with elevated IFN-γ and macrophages [165], NTM osteomyelitis is often seen in patients with immune reconstitution inflammatory syndrome after effective HAART, even when CD4+ T-cell counts were ≥ 100 cells/μl [21,64]. This could be explained if localized NTM infection or undetectable mycobacteremia already existed before HAART and effective T-cell revival. This phenomenon highlights the need for physicians to be aware of the possibility of NTM infection at all stages of AIDS, although the optimal time for prophylaxis remains to be determined [21].
Various immunosuppressive conditions
In addition to immune defects caused by mutations related to the IL-12–IFN-γ axis, steroids, anticancer chemotherapy, immunosuppressive drugs, and various conditions such as leukemia, organ transplantation, and hepatic and renal failure also impair immune function and may lead to NTM osteomyelitis [22,81,88,99,121,166]. These underlying diseases and drugs are also risk factors related to prognosis, with possible implications for therapeutic progress [6,166].
Diabetes mellitus
Diabetes mellitus (DM) can cause immune impairment, thus increasing the risk of multiple opportunistic infections, and DM is strongly associated with tuberculosis in developing countries [167]. Although NTM osteomyelitis has been reported in DM patients, it appears to be rare and its prevalence is not clear. Serum IFN-γ levels were found to be decreased in DM patients [168,169], which might contribute to the increased risk of NTM infections in these patients. IFN-γ may help to combat pathogens by contributing to the production of nitric oxide and maturation of intracellular phagosomes [170,171]. Although superoxide anions and nitric oxide have been shown to play significant roles in host resistance to MTB infection [172–176], studies of MAC showed that NTM had a strong ability to inhibit the activity of superoxide anions, and nitric oxide could only prevent the proliferation of some strains, although it could take part in the post-infection pathophysiology progress and granuloma formation [171,177,178]. DM patients produce subnormal levels of superoxide anions and nitric oxide as the result of impaired and abnormal immune function [179–181]. Still it should be remarked that effector mechanisms in macrophages active against NTM have not been fully clarified [182–184].
A case of NTM osteomyelitis associated with diabetes mellitus
A 69-year-old man found a soft, soybean-sized lump on his right upper arm on September 6, 2011. He was a retired resident living in Shaoxing, Zhejiang province, on the southeast coast of China. He had an 18-year history of DM and injected insulin daily, with good blood glucose control. Before his presentation, he had felt fatigued and the lump had enlarged slowly over a 10-day period. He underwent surgery at a local hospital to excise the lump. The pathology report indicated a sebaceous cyst, but AFB test and culture were not performed. After surgery, he started to sweat and became febrile, and small lumps appeared under the skin of his arms. The local hospital suspected tuberculosis and started antituberculosis therapy with isoniazid (INH), ethambutol (EMB), and rifampin (RIF). However, the therapy was stopped after 15 days because of rashes all over his body, and because a T-SPOT.TB test was negative. Four months later, he developed chest tightness and shortness of breath. A CBC revealed high levels of white blood cells, and a CT scan showed exudative lesions in the lung and multiple lesions on both humerus bones and the ribs. He developed respiratory failure and was transferred to the intensive care unit with tracheal intubation and ventilation. A biopsy of one rib was performed without culture and an AFB test was positive (Figure 1a). He was diagnosed with osteomyelitis caused by NTM. Caspofungin, linezolid (LZD), and moxifloxacin (MXF) were used, but changed to minocycline and clarithromycin (CLR) as a result of exfoliative dermatitis. His fever resolved after 2 months and the antibiotics were therefore stopped. However, 6 months later, the rashes and fever re-emerged, and his temperature peaked at 40°C. CLR, MXF, and 40 mg methylprednisolone once a day were then initiated, with a decrease in temperature but residual low fever.
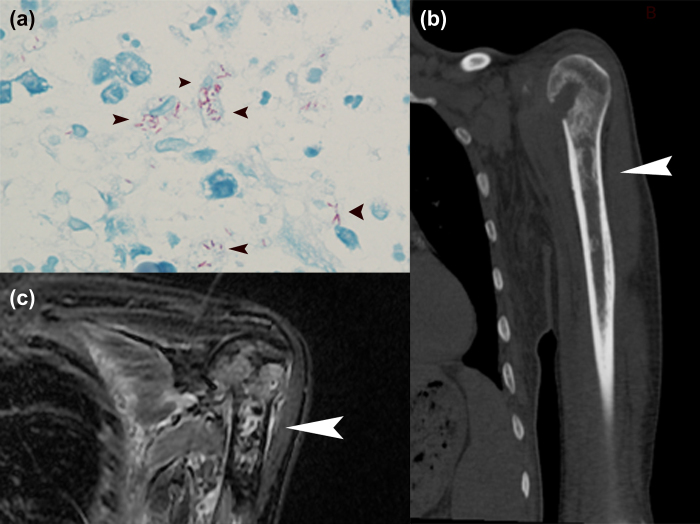
Figure 1.
(a) Acid-fast staining (AFB) test showing acid-fast bacilli in the monocytes (arrowheads). (b) CT scan of left humerus, with multiple vermiform destructions (arrowhead). (c) MRI of left humerus, T2-STIR, showing abnormal signal in the marrow (arrowhead).
The patient was transferred to our hospital on March 3, 2013. He seemed weak, with scattered rashes on his arms and legs, and multiple lumps identified by ultrasound as lymph nodes at the neck, groin, left ear, and right arm. The lumps were not tender and were about 1 cm in diameter. CBC showed neutrophils 7.2 × 109/L, eosinophils 0.65 × 109/L, hemoglobin 96 g/L, albumin 27.2 g/L, C-reactive protein (CRP) 92.2 mg/L, and ESR 103 mm/h. A CT scan showed no lung infection and multiple vermiform destructions of the left humerus (Figure 1b). MRI showed signal abnormalities of the marrow in the left scapula, and right and left humerus bones (Figure 1c).
Biopsy of the left humerus was performed and the specimen was cultured for mycobacteria. AFB-positive isolates were found 10 days later and tested by CapitalBio Mycobacterium identification microarray (CapitalBio Corp., Beijing, China) according to the manufacturer's instructions [155]. The isolate was identified as M. intracellulare. Microdilution drug susceptibility testing was carried out according to Clinical Laboratory Standard Institution recommendations [185], using MXF, levofloxacin (LVF), CLR, azithromycin (AZM), LZD, RIF, EMB, and rifabutin (RFB). Minimum inhibitory concentration (MIC) results showed that the strain was resistant to CLR (MIC > 64 μg/ml), AZM (MIC > 64 μg/ml), LZD (MIC > 64 μg/ml), RIF (MIC 4 μg/ml), EMB (MIC > 64 μg/ml), and RFB (MIC 16 μg/ml), but susceptible to MXF (MIC 0.5 μg/ml) and LVF (MIC 2 μg/ml).
PCR of the 23S rRNA gene was performed using LA Taq enzyme (Takara, Shiga, Japan) and primers 23.1 (5′-AATGGCGTAACGACTTCTC AACTGT-3′) and 23.2 (5′-GCACTAGAGGTTC GTCCGTCCC-3′) and the products were sequenced to detect mutations [148]. Sequence analysis identified an A to T substitution at the resident position 2274, and a T to C substitution at the resident position 1636 in the 23S rRNA gene. The sequence was uploaded to the GenBank database with the accession number KJ400964.
Following species identification and drug susceptibility testing in vitro, the patient was administered oral CLR 500 mg twice daily, oral EMB 750 mg once daily, and oral MXF 400 mg once daily. The patient's condition had improved 1 month later, and his temperature, CBC, and CRP were normal. However, 5 months after the therapy he complained of poor vision, and fundus examination by an ophthalmology physician showed retinal damage to both eyes. Diabetic retinopathy was considered. EMB was stopped and vitamin B was supplied daily. One month later, he again developed a low fever and his neutrophils and CRP started to increase. Despite retinal laser photocoagulation therapy in both eyes, his vision was not improved. EMB was added again to control the infection, and 1 month later his fever was controlled and his blood tests returned to normal. The patient continued to take CLR, EMB, and MXF, but his condition slowly deteriorated. MRI indicated improvements in his osteomyelitis, but after receiving anti-mycobacterial chemotherapy for 18 months, the patient suddenly died of respiratory failure. No autopsy was performed.
Reports of NTM osteomyelitis in DM patients are rare, but given the recent increase in identification of NTM as pathogens, the possibility of NTM osteomyelitis in DM patients is worthy of more attention. DM can affect cytokine levels, chemotaxis and phagocytosis of monocytes and macrophages, and can cause an abnormal delayed-type hypersensitivity response [186]. These effects are particularly serious in elderly patients with DM, and may increase the risk of intracellular infections with pathogens such as NTM and tuberculosis [186]. Osteomyelitis is a complication of DM, and is associated with increased mortality, as well as increased costs [187]. We performed a search of the English literature for reports of NTM osteomyelitis in DM patients (Table I) and identified eight cases, none of whom was HIV-positive [8,70,84,95,188–191]. The infection sites varied, but were mostly in the extremities. Three patients took immunosuppressive agents and had other serious underlying diseases, including end-stage renal disease and rheumatoid arthritis. Not all patients had a clear history of trauma or wounding, suggesting that the immunocompromised condition induced by DM might be a major risk factor for NTM infection.
Table I.
English language articles on nontuberculous mycobacteria (NTM) osteomyelitis in patients with diabetes mellitus (DM).
| Reference | Age/sex | Pathogen | Anatomic site | Underlying condition | Surgical treatment | Anti-NTM therapy | Outcome |
|---|---|---|---|---|---|---|---|
| Phoa et al. [70] | 66/M | M. scrofulaceum | Wrist | DM | None | KAN, EMB, and ETH | Improved clinically |
| Satti et al. [84] | 52/M | M. fortuitum | Bone marrow | DM | None | AMK and CIP | Cured |
| Argiris et al. [188] | 37/M | MAC | Bone marrow | DM, ESRD, renal transplant | None | AZM and EMB | Died |
| Baylor et al. [189] | 39/M | MAC | Humerus, tibia, and fibula | DM, schizophrenia | Surgical debridement | STR, CFZ, INH, EMB, RFP, PZA, CLR, and AMK | Cured |
| Iyengar et al. [95] | 58/F | M. chelonae | Finger metacarpal heads | DM, ESRD | Incision for drain, surgical debridement | CFZ and CLR | Cured |
| Conejero et al. [190] | 76/F | M. chelonae | Metatarsal and calcaneus | DM and RA | None | Intravenous IPM, CLR, and LVX | Cured |
| Halleran et al. [191] | 61/M | MAC | Wrist | DM, hypertension | Aspiration | INH and RFP | Unresolved |
| Suzuki et al. [8] | 67/M | MAC | Spine, ribs, and pelvis | DM | Surgical debridement | RFP, EMB, CLR, CS, and STR | Cured |
| This study | 69/M | M. intracellulare | Humerus and ribs | DM | Anti-mycobacterial chemotherapy | CLR, EMB, and MXF | Died |
AMK, amikacin; AZM, azithromycin; CFZ, clofazimine; CIP, ciprofloxacin; CLR, clarithromycin; CS, cycloserine; EMB, ethambutol; ESRD, end-stage renal disease; ETH, ethionamide; INH, isoniazide; IPM, imipenem; KAN, kanamycin; LVX, levofloxacin; MAC, M.avium-intracellulare complex; MXF, moxifloxacin; PZA, pyrazinamide; RA, rheumatoid arthritis; RFP, rifampicin; STR, streptomycin.
The clinical manifestations of NTM infection among DM patients vary and include fever, painless mass, fistula, and local pain [8,70,84,95,188–191]. According to his history, our patient may have initially suffered from NTM granulomatous panniculitis, with osteomyelitis developing secondary to the surgery. He had no history of trauma or wounding, but did live near a river and liked to fish, and may therefore have become infected by touching the pathogen.
The isolate from the current patient showed resistance to macrolides in vitro, and the base substitution at 2274/2275 in the 23S rRNA gene was confirmed to be associated with macrolide resistance [192,193]. Because of drug allergies, we used CLR in combination with MXF and EMB, which successfully inhibited the infection, although some side effects occurred. The current case demonstrates three points. First, macrolides might be useful in combination therapy, despite the existence of drug resistance. Second, an adequate duration of anti-mycobacterial chemotherapy is important in DM patients with NTM osteomyelitis. Third, the antibiotics used for anti-mycobacterial chemotherapy should be chosen carefully and monitored closely to avoid adverse drug events.
Conclusions
NTM osteomyelitis can have a poor prognosis and physicians should thus be aware of its clinical significance. However, the mechanisms of NTM pathogenesis remain poorly understood, and prophylactic options are currently limited. In patients with osteomyelitis caused by undetermined pathogens, care is needed to differentiate NTM infection from normal pyogenic infection or tuberculosis, through blood tests, imaging examinations, pathological examination and AFB tests, and mycobacterial culture and identification of biopsy specimens during or before surgery. In vitro drug susceptibility testing is important for anti-NTM chemotherapy, and the long duration of combined antibiotic anti-mycobacterial chemotherapy means that physicians need to monitor adverse drug events and infection progress closely.
Consent
Written informed consent was obtained from the patient and his kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Acknowledgments
Declaration of interest: The authors report no conflicts of interest. All authors are responsible for the writing of the paper. This study was funded by the National Scientific and Technological Major Project of China (grant nos 2011ZX10004-901, 2013ZX10004-904, 2014ZX10004-008), and the Fundamental Research Funds for the Central Universities.
References
- Shinnick TM, Good RC. Mycobacterial taxonomy. Eur J Clin Microbiol Infect Dis. 1994;13:884–901. doi: 10.1007/BF02111489. [DOI] [PubMed] [Google Scholar]
- Falkinham JO., 3rd Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23:529–51. doi: 10.1016/s0272-5231(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Chan ED, Kong PM, Fennelly K, Dwyer AP, Iseman MD. Vertebral osteomyelitis due to infection with nontuberculous Mycobacterium species after blunt trauma to the back: 3 examples of the principle of locus minoris resistentiae. Clin Infect Dis. 2001;32:1506–10. doi: 10.1086/320155. [DOI] [PubMed] [Google Scholar]
- Zosso C, Lienhard R, Siegrist HH, Malinverni R, Clerc O. Post liposuction infections by rapidly growing mycobacteria. Infect Dis (Lond) 2015;47:69–72. doi: 10.3109/00365548.2014.968865. [DOI] [PubMed] [Google Scholar]
- Avery RK, Eavey RD, Della Torre T, Ramos D, Pasternack MS. Bilateral otitis media and mastoiditis caused by a highly resistant strain of Mycobacterium chelonae. Pediatr Infect Dis J. 1996;15:1037–40. doi: 10.1097/00006454-199611000-00020. [DOI] [PubMed] [Google Scholar]
- Yano K, Yoshida T, Minoda Y, Horiki M, Denno K, Yoneda M, et al. Clinical outcome of the chronic flexor tenosynovitis in the hand caused by non-tuberculous mycobacterium treated by extensive tenosynovectomy and drugs. J Plast Surg Hand Surg. 2013;47:434–7. doi: 10.3109/2000656X.2013.776560. [DOI] [PubMed] [Google Scholar]
- Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43:273–90. doi: 10.1016/s0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Murai H, Miyakoshi N, Hongo M, Itoi E, Shimada Y. Osteomyelitis of the spine caused by mycobacterium avium complex in an immunocompetent patient. J Orthop Sci. 2013;18:490–5. doi: 10.1007/s00776-011-0183-7. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Mizuno Y, Nakamura I, Fukushima S, Endo K, Matsumoto T. Vertebral osteomyelitis caused by non-tuberculous mycobacteria: case reports and review. J Infect Chemother. 2013;19:972–7. doi: 10.1007/s10156-013-0550-8. [DOI] [PubMed] [Google Scholar]
- Bhatia K, Frydenberg E, Steel T, Ow-Yang M. An anterior expandable titanium cage in Mycobacterium avium vertebral osteomyelitis. J Clin Neurosci. 2011;18:431–4. doi: 10.1016/j.jocn.2010.07.108. [DOI] [PubMed] [Google Scholar]
- Yazisiz V, Erbasan F, Inan D, Ongut G, Akkaya BK, Alp A, et al. Bone marrow infection caused by Mycobacterium avium complex in a patient with systemic lupus erythematosus. Lupus. 2010;19:323–6. doi: 10.1177/0961203309346904. [DOI] [PubMed] [Google Scholar]
- Kadzielski J, Smith M, Baran J, Gandhi R, Raskin K. Nontuberculous mycobacterial osteomyelitis: a case report of Mycobacterium avium intracellulare complex tibial osteomyelitis in the setting of HIV/AIDS. Orthop J Harvard Med. 2009;11:108–11. [Google Scholar]
- Breda L, de Michele G, Nozzi M, De Sanctis S, Di Marzio D, Chiarelli F. Non-tuberculous mycobacterial osteomyelitis: an unusual cause of hip pain in immunocompetent children. Rheumatol Int. 2009;29:1487–9. doi: 10.1007/s00296-009-0844-4. [DOI] [PubMed] [Google Scholar]
- Wong NM, Sun LK, Lau PY. Spinal infection caused by Mycobacterium avium complex in a patient with no acquired immune deficiency syndrome: a case report. J Orthop Surg (Hong Kong) 2008;16:359–63. doi: 10.1177/230949900801600319. [DOI] [PubMed] [Google Scholar]
- Kahlon SS, East JW, Sarria JC. Mycobacterium-avium-intracellulare complex immune reconstitution inflammatory syndrome in HIV/AIDS presenting as osteomyelitis. AIDS Read. 2008;18:515–18. [PubMed] [Google Scholar]
- Hirakawa A, Miyamoto K, Ohno Y, Hioki A, Ogawa H, Nishimoto H, et al. Two-stage (posterior and anterior) surgical treatment of spinal osteomyelitis due to atypical mycobacteria and associated thoracolumbar kyphoscoliosis in a nonimmunocompromised patient. Spine. 2008;33:E221–4. doi: 10.1097/BRS.0b013e31816960e4. [DOI] [PubMed] [Google Scholar]
- Muller B, Kemper J, Hartwig NG, Mooi-Kokenberg EA, van Altena R, Versteegh FG. Mycobacterium avium intracellulare otomastoiditis: case report and literature review. Eur J Clin Microbiol Infect Dis. 2006;25:723–7. doi: 10.1007/s10096-006-0218-8. [DOI] [PubMed] [Google Scholar]
- Corrales-Medina V, Symes S, Valdivia-Arenas M, Boulanger C. Localized Mycobacterium avium complex infection of vertebral and paravertebral structures in an HIV patient on highly active antiretroviral therapy. South Med J. 2006;99:174–7. doi: 10.1097/01.smj.0000198645.36984.9c. [DOI] [PubMed] [Google Scholar]
- Mehta JB, Emery MW, Girish M, Byrd RP, Jr, Roy TM. Atypical Pott's disease: localized infection of the thoracic spine due to Mycobacterium avium-intracellulare in a patient without human immunodeficiency virus infection. South Med J. 2003;96:685–8. doi: 10.1097/01.SMJ.0000054604.75361.57. [DOI] [PubMed] [Google Scholar]
- Niazi S, Batra V, Zangrilli JG. Atypical mycobacterial osteomyelitis in a non-AIDS patient. Conn Med. 2002;66:387–9. [PubMed] [Google Scholar]
- Aberg JA, Chin-Hong PV, McCutchan A, Koletar SL, Currier JS. Localized osteomyelitis due to Mycobacterium avium complex in patients with Human Immunodeficiency Virus receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;35:E8–E13. doi: 10.1086/340714. [DOI] [PubMed] [Google Scholar]
- Arend SM, Janssen R, Gosen JJ, Waanders H, de Boer T, Ottenhoff TH, et al. Multifocal osteomyelitis caused by nontuberculous mycobacteria in patients with a genetic defect of the interferon-gamma receptor. Neth J Med. 2001;59:140–51. doi: 10.1016/s0300-2977(01)00152-8. [DOI] [PubMed] [Google Scholar]
- Weigl JA, Haas WH. Postoperative Mycobacterium avium osteomyelitis confirmed by polymerase chain reaction. Eur J Pediatr. 2000;159:64–9. doi: 10.1007/pl00013806. [DOI] [PubMed] [Google Scholar]
- Frosch M, Roth J, Ullrich K, Harms E. Successful treatment of Mycobacterium avium osteomyelitis and arthritis in a non-immunocompromised child. Scand J Infect Dis. 2000;32:328–9. doi: 10.1080/00365540050166045. [DOI] [PubMed] [Google Scholar]
- Flint D, Mahadevan M, Gunn R, Brown S. Nontuberculous mycobacterial otomastoiditis in children: four cases and a literature review. Int J Pediatr Otorhinolaryngol. 1999;51:121–7. doi: 10.1016/s0165-5876(99)00259-1. [DOI] [PubMed] [Google Scholar]
- Weiner BK, Love TW, Fraser RD. Mycobacterium avium intracellulare: vertebral osteomyelitis. J Spinal Disord. 1998;11:89–91. [PubMed] [Google Scholar]
- Pombo D, Woods ML, 2nd, Burgert SJ, Shumsky IB, Reimer LG. Disseminated Mycobacterium avium complex infection presenting as osteomyelitis in a normal host. Scand J Infect Dis. 1998;30:622–3. doi: 10.1080/00365549850161269. [DOI] [PubMed] [Google Scholar]
- Sheppard DC, Sullam PM. Primary septic arthritis and osteomyelitis due to Mycobacterium avium complex in a patient with AIDS. Clin Infect Dis. 1997;25:925–6. doi: 10.1086/597640. [DOI] [PubMed] [Google Scholar]
- Igram CM, Petrie SG, Harris MB. Atypical mycobacterial vertebral osteomyelitis in an immunocompetent patient. Orthopedics. 1997;20:163–6. doi: 10.3928/0147-7447-19970201-15. [DOI] [PubMed] [Google Scholar]
- Di Rocco M, Tasso L, Fondelli MP, Marino C, Romanengo M, Giacchino R, et al. Case of the month: a child with stiff neck. Eur J Pediatr. 1997;156:737–8. doi: 10.1007/s004310050702. [DOI] [PubMed] [Google Scholar]
- Weingardt JP, Kilcoyne RF, Russ PD, Johnston RJ, Nawaz S. Disseminated Mycobacterium avium complex presenting with osteomyelitis of the distal femur and proximal tibia. Skeletal Radiol. 1996;25:193–6. doi: 10.1007/s002560050062. [DOI] [PubMed] [Google Scholar]
- Kourtis AP, Ibegbu CC, Snitzer JA, Nesheim SR. Recurrent multifocal osteomyelitis due to Mycobacterium avium complex. Clin Infect Dis. 1996;23:1194–5. doi: 10.1093/clinids/23.5.1194. [DOI] [PubMed] [Google Scholar]
- Hirsch R, Miller SM, Kazi S, Cate TR, Reveille JD. Human immunodeficiency virus-associated atypical mycobacterial skeletal infections. Semin Arthritis Rheum. 1996;25:347–56. doi: 10.1016/s0049-0172(96)80020-5. [DOI] [PubMed] [Google Scholar]
- Mahan S, Jolles PR. MAI osteomyelitis. 18-Year scintigraphic follow-up. Clin Nucl Med. 1995;20:594–8. doi: 10.1097/00003072-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Jones AR, Bartlett J, McCormack JG. Mycobacterium avium complex (MAC) osteomyelitis and septic arthritis in an immunocompetent host. J Infect. 1995;30:59–62. doi: 10.1016/s0163-4453(95)92925-8. [DOI] [PubMed] [Google Scholar]
- Gallant JE, Alwood K, Chaisson RE. Osteomyelitis due to Mycobacterium kansasii and Mycobacterium avium complex in an HIV-infected patient. Infect Dis Clin Pract. 1994;3:297–99. [Google Scholar]
- Raszka WV, Jr, Trinh TT, Zawadsky PM. Multifocal M. intracellulare osteomyelitis in an immunocompetent child. Clin Pediatr (Phila) 1994;33:611–16. doi: 10.1177/000992289403301007. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Matsui H, Tsuji H. Atypical mycobacterium osteomyelitis of the fibula. Int Orthop. 1993;17:48–50. doi: 10.1007/BF00195224. [DOI] [PubMed] [Google Scholar]
- Pirofsky JG, Huang CT, Waites KB. Spinal osteomyelitis due to Mycobacterium avium-intracellulare in an elderly man with steroid-induced osteoporosis. Spine (Phila Pa 1976) 1993;18:1926–9. doi: 10.1097/00007632-199310000-00036. [DOI] [PubMed] [Google Scholar]
- Blumenthal DR, Zucker JR, Hawkins CC. Mycobacterium avium complex-induced septic arthritis and osteomyelitis in a patient with the acquired immunodeficiency syndrome. Arthritis Rheum. 1990;33:757–8. doi: 10.1002/art.1780330522. [DOI] [PubMed] [Google Scholar]
- Namey TC, Frogameni AD. Coexistent Mycobacterium intracellulare gonarthritis and patellar osteomyelitis in a patient with pulmonary sarcoidosis. A case report and literature review. Orthopedics. 1986;9:425–30. doi: 10.3928/0147-7447-19860301-17. [DOI] [PubMed] [Google Scholar]
- Collert S, Petrini B, Wickman K. Osteomyelitis caused by Mycobacterium avium. Acta Orthop Scand. 1983;54:449–51. doi: 10.3109/17453678308996600. [DOI] [PubMed] [Google Scholar]
- Cohen RJ, Samoszuk MK, Busch D, Lagios M. Occult infections with M. intracellulare in bone-marrow biopsy specimens from patients with AIDS. N Engl J Med. 1983;308:1475–6. doi: 10.1056/NEJM198306163082411. [DOI] [PubMed] [Google Scholar]
- Zvetina JR, Demos TC, Rubinstein H. Mycobacterium intracellulare infection of the shoulder and spine in a patient with steroid-treated systemic lupus erythematosus. Skeletal Radiol. 1982;8:111–13. doi: 10.1007/BF00349575. [DOI] [PubMed] [Google Scholar]
- Solheim LF, Kjelsberg F. Recurrent mycobacterial osteomyelitis. Report of a case due to Mycobacterium avium-intracellulare-scrofulaceum complex and BCG vaccination. Arch Orthop Trauma Surg. 1982;100:277–80. doi: 10.1007/BF00381669. [DOI] [PubMed] [Google Scholar]
- Bolvig L, Andresen J. Osteomyelitis due to Mycobacterium intracellulare. Pediatr Radiol. 1981;10:241–3. doi: 10.1007/BF01001591. [DOI] [PubMed] [Google Scholar]
- Bender BL, Yunis EJ. Disseminated nongranulomatous Mycobacterium avium osteomyelitis. Hum Pathol. 1980;11:476–8. doi: 10.1016/s0046-8177(80)80057-8. [DOI] [PubMed] [Google Scholar]
- Jenkin DJ, Dall G. Lesions of bone in disseminated infection due to the Mycobacterium avium-intracellulare group. Report of a case. J Bone Joint Surg Br. 1975;57:373–5. [PubMed] [Google Scholar]
- Hu LB. Mycobacterium ulcerans osteomyelitis: a closer look at the x-ray films. N Engl J Med. 1996;335:1771–2. doi: 10.1056/NEJM199612053352316. [DOI] [PubMed] [Google Scholar]
- Weed HG. Mycobacterium ulcerans and osteomyelitis. N Engl J Med. 1993:329–582. doi: 10.1056/NEJM199308193290818. [DOI] [PubMed] [Google Scholar]
- Hofer M, Hirschel B, Kirschner P, Beghetti M, Kaelin A, Siegrist CA, et al. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med. 1993;328:1007–9. doi: 10.1056/NEJM199304083281405. [DOI] [PubMed] [Google Scholar]
- Sivan M, Bose D, Athanasou N, McNally M. Mycobacterium marinum osteomyelitis of a long bone. Joint Bone Spine. 2008;75:600–2. doi: 10.1016/j.jbspin.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Wilson KC, Bielska B, Farber HW. Mycobacterium marinum osteomyelitis. Orthopedics. 2003;26:331–2. doi: 10.3928/0147-7447-20030301-17. [DOI] [PubMed] [Google Scholar]
- Shih JY, Hsueh PR, Chang YL, Chen MT, Yang PC, Luh KT. Osteomyelitis and tenosynovitis due to Mycobacterium marinum in a fish dealer. J Formos Med Assoc. 1997;96:913–16. [PubMed] [Google Scholar]
- Barton A, Bernstein RM, Struthers JK, O’Neill TW. Mycobacterium marinum infection causing septic arthritis and osteomyelitis. Br J Rheumatol. 1997;36:1207–9. doi: 10.1093/rheumatology/36.11.1207. [DOI] [PubMed] [Google Scholar]
- Clark RB, Spector H, Friedman DM, Oldrati KJ, Young CL, Nelson SC. Osteomyelitis and synovitis produced by Mycobacterium marinum in a fisherman. J Clin Microbiol. 1990;28:2570–2. doi: 10.1128/jcm.28.11.2570-2572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel G, Youssef M, Haidar R, Khater B, Kanafani ZA. Osteomyelitis at two noncontiguous sites caused by Mycobacterium marinum in an immunocompetent host: case report and literature review. J Med Liban. 2014;62:180–2. doi: 10.12816/0006221. [DOI] [PubMed] [Google Scholar]
- Williams B, Neth O, Shingadia D, Dixon G, Jupp RS, Rosendahl K, et al. Mycobacterium kansasii causing septic arthritis and osteomyelitis in a child. Pediatr Infect Dis J. 2010;29:88–9. doi: 10.1097/INF.0b013e3181b78e8b. [DOI] [PubMed] [Google Scholar]
- Yano T, Okuda S, Kato K, Kato K, Kishimoto T. Mycobacterium kansasii osteomyelitis in a patient with AIDS on highly active antiretroviral therapy. Intern Med. 2004;43:1084–6. doi: 10.2169/internalmedicine.43.1084. [DOI] [PubMed] [Google Scholar]
- Schnadig VJ, Quadri SF, Boyvat F, Borucki M. Mycobacterium kansasii osteomyelitis presenting as a solitary lytic lesion of the ulna: fine-needle aspiration findings and morphologic comparison with other mycobacteria. Diagn Cytopathol. 1998;19:94–7. doi: 10.1002/(sici)1097-0339(199808)19:2<94::aid-dc4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Capdevila O, Zurita A, Domingo E, Corbella X, Alcaide F, Monfort JL, et al. Multiple cranial osteolytic lesions due to Mycobacterium kansasii in a patient with AIDS. Scand J Infect Dis. 1998;30:305–6. doi: 10.1080/00365549850160990. [DOI] [PubMed] [Google Scholar]
- Minkin BI, Mills CL, Bullock DW, Burke FD. Mycobacterium kansasii osteomyelitis of the scaphoid. J Hand Surg Am. 1987;12:1092–4. doi: 10.1016/s0363-5023(87)80121-1. [DOI] [PubMed] [Google Scholar]
- Bakhsh WR, Mesfin A. Mycobacterium kansasii infection of the spine in a patient with sarcoidosis: a case report and literature review. J Surg Orthop Adv. 2014;23:162–5. doi: 10.3113/jsoa.2014.0162. [DOI] [PubMed] [Google Scholar]
- Kulasegaram R, Richardson D, Macrae B, de Ruiter A. Mycobacterium xenopi osteomyelitis in a patient on highly active antiretroviral therapy (HAART) Int J STD AIDS. 2001;12:404–6. doi: 10.1258/0956462011923219. [DOI] [PubMed] [Google Scholar]
- Danesh-Clough T, Theis JC, van der Linden A. Mycobacterium xenopi infection of the spine: a case report and literature review. Spine (Phila Pa 1976) 2000;25:626–8. doi: 10.1097/00007632-200003010-00015. [DOI] [PubMed] [Google Scholar]
- Clark JE, Abinun M, Flood TJ, Cant AJ. Mycobacterium xenopi osteomyelitis. Pediatr Infect Dis J. 1997;16:1011. doi: 10.1097/00006454-199710000-00027. [DOI] [PubMed] [Google Scholar]
- Molloy J, Shandilya M, Mahesh B, McShane D, El Nazir B. One to make the diagnosis. A case of non tuberculous mycobacterial mastoiditis in a nine year old female. Ir Med J. 2008;101:123–4. [PubMed] [Google Scholar]
- Elsayed S, Read R. Mycobacterium haemophilum osteomyelitis: case report and review of the literature. BMC Infect Dis. 2006;6:70. doi: 10.1186/1471-2334-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemmons RM, McAllister CK, Garces MC, Ward RL. Osteomyelitis due to Mycobacterium haemophilum in a cardiac transplant patient: case report and analysis of interactions among clarithromycin, rifampin, and cyclosporine. Clin Infect Dis. 1997;24:995–7. doi: 10.1093/clinids/24.5.995. [DOI] [PubMed] [Google Scholar]
- Phoa LL, Khong KS, Thamboo TP, Lam KN. A case of Mycobacterium scrofulaceum osteomyelitis of the right wrist. Ann Acad Med Singapore. 2000;29:678–81. [PubMed] [Google Scholar]
- Meyer JJ, Gelman SS. Multifocal osteomyelitis due to Mycobacterium szulgai in a patient with chronic lymphocytic leukemia. J Infect. 2008;56:151–4. doi: 10.1016/j.jinf.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Tappe D, Langmann P, Zilly M, Klinker H, Schmausser B, Frosch M. Osteomyelitis and skin ulcers caused by Mycobacterium szulgai in an AIDS patient. Scand J Infect Dis. 2004;36:883–5. doi: 10.1080/00365540410024736. [DOI] [PubMed] [Google Scholar]
- Luque AE, Kaminski D, Reichman R, Hardy D. Mycobacterium szulgai osteomyelitis in an AIDS patient. Scand J Infect Dis. 1998;30:88–91. doi: 10.1080/003655498750002376. [DOI] [PubMed] [Google Scholar]
- Hurr H, Sorg T. Mycobacterium szulgai osteomyelitis. J Infect. 1998;37:191–2. doi: 10.1016/s0163-4453(98)80178-3. [DOI] [PubMed] [Google Scholar]
- Hong SK, Sung JY, Lee HJ, Oh MD, Park SS, Kim EC. First case of Mycobacterium longobardum infection. Ann Lab Med. 2013;33:356–9. doi: 10.3343/alm.2013.33.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroianni A. Mycobacterium flavescens vertebral osteomyelitis in an immunocompetent host. Infez Med. 2003;11:97–101. [PubMed] [Google Scholar]
- Bar T, Mishal J, Lewkowicz A, Nahlieli O. Osteomyelitis of the mandible due to Mycobacterium abscessus: a case report. J Oral Maxillofac Surg. 2005;63:841–4. doi: 10.1016/j.joms.2004.05.229. [DOI] [PubMed] [Google Scholar]
- Kato S, Murakami H, Demura S, Yoshioka K, Hayashi H, Yokogawa N, et al. Vertebral osteomyelitis caused by Mycobacterium abscessus surgically treated using antibacterial iodine-supported instrumentation. Case Rep Orthop. 2014;2014:4. doi: 10.1155/2014/197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria JC, Chutkan NB, Figueroa JE, Hull A. Atypical mycobacterial vertebral osteomyelitis: case report and review. Clin Infect Dis. 1998;26:503–5. doi: 10.1086/517096. [DOI] [PubMed] [Google Scholar]
- Meredith FT, Sexton DJ. Mycobacterium abscessus osteomyelitis following a plantar puncture wound. Clin Infect Dis. 1996;23:651–3. doi: 10.1093/clinids/23.3.651. [DOI] [PubMed] [Google Scholar]
- Pruitt TC, Hughes LO, Blasier RD, McCarthy RE, Glasier CM, Roloson GJ. Atypical mycobacterial vertebral osteomyelitis in a steroid-dependent adolescent. A case report. Spine (Phila Pa 1976) 1993;18:2553–5. doi: 10.1097/00007632-199312000-00032. [DOI] [PubMed] [Google Scholar]
- McAvoy MJ, Carron MA, Poulik J, Altinok D, Belenky W. Sequelae of rapid growing mycobacteria otomastoiditis in a child. Arch Otolaryngol Head Neck Surg. 2009;135:602–4. doi: 10.1001/archoto.2009.52. [DOI] [PubMed] [Google Scholar]
- Kwan K, Ho ST. Mycobacterium chelonae and Mycobacterium fortuitum infection following open fracture: a case report and review of the literature. Indian J Med Microbiol. 2010;28:248–50. doi: 10.4103/0255-0857.66488. [DOI] [PubMed] [Google Scholar]
- Satti L, Abbasi S, Sattar A, Ikram A, Manzar MA, Khalid MM. Bone marrow infection with Mycobacterium fortuitum in a diabetic patient. J Coll Physicians Surg Pak. 2011;21:500–2. [PubMed] [Google Scholar]
- Cruz AT, Antekeier SB. Chronic multifocal Mycobacterium fortuitum osteomyelitis following penetrating plantar trauma. Am J Orthop (Belle Mead NJ) 2012;41:E109–11. [PubMed] [Google Scholar]
- Longardner K, Allen A, Ramgopal M. Spinal osteomyelitis due to Mycobacterium fortuitum in a former intravenous drug user. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-010326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini L, Amina S, Wang J, Calhoun JH, Mader JT. Mycobacterium tuberculosis and Mycobacterium fortuitum osteomyelitis of the foot and septic arthritis of the ankle in an immunocompetent patient. Eur J Clin Microbiol Infect Dis. 2002;21:468–70. doi: 10.1007/s10096-002-0742-0. [DOI] [PubMed] [Google Scholar]
- Tejan-Sie SA, Avery RK, Mossad SB. Mycobacterium fortuitum osteomyelitis in a peripheral blood stem cell transplant recipient. Scand J Infect Dis. 2000;32:94–6. doi: 10.1080/00365540050164317. [DOI] [PubMed] [Google Scholar]
- Miron D, El AL, Zuker M, Lumelsky D, Murph M, Floyd MM, et al. Mycobacterium fortuitum osteomyelitis of the cuboid after nail puncture wound. Pediatr Infect Dis J. 2000;19:483–5. doi: 10.1097/00006454-200005000-00023. [DOI] [PubMed] [Google Scholar]
- Gadre DV. HIV associated chronic atypical osteomyelitis by Mycobacterium fortuitum—Chelonae group – a case report. Indian J Med Sci. 1997;51:161–3. [PubMed] [Google Scholar]
- Samuels LE, Sharma S, Morris RJ, Solomon MP, Granick MS, Wood CA, et al. Mycobacterium fortuitum infection of the sternum. Review of the literature and case illustration. Arch Surg. 1996;131:1344–6. doi: 10.1001/archsurg.1996.01430240098014. [DOI] [PubMed] [Google Scholar]
- Plemmons RM, McAllister CK, Liening DA, Garces MC. Otitis media and mastoiditis due to Mycobacterium fortuitum: case report, review of four cases, and a cautionary note. Clin Infect Dis. 1996;22:1105–6. doi: 10.1093/clinids/22.6.1105. [DOI] [PubMed] [Google Scholar]
- Goodhart GL. Mycobacterium fortuitum osteomyelitis following trauma. J Orthop Trauma. 1993;7:142–5. doi: 10.1097/00005131-199304000-00007. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Watanabe R, Hayashi K, Inufusa A, Nakagawa H. Postoperative osteomyelitis due to Mycobacterium fortuitum. A case report. Arch Orthop Trauma Surg. 1992;111:178–80. doi: 10.1007/BF00388095. [DOI] [PubMed] [Google Scholar]
- Iyengar KP, Nadkarni JB, Gupta R, Beeching NJ, Ullah I, Loh WY. Mycobacterium chelonae hand infection following ferret bite. Infection. 2013;41:237–41. doi: 10.1007/s15010-012-0309-7. [DOI] [PubMed] [Google Scholar]
- Wu KC, Shu MT, Chen BN. Otomastoiditis with acute left facial nerve paralysis caused by Mycobacterium chelonae. Ear Nose Throat J. 2011;90:E18–22. doi: 10.1177/014556131109001213. [DOI] [PubMed] [Google Scholar]
- Rahman I, Bhatt H, Chillag S, Duffus W. Mycobacterium chelonae vertebral osteomyelitis. South Med J. 2009;102:1167–9. doi: 10.1097/SMJ.0b013e3181bae784. [DOI] [PubMed] [Google Scholar]
- Mullin D, Jothi S, Healy D. Mycobacterium chelonae infections involving the head and neck. Ann Otol Rhinol Laryngol. 2009;118:714–20. doi: 10.1177/000348940911801006. [DOI] [PubMed] [Google Scholar]
- Korres DS, Papagelopoulos PJ, Zahos KA, Kolia MD, Poulakou GG, Falagas ME. Multifocal spinal and extra-spinal Mycobacterium chelonae osteomyelitis in a renal transplant recipient. Transpl Infect Dis. 2007;9:62–5. doi: 10.1111/j.1399-3062.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Kim RS, Kim JS, Choi DH, Kwon DS, Jung JH. M. chelonae soft tissue infection spreading to osteomyelitis. Yonsei Med J. 2004;45:169–73. doi: 10.3349/ymj.2004.45.1.169. [DOI] [PubMed] [Google Scholar]
- Gollwitzer H, Langer R, Diehl P, Mittelmeier W. Chronic osteomyelitis due to Mycobacterium chelonae diagnosed by polymerase chain reaction homology matching. A case report. J Bone Joint Surg Am. 2004;86-A:1296–301. doi: 10.2106/00004623-200406000-00027. [DOI] [PubMed] [Google Scholar]
- Wilson S, Cascio B, Neitzschman HR. Radiology case of the month. Nail puncture wound to the foot. Mycobacterium chelonei osteomyelitis. J La State Med Soc. 1999;151:251–2. [PubMed] [Google Scholar]
- van Aarem A, Muytjens HL, Smits MM, Cremers CW. Recurrent therapy resistant mastoiditis by Mycobacterium cheilonae abscessus, a nontuberculous mycobacterium. Int J Pediatr Otorhinolaryngol. 1998;43:61–72. doi: 10.1016/s0165-5876(97)00146-8. [DOI] [PubMed] [Google Scholar]
- Thordarson DB, Patzakis MJ, Holtom P, Sherman R. Salvage of the septic ankle with concomitant tibial osteomyelitis. Foot Ankle Int. 1997;18:151–6. doi: 10.1177/107110079701800307. [DOI] [PubMed] [Google Scholar]
- Brown BA, Springer B, Steingrube VA, Wilson RW, Pfyffer GE, Garcia MJ, et al. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1999;49((Pt 4)):1493–511. doi: 10.1099/00207713-49-4-1493. [DOI] [PubMed] [Google Scholar]
- Suy F, Carricajo A, Grattard F, Cazorla C, Denis C, Girardin P, et al. Infection due to Mycobacterium thermoresistibile: a case associated with an orthopedic device. J Clin Microbiol. 2013;51:3154–6. doi: 10.1128/JCM.00925-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ingen J, Looijmans F, Mirck P, Dekhuijzen R, Boeree M, van Soolingen D. Otomastoiditis caused by Mycobacterium abscessus, The Netherlands. Emerg Infect Dis. 2010;16:166–8. doi: 10.3201/eid1601.090473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazzi MG, Chapgier A, Defilippi AC, Pistoia V, Mangini S, Savioli C, et al. Disseminated Mycobacterium scrofulaceum infection in a child with interferon-gamma receptor 1 deficiency. Int J Infect Dis. 2010;14:e167–70. doi: 10.1016/j.ijid.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Frehel C, Ryter A, Rastogi N, David H. The electron- transparent zone in phagocytized Mycobacterium avium and other mycobacteria: formation, persistence and role in bacterial survival. Ann Inst Pasteur Microbiol. 1986;137B:239–57. doi: 10.1016/s0769-2609(86)80115-6. [DOI] [PubMed] [Google Scholar]
- Kawabata Y, Semba I, Hirayama Y, Koga T, Nagao S, Takada H. Wax D of Mycobacterium tuberculosis induced osteomyelitis accompanied by reactive bone formation in Buffalo rats. FEMS Immunol Med Microbiol. 1998;22:293–302. doi: 10.1111/j.1574-695X.1998.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Broutin V, Banuls AL, Aubry A, Keck N, Choisy M, Bernardet JF, et al. Genetic diversity and population structure of Mycobacterium marinum: new insights into host and environmental specificities. J Clin Microbiol. 2012;50:3627–34. doi: 10.1128/JCM.01274-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LT, Wang CY, Lin CD, Tsai MH. Nontuberculous mycobacterial otomastoiditis: a case report. Ear Nose Throat J. 2013;92:31–3. doi: 10.1177/014556131309200110. [DOI] [PubMed] [Google Scholar]
- Portaels F, Aguiar J, Debacker M, Guedenon A, Steunou C, Zinsou C, et al. Mycobacterium bovis BCG vaccination as prophylaxis against Mycobacterium ulcerans osteomyelitis in Buruli ulcer disease. Infect Immun. 2004;72:62–5. doi: 10.1128/IAI.72.1.62-65.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherr N, Muller P, Perisa D, Combaluzier B, Jeno P, Pieters J. Survival of pathogenic mycobacteria in macrophages is mediated through autophosphorylation of protein kinase G. J Bacteriol. 2009;191:4546–54. doi: 10.1128/JB.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130:37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Corti A, Fattorini L, Thoresen OF, Ricci ML, Gallizia A, Pelagi M, et al. Upregulation of p75 tumor necrosis factor alpha receptor in Mycobacterium avium-infected mice: evidence for a functional role. Infect Immun. 1999;67:5762–7. doi: 10.1128/iai.67.11.5762-5767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TJ, Fox S, Jagger CJ, Lean JM, Chow JW. The role of prostaglandins and nitric oxide in the response of bone to mechanical forces. Osteoarthritis Cartilage. 1999;7:422–3. doi: 10.1053/joca.1998.0231. [DOI] [PubMed] [Google Scholar]
- Fox SW, Chambers TJ, Chow JW. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Physiol. 1996;270:E955–60. doi: 10.1152/ajpendo.1996.270.6.E955. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. Nitric oxide and bone. Ann N Y Acad Sci. 2010;1192:391–403. doi: 10.1111/j.1749-6632.2009.05230.x. [DOI] [PubMed] [Google Scholar]
- Turner CH, Owan I, Jacob DS, McClintock R, Peacock M. Effects of nitric oxide synthase inhibitors on bone formation in rats. Bone. 1997;21:487–90. doi: 10.1016/s8756-3282(97)00202-0. [DOI] [PubMed] [Google Scholar]
- Petitjean G, Fluckiger U, Scharen S, Laifer G. Vertebral osteomyelitis caused by non-tuberculous mycobacteria. Clin Microbiol Infect. 2004;10:951–3. doi: 10.1111/j.1469-0691.2004.00949.x. [DOI] [PubMed] [Google Scholar]
- Levin M, Newport MJ, D’Souza S, Kalabalikis P, Brown IN, Lenicker HM, et al. Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet. 1995;345:79–83. doi: 10.1016/s0140-6736(95)90059-4. [DOI] [PubMed] [Google Scholar]
- D’Souza S, Levin M, Faith A, Yssel H, Bennett B, Lake RA, et al. Defective antigen processing associated with familial disseminated mycobacteriosis. Clin Exp Immunol. 1996;103:35–9. doi: 10.1046/j.1365-2249.1996.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez LE, Wu M, Young LS. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TM, Sher A. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol. 1998;160:5428–35. [PubMed] [Google Scholar]
- Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725–34. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JL. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–51. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- Rottman M, Catherinot E, Hochedez P, Emile JF, Casanova JL, Gaillard JL, et al. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect Immun. 2007;75:5898–907. doi: 10.1128/IAI.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CG, Kullberg MC, Jankovic D, Cheever AW, Caspar P, Coffman RL, et al. Transgenic mice expressing human interleukin-10 in the antigen-presenting cell compartment show increased susceptibility to infection with Mycobacterium avium associated with decreased macrophage effector function and apoptosis. Infect Immun. 2002;70:6672–9. doi: 10.1128/IAI.70.12.6672-6679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RA, Florido M, Appelberg R. Interleukin-12 primes CD4 + T cells for interferon-gamma production and protective immunity during Mycobacterium avium infection. Immunology. 2001;103:368–74. doi: 10.1046/j.1365-2567.2001.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- Dupuis S, Doffinger R, Picard C, Fieschi C, Altare F, Jouanguy E, et al. Human interferon-gamma-mediated immunity is a genetically controlled continuous trait that determines the outcome of mycobacterial invasion. Immunol Rev. 2000;178:129–37. doi: 10.1034/j.1600-065x.2000.17810.x. [DOI] [PubMed] [Google Scholar]
- Hoflich C, Sabat R, Rosseau S, Temmesfeld B, Slevogt H, Docke WD, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. 2004;103:673–5. doi: 10.1182/blood-2003-04-1065. [DOI] [PubMed] [Google Scholar]
- Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364:2113–21. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- Browne SK, Holland SM. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol. 2010;10:534–41. doi: 10.1097/ACI.0b013e3283402b41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams LV, Waddell RD, Von Reyn CF. T-SPOT.TB Test(R) results in adults with Mycobacterium avium complex pulmonary disease. Scand J Infect Dis. 2008;40:196–203. doi: 10.1080/00365540701642179. [DOI] [PubMed] [Google Scholar]
- Hermansen TS, Thomsen VO, Lillebaek T, Ravn P. Non-tuberculous mycobacteria and the performance of interferon gamma release assays in Denmark. PLoS One. 2014;9:e93986. doi: 10.1371/journal.pone.0093986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detjen AK, Keil T, Roll S, Hauer B, Mauch H, Wahn U, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45:322–8. doi: 10.1086/519266. [DOI] [PubMed] [Google Scholar]
- Minematsu A, Sawai T, Matsutake T, Soejima Y, Naito S, Kohno S. [A case of Mycobacterium intracellulare pulmonary infection with vertebral osteomyelitis.] Kansenshogaku Zasshi. 2011;85:527–31. doi: 10.11150/kansenshogakuzasshi.85.527. [DOI] [PubMed] [Google Scholar]
- Meyers SP, Wiener SN. Diagnosis of hematogenous pyogenic vertebral osteomyelitis by magnetic resonance imaging. Arch Intern Med. 1991;151:683–7. [PubMed] [Google Scholar]
- Herlin T, Thelle T, Kragballe K, Borregaard N, Thestrup-Pedersen K. Sustained depression of monocyte cytotoxicity in a boy with disseminated nontuberculous mycobacteriosis. J Pediatr. 1981;99:264–7. doi: 10.1016/s0022-3476(81)80472-6. [DOI] [PubMed] [Google Scholar]
- Marchevsky AM, Damsker B, Green S, Tepper S. The clinicopathological spectrum of non-tuberculous mycobacterial osteoarticular infections. J Bone Joint Surg Am. 1985;67:925–9. [PubMed] [Google Scholar]
- Robicsek F, Hoffman PC, Masters TN, Daugherty HK, Cook JW, Selle JG, et al. Rapidly growing nontuberculous mycobacteria: a new enemy of the cardiac surgeon. Ann Thorac Surg. 1988;46:703–10. doi: 10.1016/s0003-4975(10)64742-x. [DOI] [PubMed] [Google Scholar]
- Smith MB, Molina CP, Schnadig VJ, Boyars MC, Aronson JF. Pathologic features of Mycobacterium kansasii infection in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2003;127:554–60. doi: 10.5858/2003-127-0554-PFOMKI. [DOI] [PubMed] [Google Scholar]
- von Graevenitz A, Punter-Streit V. Failure to recognize rapidly growing mycobacteria in a proficiency testing sample without specific request – a wider diagnostic problem? Eur J Epidemiol. 1998;14:519–20. doi: 10.1023/a:1007463630978. [DOI] [PubMed] [Google Scholar]
- Pommelet V, Vincent QB, Ardant MF, Adeye A, Tanase A, Tondeur L, et al. Findings in patients from Benin with osteomyelitis and polymerase chain reaction-confirmed Mycobacterium ulcerans infection. Clin Infect Dis. 2014;59:1256–64. doi: 10.1093/cid/ciu584. [DOI] [PubMed] [Google Scholar]
- Torriani FJ, Behling CA, McCutchan JA, Haubrich RH, Havlir DV. Disseminated Mycobacterium avium complex: correlation between blood and tissue burden. J Infect Dis. 1996;173:942–9. doi: 10.1093/infdis/173.4.942. [DOI] [PubMed] [Google Scholar]
- Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:574–6. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine EB, Karaoui LR, Mansour H. Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis. Ann Pharmacother. 2014;48:107–15. doi: 10.1177/1060028013504087. [DOI] [PubMed] [Google Scholar]
- Ispahani P, Baker M. Mycobacterial culture: how long? Lancet. 1988;1:305. doi: 10.1016/s0140-6736(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Swenson JM, Silcox VA, Bullen MG. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985;152:500–14. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- Ingram CW, Tanner DC, Durack DT, Kernodle GW, Jr, Corey GR. Disseminated infection with rapidly growing mycobacteria. Clin Infect Dis. 1993;16:463–71. doi: 10.1093/clind/16.4.463. [DOI] [PubMed] [Google Scholar]
- Chow SP, Ip FK, Lau JH, Collins RJ, Luk KD, So YC, et al. Mycobacterium marinum infection of the hand and wrist. Results of conservative treatment in twenty-four cases. J Bone Joint Surg Am. 1987;69:1161–8. [PubMed] [Google Scholar]
- Bhagat N, Read RW, Rao NA, Smith RE, Chong LP. Rifabutin-associated hypopyon uveitis in human immunodeficiency virus-negative immunocompetent individuals. Ophthalmology. 2001;108:750–2. doi: 10.1016/s0161-6420(00)00586-8. [DOI] [PubMed] [Google Scholar]
- Lee J, Armstrong DT, Ssengooba W, Park JA, Yu Y, Mumbowa F, et al. Sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob Agents Chemother. 2014;58:11–18. doi: 10.1128/AAC.01209-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaels F, Aguiar J, Debacker M, Steunou C, Zinsou C, Guedenon A, et al. Prophylactic effect of Mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli ulcer) Clin Diagn Lab Immunol. 2002;9:1389–91. doi: 10.1128/CDLI.9.6.1389-1391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis (Edinb) 2001;81:71–7. doi: 10.1054/tube.2000.0263. [DOI] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- Nightingale SD, Cameron DW, Gordin FM, Sullam PM, Cohn DL, Chaisson RE, et al. Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–33. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- Scharfstein JA, Paltiel AD, Weinstein MC, Seage GR, Losina E, Craven DE, et al. The cost-effectiveness of prophylaxis for Mycobacterium avium complex in AIDS. Int J Technol Assess Health Care. 1999;15:531–47. [PubMed] [Google Scholar]
- Good RC. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–69. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- Benson CA. Disease due to the Mycobacterium avium complex in patients with AIDS: epidemiology and clinical syndrome. Clin Infect Dis. 1994;18((Suppl 3)):S218–22. doi: 10.1093/clinids/18.supplement_3.s218. [DOI] [PubMed] [Google Scholar]
- Straus WL, Ostroff SM, Jernigan DB, Kiehn TE, Sordillo EM, Armstrong D, et al. Clinical and epidemiologic characteristics of Mycobacterium haemophilum, an emerging pathogen in immunocompromised patients. Ann Intern Med. 1994;120:118–25. doi: 10.7326/0003-4819-120-2-199401150-00004. [DOI] [PubMed] [Google Scholar]
- McNaghten AD, Adams MR, Dworkin MS. Case 23-2000: osteomyelitis in HIV-infected patients. N Engl J Med. 2001;344:66–7. doi: 10.1056/NEJM200101043440118. [DOI] [PubMed] [Google Scholar]
- Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–71. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405–12. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- Skowronski M, Zozulinska-Ziolkiewicz D, Barinow-Wojewodzki A. Tuberculosis and diabetes mellitus – an underappreciated association. Arch Med Sci. 2014;10:1019–27. doi: 10.5114/aoms.2014.46220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res. 2004;24:381–7. doi: 10.1089/1079990041535665. [DOI] [PubMed] [Google Scholar]
- Faurholt-Jepsen D, Aabye MG, Jensen AV, Range N, Praygod G, Jeremiah K, et al. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scand J Infect Dis. 2014;46:384–91. doi: 10.3109/00365548.2014.885657. [DOI] [PubMed] [Google Scholar]
- Abebe F. Is interferon-gamma the right marker for bacille Calmette-Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology. Clin Exp Immunol. 2012;169:213–19. doi: 10.1111/j.1365-2249.2012.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect Immun. 1993;61:1980–9. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MM, Balboa L, Basile JI, Lopez B, Ritacco V, de la Barrera SS, et al. Clinical isolates of Mycobacterium tuberculosis differ in their ability to induce respiratory burst and apoptosis in neutrophils as a possible mechanism of immune escape. Clin Dev Immunol. 2012;2012:152546. doi: 10.1155/2012/152546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MM, Basile JI, Lopez B, Ritacco V, Barrera L, Sasiain Mdel C, et al. Outbreaks of Mycobacterium tuberculosis MDR strains differentially induce neutrophil respiratory burst involving lipid rafts, p38 MAPK and Syk. BMC Infect Dis. 2014;14:262. doi: 10.1186/1471-2334-14-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherkat R. Enigmas of primary immunodeficiency and mycobacterial infection in our territory. Allergy Asthma Clin Immunol. 2014;10:A30. [Google Scholar]
- de Oliveira-Junior EB, Bustamante J, Newburger PE, Condino-Neto A. The human NADPH oxidase: primary and secondary defects impairing the respiratory burst function and the microbicidal ability of phagocytes. Scand J Immunol. 2011;73:420–7. doi: 10.1111/j.1365-3083.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- He H, Genovese KJ, Swaggerty CL, Nisbet DJ, Kogut MH. Differential induction of nitric oxide, degranulation, and oxidative burst activities in response to microbial agonist stimulations in monocytes and heterophils from young commercial turkeys. Vet Immunol Immunopathol. 2008;123:177–85. doi: 10.1016/j.vetimm.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Inderlied CB, Kemper CA, Bermudez LE. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BK, Falkinham JO., 3rd Superoxide dismutase activity of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Infect Immun. 1986;53:631–5. doi: 10.1128/iai.53.3.631-635.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larijani B, Mossafa N, Shoshtarizadeh P, Nouraei M, Javadi E, Shafaei A-R, et al. Phagocyte respiratory burst activity in patients with type 2 diabetes, using PMA and FMLP stimuli. Iran J Diabetes Lipid Disorders. 2003;2:53–7. [Google Scholar]
- Osar Z, Samanci T, Demirel GY, Damci T, Ilkova H. Nicotinamide effects oxidative burst activity of neutrophils in patients with poorly controlled type 2 diabetes mellitus. Exp Diabesity Res. 2004;5:155–62. doi: 10.1080/15438600490424244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–9. [PubMed] [Google Scholar]
- Bermudez LE, Young LS. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–13. [PubMed] [Google Scholar]
- Appelberg R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology. 1994;191:520–5. doi: 10.1016/S0171-2985(11)80458-4. [DOI] [PubMed] [Google Scholar]
- Eriks IS, Emerson CL. Temporal effect of tumor necrosis factor alpha on murine macrophages infected with Mycobacterium avium. Infect Immun. 1997;65:2100–6. doi: 10.1128/iai.65.6.2100-2106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ryu YJ, Lee JH, Chang JH, Shim SS. The impact of low subcutaneous fat in patients with nontuberculous mycobacterial lung disease. Lung. 2014;192:395–401. doi: 10.1007/s00408-014-9565-x. [DOI] [PubMed] [Google Scholar]
- Yuan X, Zhang T, Kawakami K, Zhu J, Zheng W, Li H, et al. Genotyping and clinical characteristics of multidrug and extensively drug-resistant tuberculosis in a tertiary care tuberculosis hospital in China. BMC Infect Dis. 2013;13:315. doi: 10.1186/1471-2334-13-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg TL, Kim EM, Simmons BA, Keasling JD, Singer SW, Soon Lee T, et al. An auto-inducible mechanism for ionic liquid resistance in microbial biofuel production. Nat Commun. 2014;5:3490. doi: 10.1038/ncomms4490. [DOI] [PubMed] [Google Scholar]
- Argiris A, Maun N, Berliner N. Mycobacterium avium complex inclusions mimicking Gaucher's cells. N Engl J Med. 1999;340:1372. doi: 10.1056/NEJM199904293401719. [DOI] [PubMed] [Google Scholar]
- Baylor P, Larson R. Mycobacterium avium septicemia with ARDS in a patient with diabetes mellitus and no other known immune-compromising condition. J Intensive Care Med. 2009;24:140–3. doi: 10.1177/0885066608330103. [DOI] [PubMed] [Google Scholar]
- Conejero R, Ara M, Lorda M, Rivera I. Mycobacterium chelonae infection in a patient being treated with adalimumab. Actas Dermosifiliogr. 2012;103:69–71. doi: 10.1016/j.adengl.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Halleran WJ, Martin NL. Nontuberculous mycobacterial arthritis: report of a chronic case. J Kans Med Soc. 1982;83:284–6. [PubMed] [Google Scholar]
- Nash KA, Inderlied CB. Genetic basis of macrolide resistance in Mycobacterium avium isolated from patients with disseminated disease. Antimicrob Agents Chemother. 1995;39:2625–30. doi: 10.1128/aac.39.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash KA, Inderlied CB. Rapid detection of mutations associated with macrolide resistance in Mycobacterium avium complex. Antimicrob Agents Chemother. 1996;40:1748–50. doi: 10.1128/aac.40.7.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]