Abstract
Background:
Bone marrow aspirate concentrate (BMAC) has emerged as a novel treatment for pathology of the knee. Despite containing a limited number of stem cells, BMAC serves as a source of growth factors that are thought to play an important role as a result of their anabolic and anti-inflammatory effects. To our knowledge, there is no systematic review regarding the outcomes of bone marrow aspirate concentrate used for the treatment of chondral defects and osteoarthritis of the knee.
Purpose:
To perform a systematic review on the outcomes of bone marrow aspirate concentrate for the treatment of chondral defects and osteoarthritis of the knee.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic review of the literature was performed using the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, PubMed, and MEDLINE from 1980 to present. Inclusion criteria were as follows: use of BMAC for treatment of chondral defects and osteoarthritis of the knee, English language, and human studies. We excluded cadaveric studies, animal studies, basic science articles, editorial articles, surveys, and studies that did not include the knee. After applying inclusion and exclusion criteria, studies were evaluated for efficacy and safety of BMAC for treatment of articular cartilage knee pathologies.
Results:
Eleven studies were considered. Of these, 5 were prospective studies, 1 was a retrospective study, 2 were case series, and 3 were case reports. Three comparative studies (2 with level 2 evidence, 1 with level 3 evidence) were found in our search; none of them were randomized. Three studies investigated the clinical efficacy of BMAC in the treatment of osteoarthritis, and 8 studies evaluated the efficacy of BMAC on focal cartilage injuries. All 3 studies regarding osteoarthritis and all 8 studies regarding focal chondral defects reported good to excellent overall outcomes with the use of BMAC.
Conclusion:
Although a growing interest for biological alternatives of treating knee pathology has been observed in the past few years, there still remains a paucity of high-quality studies. The studies included in this systematic review reported varying degrees of beneficial results with the use of BMAC with and without an additional procedure for the treatment of chondral defects and early stages of osteoarthritis. Most articles present the use of BMAC as a safe procedure and report good results.
Keywords: bone marrow aspirate concentrate, BMAC, knee, cartilage, regenerative therapy, systematic review
Bone marrow aspirate concentrate (BMAC) has emerged as an important biological tool for the orthopaedic surgeon because it is one of the few forms of delivering stem cells and growth factors currently approved by the United States Food and Drug Administration (FDA). However, in bone marrow aspirates, mesenchymal stem cells (MSCs) only represent 0.001% to 0.01% of mononuclear cells after density gradient centrifugation to remove red blood cells, granulocytes, immature myeloid precursors, and platelets.20,27 Nonetheless, BMAC serves as a source of growth factors, including platelet-derived growth factor (PDGF), transforming growth factor–beta (TGF-β), and bone morphogenetic protein (BMP)–2 and BMP-7, which are assigned to have anabolic and anti-inflammatory effects.13,21,30
The available literature regarding BMAC is limited and highly heterogeneous with respect to indications, timing, and outcomes. To our knowledge, there is no systematic review regarding the outcomes of BMAC used for the treatment of chondral defects and osteoarthritis of the knee. The purpose of this study was to systematically review the literature on BMAC outcomes for the treatment of chondral defects and osteoarthritis of the knee.
Methods
Article Identification and Selection
This study was conducted in accordance with the 2009 Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement.22 A systematic review of the literature regarding the existing evidence for outcomes for the treatment of chondral defects and osteoarthritis of the knee with BMAC was performed using the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, PubMed (1980-2014), and MEDLINE (1980-2014). The queries were performed in July 2015.
The literature search strategy included the following: search 1: (“bone marrow”[MeSH Terms] OR “bone marrow”[All Fields]) AND (“aspirate”[All Fields] OR “concentrate”[All Fields]) AND (“knee”[All Fields] OR “knee”[MeSH Terms]), search 2: (“BMAC” OR “bone marrow aspiration concentrate” OR “bone marrow aspiration”) AND (“knee” OR “knee joint” OR “knee arthritis” OR “knee osteoarthritis” OR “patellofemoral”) AND (“treatment” OR “therapy”), and search 3: bone[All fields] AND marrow[All fields] AND aspirate[All fields] AND (“knee”[Mesh Terms] OR (“knee”[All fields] AND “joint”[All fields]) OR “knee joint” [All fields]).
Inclusion criteria were as follows: BMAC for the treatment of cartilage defects or osteoarthritis, English language, and human studies. Exclusion criteria consisted of cadaveric studies, animal studies, basic science articles, editorial articles, surveys, special topics, letters to the editor, personal correspondence, studies that did not include the knee or BMAC for treatment, or studies of other pathologies not related to the cartilage.
Three investigators (J.C., C.S.D., G.M.) independently reviewed the abstracts from all identified articles. Full-text articles were obtained for review if necessary to allow further assessment of inclusion and exclusion criteria. Additionally, all references from the included studies were reviewed and reconciled to verify that no relevant articles were missing from the systematic review.
Data Collection
The level of evidence of the studies was assigned according to the classification as specified by Wright et al.35 The information was collected from the included studies. Patient demographics, follow-up, and objective and subjective outcomes were extracted and recorded. For continuous variables (eg, age, timing, follow-up, outcome scores), the mean and range were collected if reported. Data were recorded into a custom Microsoft Excel spreadsheet (Microsoft Corp) using a modified information extraction table.11
Bias
Studies classified as level of evidence 3 or 4 can potentially be affected by selection and performance bias because of the lack of randomization and prospective comparative control groups (evidence level 4), especially in populations characterized by heterogeneity of injuries. Selected studies were reviewed to ensure that authors minimized bias while recognizing the constraints present with such studies.
Results
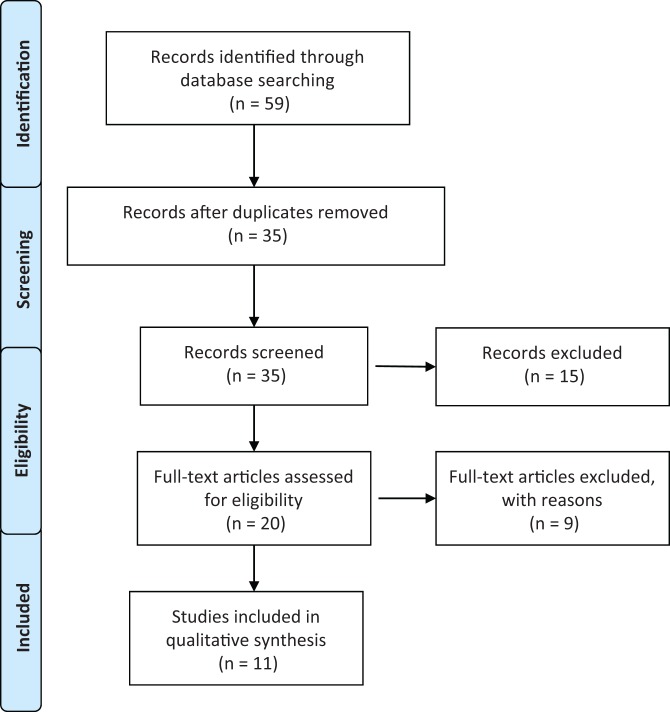
Figure 1 demonstrates the selection criteria of the studies found with our search. The systematic search performed using the previously mentioned keywords identified 20 studies. Of these, 17 were clinical articles while 3 articles used animal models and were excluded. From the clinical articles found, 5 were prospective studies,9,10,17,31,32 2 were retrospective,2,34 4 were case reports,7,14,15,23 4 were case series,3,4,9,12 1 was a surgical technique description,26 and 1 was a systematic review.5 We excluded the systematic review of treatment of chondral defects because it was specifically on the use of MSCs and not specific for BMAC nor the knee joint.5 Two of the case reports, which described BMAC as an augmentation tool for meniscal healing14 and patellar tendinopathy,23 were also excluded as well as the retrospective study regarding BMAC treatment for avascular osteonecrosis after chemotherapy.34 The surgical technique article26 was also excluded. One case report15 was excluded because the bone marrow mesenchymal cells were harvested and isolated and then expanded in the laboratory before being implanted at a later stage. After applying all exclusion criteria, 11 studies were considered for insightful data analysis.
Figure 1.
Flowchart showing selection process of systematic review.
Eight of the included studies focused on BMAC for the treatment of focal chondral defects. All studies on chondral defects included in this review reported good efficacy of BMAC in treating chondral defects, either used in combination with or without microfracture. The details of each of these studies including outcome scores and radiologic findings can be found in Tables 1 and 2.
TABLE 1.
Focal Cartilage Defect Studiesa
| Study | Study Type (N) | Age, y, Mean (Range) | Follow-up, mo (Range) | Size and Location | Treatment | Additional Factors | Results | Radiologic Findings | Second-Look Arthroscopy | Conclusion | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gobbi et al10 | P (15) | 48 (32-58) | 24 (24-38) | Mean size: 9.2 cm2; 7 patella, 6 trochlea, 4 MTP, 6 MFC, 1 LFC | BMAC covered with collagen I/III matrix | — | Significant improvement in Tegner, Lysholm, KOOS, Marx, IKDC, SF-36 scores | MRI showed complete coverage of lesions with hyaline-like cartilage in 80% | Normal to nearly normal tissue | 1-step technique with BMAC and collagen I/III matrix is a viable treatment for grade 4 knee chondral lesions | None reported |
| Gobbi et al9 | P (25) | 46.5 (32-58) | Min: 36 Mean: 41.3 | Mean size: 8.3 cm2; MFC 40.5%, patella 24.5%, trochlea 21.5% | BMAC covered with collagen I/III matrix | Ligament injuries, tibiofemoral malalignment, patellofemoral malalignment | Significant improvement in all scores: VAS, from 5.4 to 0.48; IKDC, from 37.9 to 81.7; Lysholm, from 46.4 to 86.5; Tegner, from 2.1 to 5.6 | Good stability of the implant and complete coverage of lesion in 80% of patients | Smooth newly formed tissues continuous with healthy cartilage | Treatment of large chondral defects with MSC is an effective procedure and can be performed routinely in clinical practice | None reported |
| Gobbi et al8 | P (MACI: 19, BMAC: 18) | MACI: 43 BMAC: 44.5 | Min: 36 MACI: 60 BMAC: 54 | Mean size: 5.5 cm2 (BMAC), 5.5 cm2 (MACI); PF | Comparative study: MACI vs BMAC | Patellofemoral realignment: 8 MACI, 5 BMAC HTO: 3 MACI, 5 BMAC ACLR: 1 MACI, 2 BMAC | Significant improvement in all scores; no significant difference between groups except IKDC (higher in BMAC group) | Complete filling of defects in 76% of MACI patients and 81% of the BMAC group | Hyaline-like features | Both treatments are viable and effective for large patellofemoral chondral lesions at 3-y follow-up | BMAC group: 1 MACI group: 1 Both required debridement and mobilization for intra-articular adhesions |
| Gigante et al7 | CR (1) | 37 | 24 | Size: 3 cm2; MFC | Microfracture covered with BMAC and scaffold | Microfracture | Patient asymptomatic at 24 mo | MRI at 12 months showed good defect filling with tissue signal similar to surrounding tissue. No signs of bone marrow edema | — | Covered microfracture and bone marrow concentrate is a safe and effective technique and can be adopted with an all-arthroscopic technique to treat lesions >2 cm2 | None reported |
| Enea et al4 | CS (9) | 48 | 22 | Size: 1.9-9 cm2; 7 MFC, 2 LFC | Single-stage microfracture covered with polymer-based matrix and BMAC | 1 ACL calcification removal, 1 osteochondral fixation, 1 meniscectomy, 1 trochlea resurfacing | Significant improvement in VAS pain, Lysholm, IKDC scores. Tegner score: no significant difference between pre- and postoperative but significantly better between postinjury and postoperative | Complete defect and volume filling in all patients. | 1/5 normal, 3/5 nearly normal, 1/5 abnormal. Histology showed hyaline-like repair tissue | Single-stage treatment of focal cartilage defects with microfracture and PGA-HA matrix augmented with autologous BMC is safe, improves knee function, and has potential to regenerate hyaline-like cartilage | None reported |
| Enea et al3 | CS (9) | — | 29 | Mean size: 2.6 cm2; 6 MFC, 1 LFC, 1 LFC and trochlea | Single-stage microfracture covered with collagen and BMAC | Microfracture in all patients, 1 partial meniscectomy, 1 synovectomy | Significant improvement in VAS pain, Lysholm, IKDC scores. Tegner score: no significant difference between pre- and postoperative, but significantly improved between postinjury and postoperative | Reconstitution of the original cartilage level. Bone marrow edema and/or subchondral irregularities observed in all patients | 4 patients evaluated: nearly normal, ICRS CRA grade 2. Histology: hyaline-like cartilage in 1 patient, fibrocartilage in 2, and mixed hyaline and fibrocartilage in 1 | Treatment with collagen-covered microfracture and bone marrow concentrate for focal cartilage defects in the knee is safe, improves knee function, and has potential to regenerate hyaline-like cartilage | None reported |
| Skowroński et al32 | P (54) | 18-55 | 60 | Mean size: 26.2 cm2; 59% MFC, 19% patella, 7% LFC | BMAC with collagen membrane | 7 patients had ACLR, 3 varus osteotomies, 6 patients had correction patella path | Significant improvements in Lysholm and KOOS scores in 96% of patients | — | — | 1-stage repair of large chondral lesions with BMAC is an effective treatment modality | Not reported |
| Skowroński and Rutka31 | P (46) | 26 (17-52) | 60 | >4 cm2 wide, >6 mm deep; MFC | MSC from peripheral blood vs BMC; 21 patients BMC, 25 patients MSC from peripheral blood | — | Significant improvements in all scores in both groups; VAS, Lysholm, KOOS in 86% of the patients. Treatment with MSC from peripheral blood had superior results. | Satisfactory reconstruction of the cartilaginous surface and good regenerate integration | — | Modified sandwich reconstruction is an effective treatment modality for severe osteochondral lesions. Slightly poorer results in the group treated with BMC compared with MSC from peripheral blood. | Not reported |
aACLR, anterior cruciate ligament reconstruction; BMAC, bone marrow aspirate concentrate; BMC, bone marrow concentrate; CR, case report; CS, case series; HTO, high tibial osteotomy; ICRS CRA, International Cartilage Repair Society cartilage repair assessment; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; LFC, lateral femoral condyle; MACI, matrix-induced autologous chondrocyte implantation; MFC, medial femoral condyle; MSC, mesenchymal stem cell; MRI, magnetic resonance imaging; MTP, medial tibial plateau; P, prospective; PF, patellofemoral chondral lesions; PGA-HA, polyglycolic acid–hydroxyapatite; VAS, visual analog scale.
TABLE 2.
Focal Cartilage Defect Studies: Cell Number and Type, Marrow Harvest and Concentration, Stratification of Defecta
| Study | Cell Number or Type of Cells Injected | Marrow Harvesting and Concentration | Stratification of Focal Cartilage Defect Severity |
|---|---|---|---|
| Gobbi et al10 | MSC per patient: mean ± SD, 3904 ± 1232 CFU/mL (range, 2000-5700) | Approximately 60 mL of bone marrow was harvested from the ipsilateral iliac crest and centrifuged with the BMAC Harvest Smart PreP2 System. Bone marrow concentrate was activated using batroxobin enzyme (Plateltex Act). | ICRS grade 4 |
| Gobbi et al9 | MSCs: average ± SD, 4041 ± 284 CFU/mL (range, 2500-5700) | Approximately 60 mL of bone marrow was harvested from the ipsilateral iliac crest and centrifuged with the BMAC Harvest Smart PreP2 System. Bone marrow concentrate was activated using batroxobin enzyme (Plateltex Act). | ICRS grade 4 |
| Gobbi et al8 | Not specified | Approximately 60 mL of bone marrow was harvested from the ipsilateral iliac crest and centrifuged with the BMAC Harvest Smart PreP2 System. Bone marrow concentrate was activated using batroxobin enzyme (Plateltex Act). | ICRS grade 4 |
| Gigante et al7 | Not specified | 60 mL of bone marrow blood was aspirated and processed with MarrowStim Concentration Kit, obtaining 3-4 mL of BMC. | A single 3-cm2 cartilage lesion |
| Enea et al4 | Not specified | 60 mL of bone marrow blood was aspirated and processed with MarrowStim Concentration Kit, obtaining 3-4 mL of BMC. | Lesion size ≥1.5 cm2, chondral defect Outerbridge type 3 or 4 |
| Enea et al3 | Not specified | 60 mL of bone marrow blood was aspirated and processed with MarrowStim Concentration Kit, obtaining 3-4 mL of BMC. | Lesion size ≥1.5 cm2, chondral defect Outerbridge type 3 or 4 |
| Skowroński et al32 | No description | Centrifugation of approximately 30 mL of bone marrow obtained from the ilium processed with MarrowStim Concentration Kit. | Inclusion criteria: knee cartilage lesion of 4 to 12 cm2 (mean, 6.1) classified as ICRS grade 3 or 4 |
| Skowroński and Rutka31 | Cell count in the bone marrow concentrate was in the region of 4.5 × 105 to 2.65 × 106 | 27 mL of bone marrow collected from the ilium and concentrated with MarrowStim Concentration Kit. | Inclusion criteria: a solitary osteochondral lesion in the medial femoral condyle (>4 cm2, >6 mm deep) |
aBMC, bone marrow concentrate; CFU, colony-forming unit; ICRS, International Cartilage Repair Society; MSC, mesenchymal stem cell.
Three studies evaluated the efficacy of BMAC for treatment of osteoarthritis (OA). All 3 studies report good efficacy with improvement of pain and function. Detailed results of the studies on BMAC for the treatment of OA can be found in Tables 3 and 4.
TABLE 3.
Knee Osteoarthritis Studiesa
| Study | Study Type (N) | Age, y, Mean (Range) | Follow-up, mo (Range) | Pathology | Treatment | Additional Factors | Results | Conclusion | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Centeno et al2 | R (681; 840 knees) | A: 54.3 (SD, 14.1) B: 59.9 (SD, 10.3) | A: 10.4 B: 10.7 | Knee OA | BMAC with or without adipose graft | PRP | Improved LEFS score. Mean NPS score decreased | BMAC injection for knee OA showed encouraging results. Adipose graft did not provide detectable benefit over BMAC alone. | BMAC: 6% BMAC + adipose graft: 8.9% |
| Kim et al16 | P (45; 75 knees) | 60.7 (53-80) | 8.7 (6-9) | Knee OA | BMAC injection | Arthroscopic debridement in 8%, microfracture in 6.7%, HTO in 1.3% | Significant improvement in Lysholm, IKDC, SF-36, VAS, and KOOS scores | BMAC significantly improved pain and knee function in patients with knee OA. | Joint swelling: 92% Pain: 41.3% |
| Hauser and Orlofsky 12 | CS (7 total; 6 knees) | 64 | 7.1 | Knee OA | Whole bone marrow aspirate | Injection with hyperosmotic dextrose | All patients reported improvement in pain, functionality, and quality of life | OA treatment with whole bone marrow aspirate merits further investigation. | None reported |
aBMAC, bone marrow aspirate concentrate; CS, case series; HTO, high tibial osteotomy; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; LEFS, Lower Extremity Functional Scale; NPS, Numerical Pain Scale; OA, osteoarthritis; P, prospective; PRP, platelet-rich plasma; R, retrospective; SF-36, Short Form–36; VAS, visual analog scale.
TABLE 4.
Osteoarthritis Studies: Cell Number and Type, Marrow Harvest and Concentration, Stratification of Defecta
| Publication | Cell Number or Type of Cells Injected | Marrow Harvesting and Concentration | Stratification of Focal Cartilage Defect Severity |
|---|---|---|---|
| Centeno et al2 | Approximately 10-15 mL of bone marrow aspirate was withdrawn from 6-8 sites. Isolation produced 1-3 mL of BMC injectate, which was then transported via sterile means back to the operating room. Injected with PRP and also lipoaspirate in a second group. | The aspirate was processed by hand in a sterile ISO-7 class clean room and in ISO-5 class laminar flow cabinets to isolate the buffy coat through centrifugation. | KL 2 patients were significantly more likely (2.2 times) to report ≥50% improvement on the reported outcome scale in comparison with the reference group (KL 3-4 grade). |
| Kim et al16 | Calculated estimation based off of 7 mL of bone marrow–derived mesenchymal stem cells and 10 mL of adipose tissues: 2.4 × 105 adult stem cells and 1.8 × 109 mononuclear cells. | Autologous bone marrow of 120 mL is aspirated from ASIS or PSIS of the pelvis by using SmartPReP2 Bone Marrow Procedure Pack BMAC2 kits. | KL 1, 2, 3, 4 (12, 24, 33, 6 patients, respectively) (better results KL 1-3; poor results, KL 4). |
| Hauser and Orlofsky et al12 | Whole bone marrow/not fractioned/marrow adipocytes | Not concentrated | Not reported |
aASIS, anterior superior iliac spine; BMC, bone marrow concentrate; BMAC, bone marrow aspirate concentrate; ISO, International Organization for Standardization; KL, Kellgren-Lawrence; PRP, platelet-rich plasma; PSIS, posterior superior iliac spine.
Comparative Studies
Three comparative studies (2 with level 2 evidence,8,31 1 with level 3 evidence2) were found in our search. One study compared the use of MSCs obtained from peripheral blood with MSCs obtained from bone marrow concentrate for treatment of large (>4 cm2) osteochondral lesions.31 While both treatments were reported to be effective, treatment with mesenchymal cells from peripheral blood showed superior results compared with treatment with bone marrow concentrate.31 Another study compared clinical outcomes of 2 similar groups of patients with full-thickness patellofemoral cartilage lesions treated with a scaffold and either matrix-induced autologous chondrocyte implantation (MACI) or BMAC.8 Statistical analysis of various outcome scores, magnetic resonance imaging, and standing radiographs reported significant improvements for both MACI and BMAC groups. Patients treated with BMAC showed significantly greater improvements in International Knee Documentation Committee (IKDC) scores than MACI, but in other parameters, there was no significant difference. Centeno et al2 compared the efficacy of autologous BMAC with or without an adipose-derived stem cell graft for treatment of knee osteoarthritis. They defined an adipose-derived stem cell graft as a 5- to 10-mL lipoaspirate extracted from the subcutaneous tissue on the superior buttocks or lateral thigh that was minimally processed via low-speed centrifugation or by allowing the layers to settle for several hours and then discarding the top layer. The addition of an adipose graft to the BMAC treatment was not reported to improve efficacy. However, both treatment groups received platelet-rich plasma (PRP) and plasma lysate in addition to BMAC, thereby making it difficult to determine which part of the treatment provided the most benefit.
Safety
The most common adverse events reported were swelling and pain. In 1 study, joint swelling was found in 92% of cases, while pain was reported in 41.3% of cases.16 The high incidence of swelling and pain reported in this study occurred on average 2 weeks after the injection of BMAC and adipose tissue and lasted for approximately 8 weeks.16 Centeno et al2 reported joint swelling and pain in 36 of 681 (5.3%) patients (23 in the BMAC alone group and 13 in the BMAC and adipose graft group). Additionally, Centeno et al2 reported a total of 57 adverse events (840 procedures), 3 (0.4%) of which were graded as severe. However, none of the adverse events categorized as “severe” were found to be secondary to the procedure, including 2 fatalities resulting from cancer. Some of the adverse events reported were: 2 cardiac cases, 2 cases of hematoma, 2 immune/allergic cases, and 1 renal case. Joint stiffness was reported in 2 studies,9,33 affecting 3 patients in total. Gobbi et al8 reported on 2 patients who had joint stiffness: 1 in the MACI group and another in the BMAC group. Both patients were treated with an arthroscopic lysis of adhesions with good results. One patient (2%) in a study by Skowroński et al32 was reported to have poor results due to intra-articular adhesions. Full range of motion was restored after arthroscopic lysis of adhesions. Eight of the studies included in this review reported no adverse events.
BMAC Extraction and Processing
The quantity of bone marrow aspirate extracted by most authors was 60 mL.3,4,8–10 However, Kim et al16 reported extraction of 120 mL and Skowroński et al31,32 used 30 mL of bone marrow. Gobbi et al8–10 and Kim et al16 utilized a Harvest Smart PreP2 System (Harvest Technologies) for centrifugation, and BMAC was activated using batroxobin enzyme (Plateltex Act; Plateltex SRO). Other authors3,4,7,31,32 processed their samples with MarrowStim Concentration Kit (Biomet), obtaining 3 to 4 mL of bone marrow concentrate.
Postprocedure Imaging, Second-Look Arthroscopy, and Quality of the Repair Tissue
Gobbi et al8–10 reported complete coverage of lesions seen on magnetic resonance imaging with hyaline-like cartilage in 80% to 81% of patients. They presented with normal to nearly normal tissues (hyaline cartilage–like tissues) on biopsy performed at second-look arthroscopy.8–10 Gigante et al7 reported good defect filling with tissue signal similar to surrounding tissue at 12 months with no signs of bone marrow edema. However, Enea et al3 observed bone marrow edema and subchondral irregularities in all patients. Second-look arthroscopy was performed in 5 patients, resulting in 1 normal, 3 nearly normal, and 1 abnormal result. Histology showed hyaline-like repair tissue.4
Discussion
The main finding of this review was good to excellent overall outcomes reported with the use of BMAC for the treatment of early knee osteoarthritis and moderate focal chondral defects. However, the level of evidence of the analyzed studies varied from 2 to 4.
In clinical studies, BMAC has been used to treat cartilage pathology, including both OA and focal chondral defects.7,8,10 Three studies reported BMAC to be effective in treating OA.2,12,16 However, these studies used different outcome measures and treatment protocols. Patients with moderate OA (Kellgren-Lawrence grade 2) were reported to have better clinical outcomes from BMAC administration compared with those with advanced OA (Kellgren-Lawrence grade 4).2,16 In a study by Hauser and Orlofsky,12 patients received between 2 and 6 injections of whole bone marrow with 2- to 3-month intervals. All patients reported improvement of symptoms and quality of life at follow-up. One study2 compared the treatment of osteoarthritis with injections of BMAC combined with PRP and platelet lysate, with and without adipose tissue. Subgroup analysis did not show a significant difference between the 2 groups. Importantly, of the 3 OA studies, the vast majority of patients came from 1 center.
The analyzed studies demonstrated a good effect for BMAC in treating focal cartilage defects, most of them treating large cartilage lesions (>3 cm2). The studies included report good subjective outcomes for BMAC. In some studies, BMAC was used together with microfracture and scaffolds,4,15 while in others, BMAC was used with scaffolds but without microfracture.10 In short-term follow-up, better outcomes were correlated with younger age (<45 years), smaller chondral lesion size, and fewer number of lesions, reporting good coverage of the defect as observed by magnetic resonance imaging or second-look arthroscopy9 (see Table 1). It should be noted that the 8 focal chondral studies considered in this study came from 5 centers, with the majority coming from 4 centers.
Basic science and animal model studies have reported promising results for using BMAC in treating cartilage pathology.6,29 BMAC contains MSCs, hematopoietic stem cells, platelets, growth factors, and cytokines. The anti-inflammatory and immunomodulatory properties of bone marrow stem cells can facilitate regeneration of tissue. MSCs have been reported to enhance the quality of cartilage repair by increasing aggrecan content and tissue firmness.28
It is still not clear how BMAC can be best utilized for the treatment of different conditions and which of the components of BMAC are predominantly responsible for the desired effect. Cassano, Fortier, and colleagues reported that BMAC has a significantly greater amount of monocytes and interleukin-1 receptor antagonist (IL-1RA) (unpublished data). IL-1RA (inhibits IL-1 catabolism) is thought to be responsible for the beneficial effects of the biologic autologous conditioned serum.33 The number of MSCs in bone marrow aspirates varies depending on the location of harvest, sex, and patient age, but overall, it constitutes a small quantity. In an experimental study by Lavasani et al,18 the authors suggested that the therapeutic effects of the MSCs might be mediated by secreted factors. However, the mechanisms by which MSCs potentially act remain the subject of further investigation. Some papers report that there is a lower MSC count and chondrogenic capacity in the elderly population,25 but the age limit has not been well defined.24 Other factors such as comorbidities and medication can affect the quality of bone marrow aspirates. The dose response and optimal dose for treating cartilage pathology requires further research.
The number of treatments or injections needed to obtain the intended effect was not thoroughly examined in the studies. For focal cartilage treatment, the patients who underwent a single BMAC treatment reported good results. In the studies evaluating efficacy for the treatment of OA,2,12,16 most of the outcome scores demonstrated significant improvement. However, the number of injections varied and the use of other biologic injections was not standardized. Furthermore, the relatively short follow-up in most of these studies leaves concerns regarding the durability of these treatments.
Different augmentation methods have been used in conjunction with BMAC, including adipose tissue grafts, PRP, hyaluronic acid, and collagen matrices. The best method to potentially augment BMAC remains to be determined. PRP has been reported to have a positive healing effect in treating degenerative knee pathologies.19 It is not clear whether the effects seen in the BMAC studies are a result of BMAC, adjuvant therapies, or the synergic effect. Most of the studies analyzed used a collagen matrix in association with the BMAC. The need for a graft in BMAC treatment and the optimal graft are areas that need further investigation.
The safety of using mesenchymal stem cells remains an issue. There is a concern that these cells can further develop into an unwanted lineage as oncologic cells.1 Factors that influence the differentiation of the mesenchymal stem cells are still poorly understood. Centeno et al2 reported the frequency of adverse effects after the procedure to be 6% for BMAC and 8.9% for BMAC with adipose graft. Self-limited pain and swelling were the most commonly reported adverse events. Although the authors did not define “severity,” 0.4% of adverse effects were considered severe, but it was not possible to establish a causative relationship with the procedure. Most studies in this search have short follow-up and include few patients; therefore, complications regarding cell differentiation might not be easily detected. Furthermore, 2 of the larger studies did not report on postprocedure complications.
In spite of a growing interest for the use of biological alternatives for treating knee pathology in past years, few studies were found regarding the use of BMAC during this systematic review. The current knowledge on this subject is still preliminary, as demonstrated by studies conducted with few patients, short-term follow-up, different outcome measures, and generally poor methodology. Most of the studies used different scoring systems at follow-up, making it difficult to compare results between them. To further the existing knowledge of BMAC, randomized studies with placebo or control groups are essential.
The authors recognize that this systematic review has limitations. First, there was little uniformity in reporting subjective and objective outcomes for BMAC treatment. In addition, BMAC treatment was used as an adjuvant therapy in many cases, which impedes the ability to isolate the efficacy of BMAC used as a monotherapy. All the included studies had additional cartilage procedures performed, and many had additional realignment or ligament surgery as well. Additionally, no placebo or control groups were used, making comparative analysis very difficult. The relatively short follow-up reported in most of the studies impedes assessment of the real outcome of this procedure in the long term. As with any systematic review, it is possible that relevant articles or patient subgroups were not identified with our search terms and literature review.
Conclusion
BMAC treatment appears to be a safe procedure that is growing exponentially, most likely because it represents one of the few categories allowed by the FDA to deliver stem cells (minimally manipulated). All the studies included in this systematic review reported good results, but they used different outcome measures and this heterogeneity does not allow for direct comparison.
There is a need for well-conducted randomized controlled trials with large sample sizes and defined end points to further evaluate the efficacy of BMAC for the treatment of knee pathologies. Such studies would help elucidate the safety, duration, aspirate amount, dose, need for a scaffold, and efficacy of BMAC treatment. While BMAC is used by many centers around the world, there remains a lack of level 1 or 2 evidence studies to support its use; therefore, we recommend careful usage of this modality until there is stronger evidence available.
Footnotes
One or more of the authors has disclosed the following potential conflict of interest or source of funding: R.F.L. is a consultant for and receives royalties from Arthrex, Ossur, and Smith & Nephew.
References
- 1. Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. [DOI] [PubMed] [Google Scholar]
- 2. Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Gigante A. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22:30–35. [DOI] [PubMed] [Google Scholar]
- 4. Enea D, Cecconi S, Calcagno S, et al. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20:562–569. [DOI] [PubMed] [Google Scholar]
- 5. Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21:1717–1729. [DOI] [PubMed] [Google Scholar]
- 6. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927–1937. [DOI] [PubMed] [Google Scholar]
- 7. Gigante A, Cecconi S, Calcagno S, Busilacchi A, Enea D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc Tech. 2012;1:e175–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648–657. [DOI] [PubMed] [Google Scholar]
- 10. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2:286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris JD, Quatman CE, Manring MM, Siston RA, Flanigan DC. How to write a systematic review. Am J Sports Med. 2014;42:2761–2768. [DOI] [PubMed] [Google Scholar]
- 12. Hauser RA, Orlofsky A. Regenerative injection therapy with whole bone marrow aspirate for degenerative joint disease: a case series. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Indrawattana N, Chen G, Tadokoro M, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. [DOI] [PubMed] [Google Scholar]
- 14. James EW, LaPrade CM, Feagin JA, LaPrade RF. Repair of a complete radial tear in the midbody of the medial meniscus using a novel crisscross suture transtibial tunnel surgical technique: a case report. Knee Surg Sports Traumatol Arthrosc. 2015;23:2750–2755. [DOI] [PubMed] [Google Scholar]
- 15. Kasemkijwattana C, Hongeng S, Kesprayura S, Rungsinaporn V, Chaipinyo K, Chansiri K. Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: two cases report. J Med Assoc Thai. 2011;94:395–400. [PubMed] [Google Scholar]
- 16. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24:1505–1511. [DOI] [PubMed] [Google Scholar]
- 17. Kim YS, Choi YJ, Koh YG. Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes. Am J Sports Med. 2015;43:2293–2301. [DOI] [PubMed] [Google Scholar]
- 18. Lavasani M, Robinson AR, Lu A, et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marmotti A, Rossi R, Castoldi F, Roveda E, Michielon G, Peretti GM. PRP and articular cartilage: a clinical update. Biomed Res Int. 2015;2015:542502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–886. [DOI] [PubMed] [Google Scholar]
- 21. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27:1033–1042. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 23. Pascual-Garrido C, Rolon A, Makino A. Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int. 2012;2012:953510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. 2010;18:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. 2010;18:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piontek T, Ciemniewska-Gorzela K, Szulc A, Slomczykowski M, Jakob R. All-arthroscopic technique of biological meniscal tear therapy with collagen matrix. Pol Orthop Traumatol. 2012;77:39–45. [PubMed] [Google Scholar]
- 27. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 28. Sampson S, Botto-van Bemden A, Aufiero D. Autologous bone marrow concentrate: review and application of a novel intra-articular orthobiologic for cartilage disease. Phys Sportsmed. 2013;41(3):7–18. [DOI] [PubMed] [Google Scholar]
- 29. Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy. 2009;25:1391–1400. [DOI] [PubMed] [Google Scholar]
- 30. Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. [DOI] [PubMed] [Google Scholar]
- 31. Skowroński J, Rutka M. Osteochondral lesions of the knee reconstructed with mesenchymal stem cells—results. Ortop Traumatol Rehabil. 2013;15:195–204. [DOI] [PubMed] [Google Scholar]
- 32. Skowroński J, Skowroński R, Rutka M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane—results. Ortop Traumatol Rehabil. 2013;15:69–76. [DOI] [PubMed] [Google Scholar]
- 33. Wehling P, Moser C, Frisbie D, et al. Autologous conditioned serum in the treatment of orthopedic diseases: the orthokine therapy. Biodrugs. 2007;21:323–332. [DOI] [PubMed] [Google Scholar]
- 34. Werner A, Jager M, Schmitz H, Krauspe R. Joint preserving surgery for osteonecrosis and osteochondral defects after chemotherapy in childhood. Klin Padiatr. 2003;215:332–337. [DOI] [PubMed] [Google Scholar]
- 35. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85-A:1–3. [PubMed] [Google Scholar]