Abstract
Background
Angiotensin converting enzyme (ACE) inhibitor and angiotensin receptor blocker (ARB) may induce acute kidney injury (AKI). The aim of this study was to evaluate the role of the resistive index (RI), which reflects renal artery resistance on renal duplex ultrasonography, as a predictor of AKI in chronic kidney disease (CKD) patients who are prescribed an ACE inhibitor or ARB.
Methods
We screened 105 CKD patients evaluated with renal duplex ultrasonography from 2008 to 2012. We excluded patients not treated with ACE inhibitor or ARB and diagnosed with renal artery stenosis. Finally, we retrospectively analyzed the medical records of 54 patients. AKI was defined as increased serum creatinine by >30% compared with baseline after starting ACE inhibitor or ARB treatment.
Results
The mean age of the patients was 60.5±13.0 years, serum creatinine level was 1.85±0.85 mg/dL and 22.2% of the patients had AKI after the use of an ACE inhibitor or ARB. The RI (P=0.006) and the percentages of patients with diabetes (P=0.008) and using diuretics (P=0.046) were higher in the AKI group. The area under the receiver operating characteristics curve for the prediction of AKI was 0.736 (95% confidence interval=0.587–0.885, P=0.013), and RI≥0.80 predicted AKI with 83.3% sensitivity and 61.9% specificity. In the multivariate analysis, RI≥0.80 was an independent prognostic factor [Exp (B)=8.03, 95% confidence interval=1.14–56.74, P=0.037] for AKI.
Conclusion
RI≥0.80 on the renal duplex ultrasonography may be a helpful predictor for AKI in CKD patients who are prescribed an ACE inhibitor or ARB.
Keywords: Acute kidney injury, Angiotensin converting enzyme inhibitors, Angiotensin II receptor blockers, Diuretics, Duplex Doppler ultrasonography
Introduction
Angiotensin converting enzyme (ACE) inhibitor and angiotensin II receptor blocker (ARB) slow the rate of decline of kidney function and prevent cardiovascular disease in chronic kidney disease (CKD) patients [1]. The Kidney Disease: Improving Global Outcomes (KDIGO) recommend that ACE inhibitor or ARB should be used in CKD patients with urine albumin excretion >300 mg/day, regardless of the presence of diabetes [2]. Therefore, physicians commonly prescribe ACE inhibitor and ARB to not only control hypertension but also to reduce proteinuria. However, ACE inhibitor and ARB cause renal hypoperfusion and can occasionally induce an episode of acute kidney injury (AKI), especially when used in patients with ischemic renovascular disease. However, there are no definite predictors of AKI from ACE inhibitor or ARB. Having a predictor for the occurrence of AKI after using an ACE inhibitor or ARB might be very useful.
Renal duplex ultrasonography is an accurate noninvasive diagnosis tool to evaluate significant renovascular disease in patients with renal dysfunction [3], [4]. The renal resistive index (RI) by duplex ultrasonography has been found to be a reliable marker for evaluating the resistance of the renal artery and a lower RI is associated with less resistance to flow [5], [6]. Renal RI is also a promising tool to assess the risk of AKI in critically ill patients with severe sepsis [7]. A renal RI>0.71 was helpful for distinguishing acute tubular necrosis from prerenal AKI in one study. In addition, a renal RI>0.80 was a predictor of worsened renal function and progression to renal replacement in newly diagnosed CKD patients [8]. However, there are no studies on whether renal RI by duplex ultrasonography can help predict an event of AKI caused by an ACE inhibitor and ARB treatments.
The aim of this study was to evaluate the role of RI, which reflects renal artery resistance on color Doppler ultrasonography as a predictor of AKI in CKD patients who are prescribed an ACE inhibitor or ARB.
Methods
Patient inclusion and data collection
This single center study was conducted from January 2008 to July 2012. We screened 105 CKD patients (Stage 1–5) who were evaluated with renal duplex ultrasonography. We included patients aged >20 years who were treated with an ACE inhibitor or ARB. We excluded patients with a single kidney, patients with renal replacement therapy, patients diagnosed with renal artery stenosis, and patients with clinical evidence of renovascular stenosis.
Finally, we retrospectively analyzed the medical records of 54 patients, including the patients’ characteristics, underlying disease, laboratory findings, and medications, such as diuretics. We investigated the sex, age, blood pressure, diabetes, hypertension, ischemic heart disease, cerebrovascular accidents, and peripheral artery disease of the patients. Their renal function was evaluated by serum creatinine and the glomerular filtration rate as estimated by the modified modification of diet in renal disease equation (eGFR). We did not analyze the medical records, which were not related with AKI caused by an ACE inhibitor or ARB.
Definition of AKI caused by ACE inhibitor or ARB
We defined AKI caused by an ACE inhibitor or ARB as showing any one criterion of two diagnosis criteria: (1) increased serum creatinine by >30% within 1 month after being treated with an ACE inhibitor or ARB; and (2) slowly increased serum creatinine within 3 months of >30% without other cause, rapidly recovered renal function of >30% after stopping ACE inhibitor or ARB medication and persistently maintained renal function for >3 months. All AKI patients stopped ACE inhibitor or ARB medication and took calcium channel blocker.
Renal duplex ultrasonography
The renal duplex ultrasonography was done within 6 months at the point of checking baseline serum creatinine levels for the evaluation of renal artery stenosis and renal blood flow. Renal duplex ultrasonography was performed by one radiologist using a Siemens Sequoia 512 (Siemens Medical Solutions USA, Issaquah, WA, USA).
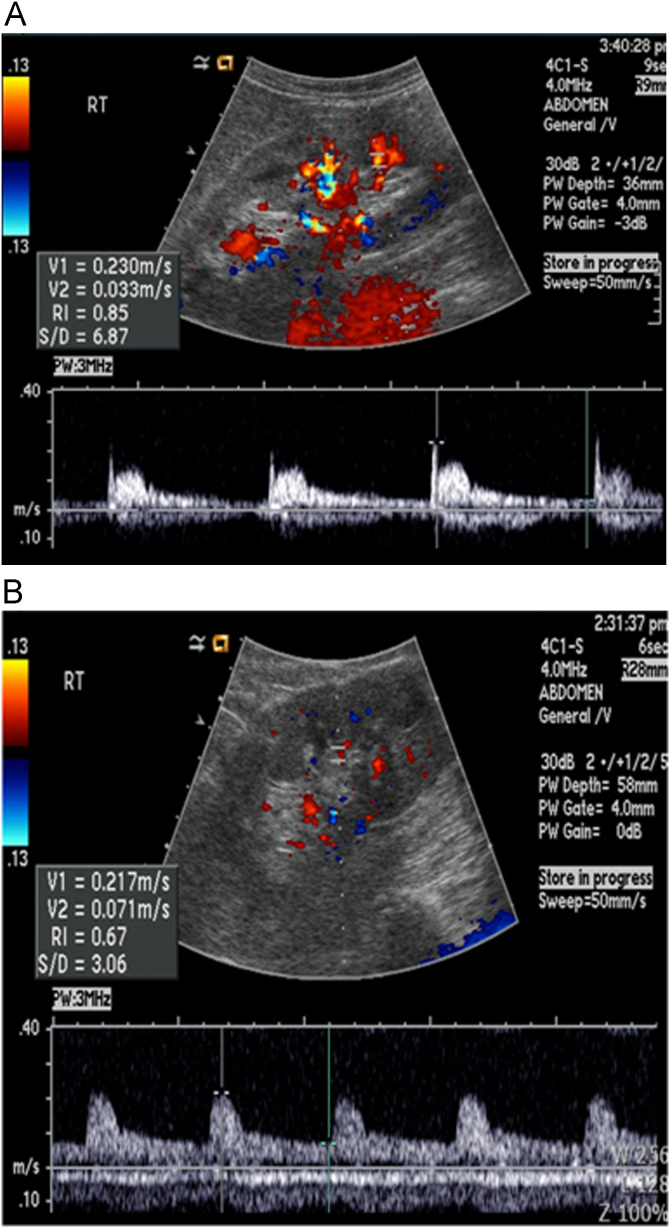
The renal RI was calculated as [1–(end diastolic frequency shift/peak systolic frequency shift)] using Doppler samples from the segmental and interlobar arteries of the left or right kidney with the higher main renal artery peak systolic velocity. Using a 3-mm Doppler sample, the parenchymal peak systolic and end diastolic frequency shifts (kHz) of the segmental and interlobar arteries were obtained (Fig. 1). The RI was evaluated at least three times in the right and left kidneys. We selected an RI cutoff value of 0.8 because renal RI >0.80 reliably identified patients at risk for progressive renal disease in previous study [8].
Figure 1.
Renal duplex ultrasonography. Findings in patients with (A) higher resistive index and (B) normal resistive index.
Statistical analysis
The data are presented as the mean±standard deviation. Fisher exact test was used to compare categorical data between the two groups. Comparisons of parametric data according to AKI and renal RI were analyzed with an independent t test. The nonparametric Mann–Whitney U test was used to compare data with a non-normal distribution. The Spearman analysis was used for the correlations with renal RI. The renal RI cutoff values for AKI events were determined by the area under the curve values through a receiver operating characteristics (ROC) curve. Univariate and multivariate logistic regression analyses were used to identify factors that affect AKI. The variables included age, sex, renal RI, and diuretic use. A P value <0.05 was considered significant. All statistical calculations were performed with SPSS software version 19.0 (IBM Corporation, Armonk, NY, USA).
Results
General patients characteristics
The patient characteristics are summarized in Table 1. Of the 54 patients, 70.4% were male, 57.4% had diabetes, and the mean age of the patients was 60.5±13.0 years. The causes of CKD were diabetic nephropathy (57.4%), hypertensive nephropathy (20.9%), glomerulonephritis including lupus nephritits (16.7%), and others (5.0%). The percentage of CKD Stage 1 was 1.9%, CKD Stage 2 was 18.5%, CKD Stage 3 was 50.0%, CKD Stage 4 was 25.9%, and CKD Stage 5 was 3.7%. The mean serum creatinine level was 1.85±0.85 mg/dL (range, 0.6–4.8 mg/dL), and the follow-up period was 36.0±21.5 months (Table 1). Fifty-two patients (90.2%) were treated with an ACE inhibitor or ARB for the control of hypertension, and two patients were treated for the control of proteinuria. Most patients (70.4%) were treated with ARB, 7.4% were treated with ACE inhibitor, and 22.2% received combination therapy with both ACE inhibitor and ARB. Thirty patients were treated with diuretics such as furosemide (43.3%), torsemide (10.0%), and spironolactone, hydrochlorothiazide, metolazone, or two diuretics (36.7%).
Table 1.
Characteristics of patients with and without AKI
| All patients (n= 54) | No AKI (n= 42) | AKI (n= 12) | P | |
|---|---|---|---|---|
| Sex (male) | 38 (70.4) | 31 (73.8) | 7 (58.3) | 0.309 |
| Age (y) | 60.5±13.0 | 59.7±14.0 | 63.4±8.5 | 0.382 |
| Medical history | ||||
| DM | 31 (57.4) | 20 (47.6) | 11 (91.7) | 0.008 |
| HTN | 49 (90.7) | 39 (92.9) | 10 (83.3) | 0.306 |
| IHD | 13 (24.1) | 9 (21.4) | 4 (33.3) | 0.453 |
| CVA | 11 (20.4) | 8 (19.0) | 3 (25.0) | 0.693 |
| PAD | 5 (9.3) | 4 (9.5) | 1 (8.3) | 0.900 |
| Baseline renal function | ||||
| Creatinine (mg/dL) | 1.85±0.85 | 1.87±0.83 | 1.80±0.94 | 0.811 |
| RUP/Cr (g/g) | 3.75±3.14 | 3.32±3.10 | 6.11±2.52 | 0.103 |
| eGFR (mL/min/1.73 m2) | 45.3±20.7 | 44.3±18.6 | 48.7±27.9 | 0.544 |
| Right kidney size (cm) | 10.43±1.56 | 10.45±1.72 | 10.36±0.89 | 0.859 |
| Systemic factor | ||||
| Hb (g/dL) | 11.94±2.19 | 12.05±2.30 | 11.55±1.81 | 0.492 |
| Albumin (g/dL) | 3.94±0.55 | 3.92±0.55 | 4.00±0.56 | 0.660 |
| SBP (mmHg) | 139.5±27.4 | 137.8±28.0 | 145.5±25.4 | 0.400 |
| DBP (mmHg) | 78.6±16.0 | 78.9±16.4 | 77.5±15.0 | 0.788 |
| PP (mmHg) | 60.9±21.6 | 58.9±22.2 | 68.0±18.1 | 0.201 |
| Cardiac EF (%) | 54.8±10.7 | 54.0±11.6 | 56.6±8.6 | 0.554 |
| Medication | ||||
| ACE inhibitor | 4 (7.4) | 2 (4.8) | 2 (16.7) | 0.210 |
| ARB | 38 (70.4) | 30 (71.4) | 8 (66.7) | 0.734 |
| ACE inhibitor+ARB | 12 (22.2) | 10 (23.8) | 2 (16.7) | 0.714 |
| Diuretics | 30 (55.6) | 20 (47.6) | 10 (83.8) | 0.046 |
| RI | 0.78±0.09 | 0.76±0.09 | 0.85±0.08 | 0.006 |
| RRT | 21 (38.9) | 13 (31.0) | 8 (66.7) | 0.042 |
Data are expressed as n (%) or mean ± SD.
ACE inhibitor, angiotensin converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin II receptor blocker; Combination, ACE inhibitor and ARB combination; Cr, creatinine; CVA, cerebro-vascular accident; DBP, diastolic blood pressure; DM, diabetes mellitus; EF, ejection fraction; eGFR, glomerular filtration rate estimated by the modified modification of diet in renal disease equation; Hb, hemoglobin; HTN, hypertension; IHD, ischemic heart disease; PAD, peripheral artery disease; PP, pulse pressure; RI, resistive index; RRT, renal replacement therapy; RUP, random urine protein; SBP, systolic blood pressure.
Patient characteristics according to AKI
Based on the definitions, 22.2% of the patients had AKI, 12.9 % of the patients had AKI after the use of an ACE inhibitor or ARB within 1 month, and 9.3% of the patients showed slow decline of renal function and rapidly recovered from AKI after stopping ACE inhibitor or ARB treatment. The percentages of patients with diabetes (P=0.008), renal progression to end-stage renal disease needing renal replacement therapy (P=0.042), and diuretic use (P=0.046) were higher in the AKI group than in the non-AKI group. The occurrence of AKI did not differ between patients with and without combination therapy of an ACE inhibitor and an ARB or between patients with and without, cardiovascular disease, including ischemic heart disease. There were no significant differences with respect to the age, sex, systolic blood pressure, diastolic blood pressure, pulse pressure, hemoglobin, creatinine and albumin levels, and ejection fraction of the enrolled patients according to AKI status. The AKI group showed a significantly higher renal RI (0.85±0.08 vs. 0.76±0.09, P=0.006) than the non-AKI group. The AKI group showed a higher renal RI (0.78±0.13 vs. 0.72±0.09) than the non-AKI group among patients taking ACE inhibitor or ARB without diuretics but was not significant. The AKI group showed a lower eGFR decrease for the periods of 24 months (−6.93±3.44 vs. −12.43±3.14, mean±standard error) than the non-AKI group among patients followed-up over 24 months but was not significant.
Patient characteristics according to renal RI
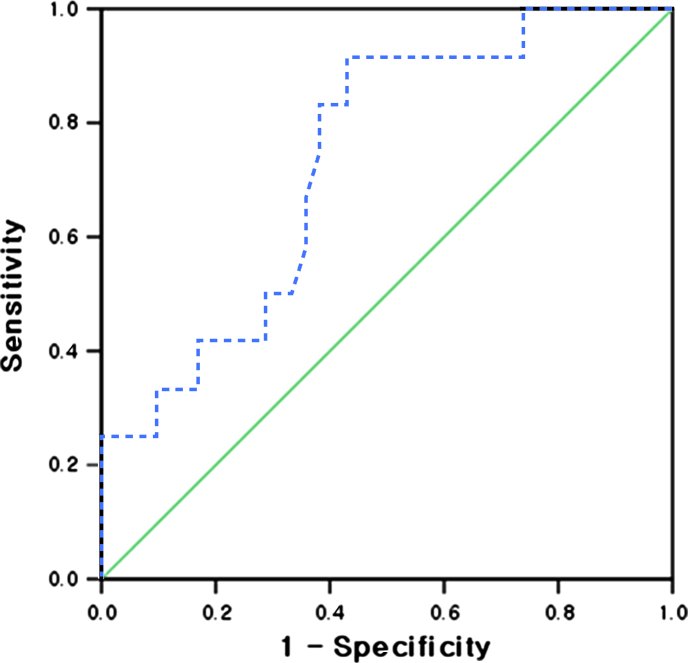
The area under the ROC curve for the prediction of AKI was 0.736 (95% confidence interval=0.587–0.885, P=0.013), and a renal RI≥0.80 predicted AKI with 83.3% sensitivity and 61.9% specificity (Fig. 2). Age (r=0.381, P=0.004), pulse pressure (r=0.451, P=0.001), and diastolic blood pressure (r=−0.375, P=0.005) were significantly correlated with the renal RI. The percentages of patients with diabetes (P<0.001) and ischemic heart disease (P=0.026) were higher in the group with a renal RI≥0.80 than in the group with a renal RI<0.80. Age (P=0.001) and pulse pressure (P=0.002) were higher and the diastolic blood pressure (P<0.001) was lower in the group with a renal RI≥0.80 than in the group with a renal RI<0.80 (Table 2).
Figure 2.
The receiver-operating characteristics (ROC) curve for the prediction of acute kidney injury (AKI). The area under the ROC curve was 0.736 (95% confidence interval= 0.587–0.885, P= 0.013), and a renal RI≥0.80 predicted AKI with 83.3% sensitivity and 61.9% specificity.
Table 2.
Characteristics of patients according to resistive index (RI) value
| RI<0.8 (n= 28) | RI≥0.8 (n= 26) | P | |
|---|---|---|---|
| Sex (male) | 20 (71.4) | 18 (69.2) | 1.000 |
| Age (y) | 55.25±13.55 | 66.15±9.70 | 0.001 |
| Medical history | |||
| DM | 9 (32.1) | 22 (84.6) | <0.001 |
| HTN | 26 (92.9) | 23 (88.5) | 0.663 |
| IHD | 3 (10.7) | 10 (38.5) | 0.026 |
| CVA | 5 (17.9) | 6 (23.1) | 0.741 |
| PAD | 1 (3.6) | 4 (15.4) | 0.184 |
| Baseline renal function | |||
| Creatinine (mg/dL) | 1.94±1.00 | 1.76±0.64 | 0.458 |
| RUP/Cr (g/g) | 3.51±3.36 | 4.20±2.81 | 0.584 |
| eGFR (mL/min/1.73 m2) | 46.7±24.0 | 43.7±16.7 | 0.606 |
| Right kidney size (cm) | 10.29±1.25 | 10.57±1.85 | 0.359 |
| Systemic Factor | |||
| Hb (g/dL) | 12.47±2.37 | 11.38±1.88 | 0.070 |
| Albumin (g/dL) | 3.98±0.61 | 3.89±0.50 | 0.570 |
| SBP (mmHg) | 138.3±22.7 | 140.8±32.1 | 0.739 |
| DBP (mmHg) | 85.9±12.0 | 70.6±16.1 | <0.001 |
| PP (mmHg) | 52.3±18.9 | 70.1±20.7 | 0.002 |
| Cardiac EF | 57.2±8.7 | 53.5±11.7 | 0.320 |
| Medication | |||
| ACE inhibitor (%) | 2(7.1) | 2 (7.7) | 1.000 |
| ARB | 19 (67.9) | 19 (73.1) | 0.770 |
| ACE inhibitor+ARB | 7 (25.0) | 5 (19.2) | 0.747 |
| Diuretics | 11 (39.3) | 19 (73.1) | 0.016 |
| RI | 0.70±0.05 | 0.86±0.04 | <0.001 |
| ESV | 0.26±0.82 | 0.28±060 | 0.411 |
| EDV | 0.09±0.66 | 0.04±0.16 | 0.009 |
| AKI | 2 (7.1) | 10 (38.5) | 0.008 |
Data are expressed as n (%) or mean ± SD.
ACE inhibitor, angiotensin converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin II receptor blocker; Combination, ACE inhibitor and ARB combination; Cr, creatinine; CVA, cerebro-vascular accident; DBP, diastolic blood pressure; DM, diabetes mellitus; EDV, end diastolic velocity; EF, ejection fraction; eGFR, glomerular filtration rate estimated by the modified modification of diet in renal disease equation; ESV, end systolic velocity; Hb, hemoglobin; HTN, hypertension; IHD, ischemic heart disease; PAD, peripheral artery disease; PP, pulse pressure; RUP, random urine protein; SBP, systolic blood pressure.
In the multivariate analysis, a renal RI≥0.80 was an independent prognostic factor [Exp (B)=8.03, 95% confidence interval=1.14–56.74, P=0.037) for AKI in patients who were prescribed ACE inhibitor or ARB (Table 3).
Table 3.
Logistic regression analysis for factors that affect AKI
| Variable | Exp(B) (95% CI) | P |
|---|---|---|
| Sex, male | 1.60 (0.35–7.39) | 0.546 |
| Age | 0.98 (0.93–1.05) | 0.713 |
| RI | 8.03 (1.14–56.74) | 0.037 |
| Diuretics use | 0.37 (0.06–2.25) | 0.282 |
AKI, acute kidney injury; CI, confidence interval; Exp(B), exponentiation of the B coefficient, which is an odds ratio; RI, resistive index.
Discussion
ACE inhibitor or ARB treatment definitely induces AKI in CKD patients under conditions with identifiable precipitating risk factors such as hypovolemia and sepsis [9]. However, ACE inhibitor or ARB may cause renal hypoperfusion and lead to AKI in the absence of definite risk factors in CKD patients with atherosclerotic renovascular disease. The existence of microvascular renal arteriolar narrowing is possible but not demonstrable on renal angiography using magnetic resonance imaging or conventional angiography in CKD patients. The renal RI on duplex ultrasonography can reflect the resistance of the renal artery and provide information regarding ischemic nephropathy. Therefore, high renal RI on duplex ultrasonography may indicate narrowed interlobar artery and segmental artery derived from atherosclerosis. We suspect that hemodynamic and prerenal mechanisms are related in AKI patients with high renal RI. First, vasodilatation capacity of narrowed renal afferent arteriole is impaired and reduced when ACE inhibitor and/or ARB administration relieve vasoconstriction of renal efferent arteriole. Second, AKI after ACE inhibitor and/or ARB administration may be related to prerenal AKI because AKI is associated with diuretics use in this study. In this retrospective study, we observed that a renal RI≥0.80 on duplex ultrasonography was a predictor of AKI caused by an ACE inhibitor or ARB treatment in CKD patients. To the best of our knowledge, this report is the first to show that a renal RI≥0.80 on duplex ultrasonography may be informative to predict AKI caused by an ACE inhibitor or ARB. Further prospective studies are necessary to confirm the role of renal RI on duplex ultrasonography as a good predictor for AKI caused by an ACE inhibitor or ARB therapy.
The renal RI on duplex ultrasonography can reflect the degree of renal damage in CKD, and a renal RI≥0.80 reliably identifies patients at risk for progressive renal disease [8]. The presence of renovascular disease assessed using renal duplex sonography was also associated with severe hypertension and decreased renal function [10], [11]. Furthermore, a high renal RI was associated with an increased risk of subsequent cardiovascular disease events and mortality [12], [13], [14]. In this study, the percentage of renal progression to renal replacement was higher in the AKI group with a higher renal RI. Therefore, the renal RI on duplex ultrasonography may be helpful for the prediction of CKD progression. We recommend renal duplex ultrasonography in place of kidney ultrasonography for the evaluation of CKD with respect to renal progression, the risk for cardiovascular disease, and the prediction of AKI caused by ACE inhibitor or ARB.
Conditions such as significant hypotension, a markedly decreased heart rate, and a perinephric fluid collection can increase the renal RI [15], [16]. The renal RI measurement should be avoided in the conditions with perinephric hematoma, systolic blood pressure <90 mmHg or heart rate <50 beats/minute. Mild edema or slight dehydration not affecting blood pressure may have a minimal influence on RI measurements. Therefore, renal RI may reflect well intrarenal vascular resistance in CKD patients with mild volume derangement. There was no case with conditions affecting renal RI in this study. We measured renal RI at the point of AKI development in patients with AKI. The sequential renal RI measurement was not done after recovering renal function in this study. However, we suspect that sequential renal RI may not be changed after recovering renal function based on high RI of AKI patients meaning ischemic nephropathy caused by atherosclerosis. Further prospective studies are necessary to elucidate the sequential change of renal RI in AKI caused by an ACE inhibitor or ARB.
Vasodilatation of the efferent glomerular arterioles caused by an ACE inhibitor or ARB results in a decreased intraglomerular pressure and reductions in both GFR and urine albumin excretion for long-term renoprotection. A reversible reduction in the serum creatinine level of up to 30% can be regarded as a physiological response caused by an ACE inhibitor or ARB treatment. One cohort analysis of elderly diabetic patients showed an increased ratio of end-stage renal disease after the use of ACE inhibitor for 3 years or longer [17]. The CHARM (Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity) trials also revealed a higher rate of the doubling of the serum creatinine level in the candesartan-treated group compared with the placebo-treated group. In addition, a recent study showed that the cessation of ACE inhibitor or ARB increased GFR up to 50% of the baseline level in advanced CKD patients [18]. Our study also showed that the serum creatinine level was decreased more than 30% after stopping ACE inhibitor or ARB therapy in five CKD patients with a high renal RI. Therefore, whether renal function is recovering after the cessation of ACE inhibitor or ARB treatment should be considered when the patient's renal function is rapidly declining without other precipitating risk factors.
One study showed that of 100 CKD patients with reversible AKI following the discontinuation of ACE inhibitors and/or ARB, 75% were older than 65 years, 63% older than 70 years, and 23% older than 80 years old [9]. In this study, the mean age of the AKI group was not significantly higher than that of the non-AKI group. However, the mean age of patients with a renal RI≥0.8 was >65 years and significantly higher than that of patients with a renal RI<0.8. Therefore, we suggest that physicians should use ACE inhibitor and/or ARB with caution, especially in patients aged >65 years. Patients receiving diuretics and with diabetes were also at high risk for AKI caused by an ACE inhibitor and/or ARB in this study. This result may be explained by a high atherosclerotic risk of diabetes and the risk of decreased plasma volume caused by diuretics. Our study also showed that age, higher pulse pressure, lower diastolic blood pressure, diabetes, and ischemic heart disease related with atherosclerosis were associated with a higher renal RI. Therefore, patients with these clinical findings should be evaluated with renal duplex ultrasonography.
This study has several limitations. First, the sample size is small and the study design is retrospective. Second, we measured an renal RI at one point without sequential renal RI measurement, although there is no case with conditions affecting renal RI in this study. Despite these limitations, we found that a renal RI≥0.80 on Doppler ultrasonography could be an indicator of the possible occurrence of AKI caused by ACE inhibitor or ARB treatment. In addition, we found that elderly patients, patients taking diuretics, patients with ischemic heart disease, patients with diabetes and patients with high pulse pressure and low diastolic pressure may be at risk of having high renal RI.
In conclusion, a renal RI≥0.80 on Doppler ultrasonography may be a helpful predictor for AKI in CKD patients who are prescribed ACE inhibitors or ARBs. In particular, we suggest that physicians watch for events of AKI in diabetic CKD patients taking diuretics and with a high renal RI after prescribing an ACE inhibitor or ARB.
Conflict of interest
No conflict of interest has been declared.
Acknowledgments
This study was supported by research funds from Dong-A University (Busan, Korea).
References
- 1.Ripley E. Complementary effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in slowing the progression of chronic kidney disease. Am Heart J. 2009;157(6 Suppl):S7–S16. doi: 10.1016/j.ahj.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler D.C., Becker G.J. Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int. 2013;83:377–383. doi: 10.1038/ki.2012.425. [DOI] [PubMed] [Google Scholar]
- 3.Hansen K.J., Tribble R.W., Reavis S.W., Canzanello V.J., Craven T.E., Plonk G.W., Jr, Dean R.H. Renal duplex sonography: evaluation of clinical utility. J Vasc Surg. 1990;12:227–236. doi: 10.1067/mva.1990.22791. [DOI] [PubMed] [Google Scholar]
- 4.Motew S.J., Cherr G.S., Craven T.E., Travis J.A., Wong J.M., Reavis S.W., Hansen K.J. Renal duplex sonography: main renal artery versus hilar analysis. J Vasc Surg. 2000;32:462–471. doi: 10.1067/mva.2000.108643. [DOI] [PubMed] [Google Scholar]
- 5.Darmon M., Schortgen F., Vargas F., Liazydi A., Schlemmer B., Brun-Buisson C., Brochard L. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 2011;37:68–76. doi: 10.1007/s00134-010-2050-y. [DOI] [PubMed] [Google Scholar]
- 6.Wan L., Yang N., Hiew C.Y., Schelleman A., Johnson L., May C., Bellomo R. An assessment of the accuracy of renal blood flow estimation by Doppler ultrasound. Intensive Care Med. 2008;34:1503–1510. doi: 10.1007/s00134-008-1106-8. [DOI] [PubMed] [Google Scholar]
- 7.Schnell D., Deruddre S., Harrois A., Pottecher J., Cosson C., Adoui N., Benhamou D., Vicaut E., Azoulay E., Duranteau J. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock. 2012;38:592–597. doi: 10.1097/SHK.0b013e318271a39c. [DOI] [PubMed] [Google Scholar]
- 8.Radermacher J., Ellis S., Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703. doi: 10.1161/hy0202.103782. [DOI] [PubMed] [Google Scholar]
- 9.Onuigbo M.A., Onuigbo N.T. Late-onset renal failure from angiotensin blockade (LORFFAB) in 100 CKD patients. Int Urol Nephrol. 2008;40:233–239. doi: 10.1007/s11255-007-9299-2. [DOI] [PubMed] [Google Scholar]
- 10.Zierler R.E., Bergelin R.O., Isaacson J.A., Strandness D.E., Jr Natural history of atherosclerotic renal artery stenosis: a prospective study with duplex ultrasonography. J Vasc Surg. 1994;19:250–257. doi: 10.1016/s0741-5214(94)70100-8. [DOI] [PubMed] [Google Scholar]
- 11.Tullis M.J., Zierler R.E., Caps M.T., Bergelin R.O., Cantwell-Gab K., Strandness D.E. Clinical evidence of contralateral renal parenchymal injury in patients with unilateral atherosclerotic renal artery stenosis. Ann Vasc Surg. 1998;12:122–127. doi: 10.1007/s100169900127. [DOI] [PubMed] [Google Scholar]
- 12.Edwards M.S., Craven T.E., Burke G.L., Dean R.H., Hansen K.J. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med. 2005;165:207–213. doi: 10.1001/archinte.165.2.207. [DOI] [PubMed] [Google Scholar]
- 13.Edwards M.S., Hansen K.J., Craven T.E., Cherr G.S., Bleyer A.J., Burke G.L., Dean R.H. Relationships between renovascular disease, blood pressure, and renal function in the elderly: a population-based study. Am J Kidney Dis. 2003;41:990–996. doi: 10.1016/s0272-6386(03)00196-3. [DOI] [PubMed] [Google Scholar]
- 14.Crutchley T.A., Pearce J.D., Craven T.E., Stafford J.M., Edwards M.S., Hansen K.J. Clinical utility of the resistive index in atherosclerotic renovascular disease. J Vasc Surg. 2009;49:148–155. doi: 10.1016/j.jvs.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Pozniak M.A., Kelcz F., Stratta R.J., Oberley T.D. Extraneous factors affecting resistive index. Invest Radiol. 1988;23:899–904. doi: 10.1097/00004424-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Mostbeck G.H., Gössinger H.D., MalIek A., Siostrzonek P., Schneider B., Tscholakoff D. Effect of heart rate on Doppler measurements of resistive index in renal arteries. Radiology. 1990;175:511–513. doi: 10.1148/radiology.175.2.2183288. [DOI] [PubMed] [Google Scholar]
- 17.Suissa S., Hutchinson T., Brophy J.M., Kezouh A. ACE-inhibitor use and the long-term risk of renal failure in diabetes. Kidney Int. 2006;69:913–919. doi: 10.1038/sj.ki.5000159. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A.K., Kamath N.S., El Kossi M., El Nahas A.M. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2010;25:3977–3982. doi: 10.1093/ndt/gfp511. [DOI] [PubMed] [Google Scholar]