Abstract
Background
Cardiovascular disease is the main cause of mortality in dialysis patients. Carotid intima–media thickness (CIMT) is used as a surrogate marker of early atherosclerosis. Atherosclerosis can cause vascular access failure. The purpose of this study was to define the clinical features of atherosclerosis in hemodialysis patients based on CIMT and to define the relationship between CIMT and access failure.
Methods
In this cross-sectional study, the CIMT of 60 patients on hemodialysis was examined using B-mode Doppler ultrasonography between May 2012 and November 2012. Carotid atherosclerosis was defined as a CIMT≥0.9 mm or the incidence of atherosclerotic plaques.
Results
The patients’ mean age was 54.5±10.6 years, and 60% of the patients were male. The CIMT was 0.81±0.47 mm (range, 0.35–2.50 mm). The group with atherosclerosis was characterized by older age compared with those without atherosclerosis. Patients with atherosclerosis showed much shorter durations of access patency than their counterparts in the nonatherosclerosis group (hazard ratio, 2.822; 95% confidence interval, 1.113–7.156; P=0.029). Moreover, being overweight was associated with a 2.47-fold (95% confidence interval, 1.101–5.548) increased primary access failure.
Conclusion
This study shows that atherosclerosis is associated with older age. Patients who are overweight and have atherosclerosis may have shortened access patency.
Keywords: Atherosclerosis, Carotid intima–media thickness, Hemodialysis, Hemodialysis vascular access
Introduction
Cardiovascular disease is a principal cause of morbidity and mortality in dialysis patients. The United States Renal Data System reports that cardiovascular disease accounts for 30–40% of all deaths [1]. Cardiovascular disease ranks as the most common cause (46.8%) of death among Korean dialysis patients according to the registry committee of the Korean Society of Nephrology in 2011 [2]. Atherosclerosis is a condition in which an artery wall thickens as a result of the accumulation of fatty materials. Atherosclerosis is the dominant cause of cardiovascular disease including myocardial infarction, heart failure, stroke, and claudication [3]. Kawagishi et al [4] reported that hemodialysis (HD) patients show advanced atherosclerosis in the carotid artery compared with age-matched healthy controls. Assessment of the carotid intima–media thickness (CIMT) using B-mode ultrasound is a useful clinical tool for the measurement of atherosclerosis. A CIMT≥0.9 mm has been shown to be a marker of generalized atherosclerosis and is associated with cardiovascular risk factors [5], [6]. In previous studies, HD patients were shown to exhibit increased intima–media thickness (IMT), common carotid plaque, arterial stiffness, and coronary artery calcification [7], [8], [9], [10].
Vascular access dysfunction is a major cause of morbidity and hospitalization in HD patients. The main cause of arteriovenous dysfunction is thrombosis, secondary to disproportionate intimal hyperplasia in the venous outflow tract. However, atherosclerotic lesions including calcification may also cause arterial problems and vascular stiffness. Calcified arteries might not remodel appropriately to provide an adequate flow to the fistula [11]. And extensive calcification in the intima and media of venous segment might result in reduced venous compliance by limiting the outward remodeling of arteriovenous fistula (AVF) maturation [11], [12]. There have been only a few studies that ascertained the association between atherosclerosis and vascular access failure (VAF) [13].
Our study objectives were: (1) to analyze clinical features of atherosclerosis in HD patients; and (2) to assess the relationship between atherosclerosis and vascular access failure.
Methods
Participants
Between May 2012 and November 2012, we evaluated 60 HD patients with AVFs and grafts. Each participant gave informed consent to participate in the study. This study protocol was approved by the Soonchunhyang University Hospital Institutional Review Board (Bucheon, South Korea; SCHBC-IRB-2012-65). The enrolled patients received 12–15 hours of HD each week by using a bicarbonate dialysate. The clinical history of the patients was obtained from their medical records. CIMT measurement was performed on the day of HD. Carotid atherosclerosis was defined as a CIMT ≥0.9 mm or the incidence of plaques.
AVF failure episodes were defined as a need for percutaneous transluminal angioplasty and/or surgery, and each episode was recorded.
CIMT measurement
CIMT was measured prior to or during HD by a single nephrologist who used B-mode ultrasonography [LOGIQ e ultrasound system (GE Healthcare, Milwaukee, WI, USA)] with a high-resolution 4–12 MHz imaging transducer. For the carotid artery examination, all participants were instructed to lay supine with their necks extended in mild lateral rotation. The right common carotid artery, bifurcation, and internal carotid artery at the bulb level were scanned for plaque and measured for IMT. The areas measured were 10 mm and 20 mm after the carotid bifurcation in the right internal carotid artery. Then, the mean IMT was obtained by averaging the two measurements. The CIMT was defined as the distance from the leading edge of the first echogenic line (lumen–intima interface) to the leading edge of the second line (media–adventitia interface).
When plaque was present in the segment used for measuring the mean IMT, the plaque thickness was averaged to the mean IMT measurement. Plaque was designated as a focal intima–media thickening of ≥1.1 mm.
Laboratory measurements
Serum intact fibroblast growth factor (FGF) 23 was measured using a two-site monoclonal antibody enzyme-linked immunosorbent assay (ELISA) system (ELISA assay; Kainos Laboratory, Tokyo, Japan; reference range=8.2–54.3 pg/mL). All other laboratory variables, including calcium, phosphate, and intact parathyroid hormone (PTH), were determined using conventional laboratory techniques after 8-hour fasting. The level of glycosylated hemoglobin (HbA1c) was determined in diabetic patients. Fasting blood samples were collected on the day of dialysis just prior to starting the dialysis.
Statistical analysis
We used the SPSS software for all analyses (version 14.0; SPSS Inc., Chicago, IL, USA). In Table 1, Table 2, the demographic and hemodynamic data are presented as mean±standard deviation (SD), median (interquartile range), or the percentage of the total. The multiple regression model was used for the correlation analysis of CIMT and variables. The difference in frequency was tested using Pearson Chi-square analysis. Differences between the groups regarding the numerical data were assessed using the Mann–Whitney test. The Kaplan–Meier method was used to evaluate the time-to-event distribution. We calculated adjusted hazard ratios (HRs) with 95% confidence intervals (95% CIs) for patency loss using Cox regression analysis. A P value <0.05 was considered statistically significant.
Table 1.
Clinical and biochemical characteristics of the study patients
| Characteristics | Value |
|---|---|
| Age (y) | 54.52±10.63 |
| Sex (M/F) | 36/24 (60.0/40.0) |
| BMI (kg/m2) | 22.63 (202.485–246.62) |
| Systolic blood pressure (mmHg) | 134.70±19.327 |
| Diastolic blood pressure (mmHg) | 76.105±13.22 |
| Underlying disease | |
| Hypertension | 51 (80.00) |
| Diabetes mellitus | 26 (43.33) |
| Angina | 7 (11.67) |
| Peripheral arterial occlusive disease | 9 (15.00) |
| Stroke | 4 (6.67) |
| Smoking | 17 (28.33) |
| Albumin (g/dL) | 3.90 (3.60–4.20) |
| Calcium (mg/dL) | 8.60 (3.60–9.10) |
| Phosphate (mg/dL) | 4.80 (4.253–6.253) |
| PTH (pg/mL) | 122.30 (52.40–210.70) |
| FGF23 (pg/mL) | 1532.12 (423.325–2122.56) |
| Duration of hemodialysis (mo) | 52.00 (20.30–85.50) |
| Duration of access survival (mo) | 26.00 (6.80–60.00) |
| Duration from hemodialysis to first access revision (mo) | 17.00 (2.00–52.00) |
Data are presented as mean±SD, n (%) or median (IQR).
BMI, body mass index; FGF, fibroblast growth factor; IQR, interquartile range; PTH, parathyroid; SD, Standard deviation.
Table 2.
Comparison of clinical characteristics between individuals with atherosclerosis and without atherosclerosis
| Characteristics | Patients with atherosclerosis (IMT≥0.9 mm) (n=14) | Patients without atherosclerosis (IMT<0.9 mm) (n=46) | P |
|---|---|---|---|
| Age (y) | 62.21±11.25 | 52.17±9.36 | 0.008 |
| Diabetes mellitus | 9 (64.293) | 17 (37.00) | 0.071 |
| Cardiovascular disease | 3 (21.43) | 4 (8.70) | 0.337 |
| Peripheral arterial disease | 4 (28.576) | 5 (10.879) | 0.193 |
| Stroke | 1 (7.14) | 3 (6.52) | 1.000 |
| ESR (mm/h) | 42.50 (24.50–120.00) | 31.00 (10.00–66.00) | 0.054 |
| CRP (mg/dL) | 0.25 (0.13–1.28) | 0.13 (0.06–0.55) | 0.209 |
| HbA1C | 5.80 (5.15–6.45) | 7.50 (6.10–8.90) | 0.004 |
| Total cholesterol (mg/dL) | 135.50 (110.50–159.75) | 121.00 (109.00–162.25) | 0.727 |
| HDL-cholesterol (mg/dL) | 37.00 (30.00–45.00) | 42.00 (36.00–50.00) | 0.141 |
| LDL-cholesterol (mg/dL) | 93.00 (57.50–107.00) | 60.00 (48.50–84.25) | 0.063 |
| Triglycerides (mg/dL) | 129.00 (69.50–146.00) | 75.50 (48.75–114.50) | 0.081 |
| Albumin (g/dL) | 3.75 (3.28–4.00) | 3.90 (3.60–4.20) | 0.079 |
| Calcium (mg/dL) | 8.30 (7.55–9.00) | 8.70 (8.20–9.10) | 0.195 |
| Phosphate (mg/dL) | 4.55 (3.65–5.80) | 4.85 (4.48–6.38) | 0.173 |
| PTH (pg/mL) | 82.56 (46.04–137.50) | 174.95 (66.57–286.45) | 0.057 |
| FGF23 (pg/mL) | 1686.17 (187.68–2122.57) | 1415.10 (448.07–2122.57) | 0.906 |
| AVF | 10 (71.43) | 32 (69.576) | 1.000 |
| Incidence of access failure | 7 (50.00) | 24 (52.172) | 0.887 |
| Duration from hemodialysis to first access failure (mo) | 3.00 (1.00–24.00) | 23.00 (2.25–60.00) | 0.076 |
Data are presented as mean±SD, n (%) or median (IQR).
AVF, arteriovenous fistula; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FGF, fibroblast growth factor; HbA1C, glycosylated hemoglobin; HDL, high-density lipoprotein; IMT, intima-media thickness; IQR, interquartile range; LDL, low-density lipoprotein; PTH, parathyroid hormone; SD, standard deviation.
Results
Patient characteristics
The baseline demographic and the clinical and biochemical parameters of the 60 patients are reported in Table 1. The mean age of the patients in this study was 54.5±10.6 years, and 60% of the patients were male. Hypertension was present in 51 (85%), diabetes mellitus in 26 (43.3%), and smoking in 17 (28.3%) of the patients. The mean duration of HD was 65.78±60.56 months. The mean access survival was 62.15±54.57 months. In patients with access failure, the mean time to first access failure was 34.90±44.57 months.
CIMT and factors associated with CIMT
The median CIMT was 0.81±0.47 mm (range, 0.35–2.50 mm). Of the 60 patients, 12 had plaques. Based on the definition of atherosclerosis (CIMT≥0.9 mm), we divided the patients into two groups: atherosclerosis group (n=14) and nonatherosclerosis group (n=46) [14]. In the atherosclerosis group, 10 patients had native AVF (71.4%, 10 lower arm), whereas 32 patients were dialyzed via native AVF in the nonatherosclerosis group (69.6%, 31 lower arm, 1 upper arm). There was no significant difference in terms of access type (P=1.00). Patients in the atherosclerosis group were older than those in the nonatherosclerosis group. Furthermore, patients in the atherosclerosis group had lower levels of HbA1C and a higher frequency of plaques (Table 2). The atherosclerosis group had higher levels of total cholesterol, low-density lipoprotein cholesterol, and triglyceride, and a lower level of high-density lipoprotein cholesterol and albumin than the nonatherosclerosis group, but the differences were not statistically significant. The inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were also higher in the atherosclerosis group, but again did not reach statistical significance.
Of these factors, CIMT showed a positive correlation with ESR (β=0.005, P=0.006; Table 3). Age showed a positive correlation but not a statistically significant relationship with CIMT (β=0.011, P=0.064).
Table 3.
Correlations between IMT and laboratory findings by multiple regression analysis
| Characteristics | β | P |
|---|---|---|
| Age⁎ | 0.011 | 0.064 |
| BMI† | 0.116 | 0.333 |
| Systolic Blood pressure‡ | 0.153 | 0.334 |
| Diastolic Blood pressure‡ | 0.008 | 0.081 |
| ESR‡ | 0.005 | 0.006* |
| HbA1C‡ | −0.383 | 0.409 |
| CRP‡ | 0.010 | 0.869 |
| Total cholesterol‡ | 0.601 | 0.482 |
| HDL-cholesterol‡ | −7.988E−5 | 0.983 |
| LDL-cholesterol‡ | 0.003 | 0.179 |
| Triglycerides‡ | 0.000 | 0.703 |
| Albumin‡ | 0.049 | 0.713 |
| Calcium‡ | 0.050 | 0.273 |
| Phosphate‡ | −0.039 | 0.284 |
| PTH‡ | 0.000 | 0.295 |
| FGF23‡ | 7.441E−5 | 0.244 |
BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FGF, fibroblast growth factor; HbA1C, glycosylated hemoglobin; HDL, High-density lipoprotein; IMT, intima-media thickness; LDL, Low density lipoprotein; PTH, parathyroid hormone.
Adjusted for sex.
Adjusted for age, sex, hypertension, diabetes mellitus, cardiovascular disease.
Adjusted for age, sex, BMI, hypertension, diabetes mellitus, cardiovascular disease.
Impact of atherosclerosis on VAF
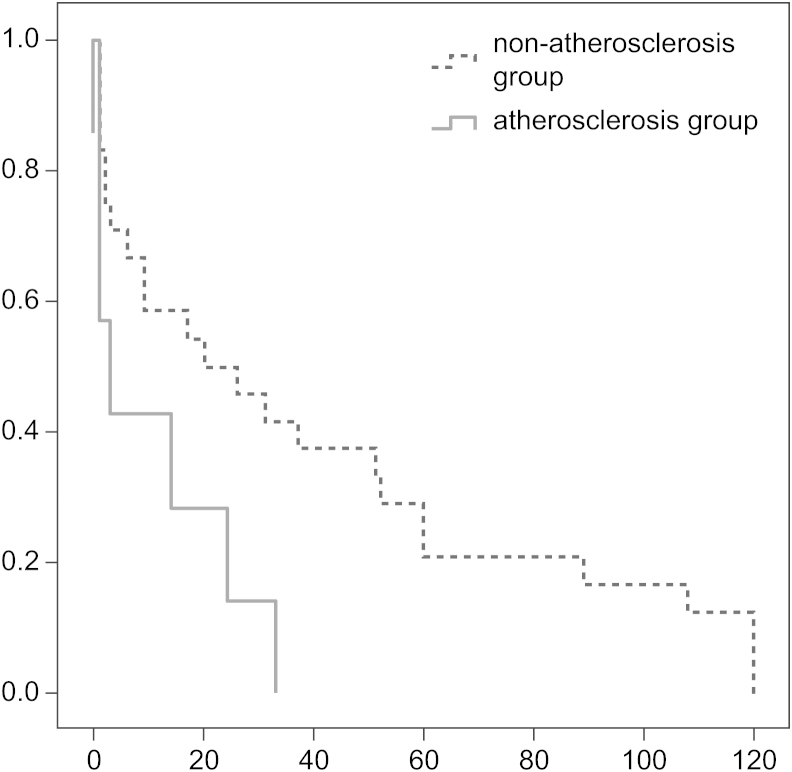
Table 4 shows the adjusted HRs of the risk factors for primary access patency. Patients with atherosclerosis showed much shorter durations of access patency than those in the nonatherosclerosis group (HR=2.822, CI 1.113–7.156; P=0.029). Kaplan–Meier curves also showed shorter access patency in the atherosclerosis group with statistical significance (P=0.027, Fig. 1). Furthermore, being overweight was associated with a 2.47-fold (95% CI 1.101–5.548, P=0.028) increased primary access failure. We did not find an association between access patency and hypertension, diabetes mellitus, and CRP (Table 4).
Table 4.
Correlations between vascular access failure and laboratory findings
| Characteristics | Duration from hemodialysis to first access failure |
||
|---|---|---|---|
| P | HR (95% CI) | ||
| Age⁎ | ≥ 65 versus<65y | 0.615 | |
| Sex† | Female versus male | 0.329 | |
| CIMT‡ | CIMT ≥0.09 versus<0.09 mm | 0.029 | 2.822 (1.113–7.156) |
| Hypertension§ | Yes versus No | 0.674 | |
| Diabetes mellitus║ | Yes versus No | 0.784 | |
| Angina‡ | Yes versus No | 0.515 | |
| Peripheral arterial occlusive disease‡ | Yes versus No | 0.765 | |
| Stroke‡ | Yes versus No | 0.067 | |
| BMI‡ | BMI≥23 versus<23 kg/m2 | 0.028 | 2.472 (1.101–5.548) |
| Leukocytosis‡ | WBC≥104 versus<104 /μL | 0.259 | |
| CRP‡ | ≤ 0.05 versus>0.05 mg/dL | 0.938 | |
| Hypercholesterolemia‡ | Total cholesterol≥200 versus<200 mg/dL | 0.252 | |
BMI, body mass index; CI, confidence interval; CIMT, carotid intima–media thickness; CRP, C-reactive protein; HR, hazard ratio; WBC, white blood cell.
Adjusted for sex.
Adjusted for age.
Adjusted for age, sex, history of hypertension, diabetes mellitus.
Adjusted for age, sex, history of diabetes mellitus.
Adjusted for age, sex, history of hypertension.
Figure 1.
Kaplan-Meier curves for access patency. Atherosclerosis group had shorter access patency than non-atherosclerosis group (p=0.027).
Discussion
Cardiovascular disease due to accelerated atherosclerosis is the principal cause of morbidity and mortality in HD patients [15]. An increased CIMT is regarded as an early sign of atherosclerosis. CIMT is related to cardiovascular disease affecting distant vascular beds, such as the cerebral, peripheral, and coronary artery vascular beds [16]. High-resolution B-mode ultrasonography of the carotid arteries provides measures of IMT and atherosclerotic plaques. This is an inexpensive, mobile, and easily available method, with few, if any, complications or side effects [17].
In previous studies, HD patients were reported to show advanced atherosclerosis in the carotid arteries compared with age-matched healthy control participants [4], [18]. Moreover, increased CIMT might be an independent predictor of cardiovascular mortality in the HD population [19]. In Korea, Song et al [20] studied the association of inflammatory markers and CIMT in continuous ambulatory peritoneal dialysis patients. To our knowledge, the present study is the first to evaluate the CIMT in Korean HD patients by using high-resolution B-mode ultrasound.
The mean IMT of the participants in the present study was 0.81±0.47 mm, whereas the reported mean CIMT of healthy people in Korea is 0.68±0.17 mm [21]. Increased IMT is associated with age, hypercholesterolemia, hypertension, and smoking. Hypertension has been associated with increased CIMT [13], [14], [22]. However, we found no such link with blood pressure in our patients when we compared the blood pressure between the atherosclerosis and the nonatherosclerosis groups. In our study, we did not analyze the effect of antihypertensive medications, and the number of the study patients was small. Moreover, blood pressure in HD patients often varies because of autonomic neuropathy [22]. Therefore, it is difficult to evaluate the correlation between blood pressure and atherosclerosis in HD patients.
Stenvinkel et al [18] reported that atherosclerosis in advanced chronic renal disease was caused by the synergistic effects of malnutrition, inflammation, oxidative stress, and genetic components. Our study showed that the group with atherosclerosis had higher levels of ESR and CRP; however, statistical significance was not reached.
Secondary hyperparathyroidism with hyperphosphatemia and increased calcium-phosphate product is a cause of vascular calcification [23]. PTH causes an increase of endothelial microparticles [24], and the increased endothelial microparticles may cause changes in the arterial structure, including atherosclerosis [25], [26]. In our study, IMT showed no association with calcium, phosphate, and PTH levels. This may be because our study did not analyze medications, including vitamin D analogs, or include a history of parathyroidectomy.
VAF is a common problem in HD patients and is associated with increased morbidity and hospitalization [27]. The main cause of VAF is stenosis at the arteriovenous anastomosis due to abnormal neointimal proliferation and extracellular matrix deposition [28]. However, arterial problems with atherosclerotic lesions and vascular calcification also cause VAF [11], [29]. Campos et al [30] noted that causes of early AVF failure may include atherosclerotic disease. Furthermore, a recent study suggested that well-known risk factors for atherosclerosis such as cardiovascular disease and fetuin-A levels might play an important role in the development of stenotic lesions in AVFs in dialysis patients [13]. Hofstra et al [31] stated that stenotic lesions in AVFs and peripheral bypass grafts had atherosclerotic histopathology. Chen et al [32] reported that HD patients with peripheral arterial disease had higher VAF rates.
Previous studies on the risk factors of arteriovenous dysfunction have shown conflicting results [13], [33], [34], [35]. Clinical risk factors are increased age, female gender, diabetes, stroke, cardiovascular disease, and prior catheter use [13], [36], [37], [38]. In this study, we showed that the duration of access patency had an inverse relationship with atherosclerosis and body mass index (BMI). Our findings suggest that increased CIMT may be one of the factors of access failure in HD patients. Kim et al [39] found that increased radial artery IMT is closely associated with early failure of radiocephalic AVF in HD patients. Atherosclerosis may cause luminal narrowing and loss of vascular elasticity, which increases arterial stiffness. These factors restrict the outward remodeling of AVF maturation [11], [39]. However, further study is required to evaluate the impact of atherosclerosis on primary access failure.
The relationship between obesity and vascular access outcomes remains controversial. However, many studies suggested that increased BMI is a risk factor for the absence of a functioning fistula [36], [38], [40], [41], [42]. Marchi et al [37] suggested pathogenetic mechanisms for fistula failure in the obese subpopulation. They demonstrated that patients with fistula dysfunction have a higher level of monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), hyperinsulinemia, hyperlipidemia, plasminogen activator inhibitor type-1 (PAI-1), and factor VII than patients without fistula dysfunction [37]. We know that neointimal hyperplasia is primarily responsible for primary and secondary fistula failure and that MCP-2, IL-6, and tumor necrosis factor-α regulate smooth muscle migration and endothelial dysfunction. There are increased levels of these cytokines, hyperinsulinemia and dyslipidemia, as well as upregulation of PAI-1 in obese patients [37], [43]. Plumb et al [40] observed that excess axillary tissue in obese patients resulted in compression of the venous outflow of the arm in the adducted position, suggesting a potential cause of this HD vascular access failure [40]. It could be another explanation to early failure in patients with high BMI.
Although not mentioned in the results, HbA1C was significantly lower in the group with atherosclerosis. This result is related to the treatment condition of diabetic patients. In the atherosclerosis group, 50% of diabetic patients were well controlled (HbA1C<6.0%) without treatment, whereas in the nonatherosclerosis group, 25% of diabetic patients were well controlled without treatment. Recent studies reported that HbA1C and carotid IMT have a positive relationship [44]. We think that the discrepancy between this study and the previous studies is attributable to the small number of the study sample including diabetic patients. Thus, further large-scale studies are required to verify this issue.
This study has several limitations. First, this study was performed only on a minimum number of patients, as all participants came from a single regional hospital. Second, we measured the intima thickness only in the right common carotid artery. Chatzizisis et al [45] showed that the left carotid IMT is more susceptible to atherosclerotic disease. Third, we did not include the patients’ medications and possible history of parathyroidectomy in our analysis.
In conclusion, this is the first report of the clinical characteristics of atherosclerosis in Korean HD patients. This study shows that atherosclerosis is associated with older age. Moreover, vascular access patency is negatively correlated with CIMT and BMI. We recommend that the vascular access monitoring be performed more carefully in HD patients with atherosclerosis and overweight.
Conflict of interest
None to declare.
References
- 1.2012 Atlas of CKD & ESRD: Available at: http://www.usrds.org/2012/pdf/v2_ch5_12.pdf [Date accessed: 16 September 2012]
- 2.Committee ER: Korean Society of Nephrology: Current renal replacement therapy in Korea — Insan memorial dialysis registry, 2012. Kidney Res Clin Pract 31:S7–S24, 2012
- 3.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117–129. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawagishi T, Nishizawa Y, Konishi T, Kawasaki K, Emoto M, Shoji T, Akizawa T, Tabata T, Morii H. High resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int. 1995;48:820–826. doi: 10.1038/ki.1995.356. [DOI] [PubMed] [Google Scholar]
- 5.Bots M, Hofman A, Grobbee D:Common carotid intima–media thickness and cardiovascular disease in the Rotterdam Study: a cross-sectional analysis. In: Koenig W, Hombach V, MG Bond, DM Kramsch, editors. Progression and Regression of Atherosclerosis. Vienna, Austria: Blackwell Scientific Publishers, 118–123, 1995
- 6.Glagov S, Zarins C, Giddens D, Ku D. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 7.Goodman W, Goldin J, Kuizon B. Coronary–artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 8.Oh J, Wunsch R, Turzer M. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 9.Goodthoff J, Gruppen M, Offringa M. Increased arterial stiffness in young adults with end-stage renal disease since childhood. J Am Soc Nephrol. 2002;13:2953–2961. doi: 10.1097/01.asn.0000037677.16961.df. [DOI] [PubMed] [Google Scholar]
- 10.Savage T, Clarke A, Giles M, Tomson C, Raine A. Calcified plaque is common in the carotid and femoral arteries of dialysis patients without clinical vascular disease. Nephrol Dial Transplant. 1998;13:2004–2012. doi: 10.1093/ndt/13.8.2004. [DOI] [PubMed] [Google Scholar]
- 11.Mercado C, Salman L, Krishnamurthy G, Choi K, Artikov S, Thomas I, Merrill D, Asif A. Early and late fistula failure. Clin Nephrol. 2008;69:77–83. doi: 10.5414/cnp69077. [DOI] [PubMed] [Google Scholar]
- 12.Rothuizen T, Wong C, Quax P, Av Zonneveld, Rabelink T, Rotmans J. Arteriovenous access failure: more than just intimal hyperplasia? Nephrol Dial Transplant. 2013;28:1085–1092. doi: 10.1093/ndt/gft068. [DOI] [PubMed] [Google Scholar]
- 13.Ocak G, Rotmans J, Vossen C, Rosendaal F, Krediet R, Boeschoten E, Dekker F, Verduijn M. Type of arteriovenous vascular access and association with patency and mortality. BMC Nephrol. 2013;47:79–86. doi: 10.1186/1471-2369-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grobbee D, Bots M. Carotid artery intima–media thicknesses as an indicator of generalized atherosclerosis. J Int Med. 1994;236:567–573. doi: 10.1111/j.1365-2796.1994.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 15.Joki N, Hase H, Ishikawa H, Fukazawa C, Nakamura R, Imamura Y, Tanaka Y, Saijyo T, Fukazawa M, Yamaguchi T. Coronary artery disease as a definitive risk factor of short-term outcome after starting hemodialysis in diabetic renal failure patients. Clin Nephrol. 2001;55:109–114. [PubMed] [Google Scholar]
- 16.Burke G, Evans G, Riley W, Sharrett A, Howard G, Barnes R, Rosamond W, Rautaharju P RS RC, Heiss G. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 17.Bots M, Hofman A, PD Jong, Grobbee D. Common carotid intima–media thickness as an indicator of atherosclerosis at other sites of the carotid artery. The Rotterdam Study. Ann Epidemiol. 1996;6:147–153. doi: 10.1016/1047-2797(96)00001-4. [DOI] [PubMed] [Google Scholar]
- 18.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 19.Nishizawa Y, Shoji T, Maekawa K, Nagasue K, Okuno S, Kim M, Emoto M, Ishimura E, Nakatani T, Miki T, Inaba M. Intima–media thickness of carotid artery predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2003;41:S76–S79. doi: 10.1053/ajkd.2003.50090. [DOI] [PubMed] [Google Scholar]
- 20.Song H, Song Y, Ahn C, Kang S, Choi K, Ha S, Lee H, Lee H, Han D. Relationship between inflammatory markers and high resolution B-mode carotid artery ultrasonography in continuous ambulatory peritoneal dialysis(CAPD) patients. Korean J Nephrol. 2002;21:285–294. [Google Scholar]
- 21.Center CCD: Request for registration of standardization of Korean carotid intima–media thickness data 2011. Available at: http://www.google.co.kr/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=1&ved=0CCoQFjAA&url=http%3A%2F%2Fwww.srd.re.kr%2Ffile_down.php3%3Ffile_mask%3D20111111000005500%26file_name%3DI_%25C7%25D1%25B1%25B9%25C0%25CE%25B0%25E6%25B5%25BF%25B8%25C6%25B5%25A5%25C0%25CC%25C5%25CD_%25C2%25FC%25C1%25B6%25C7%25A5%25C1%25D8%25B5%25EE%25B7%25CF%25BF%25E4%25C3%25BB%25BC%25AD(%25BD%25C9%25B3%25FADC)111110.pdf%26file_dir%3Dannouncement_file%2Fannounce&ei=JT1_Ut82zNeSBZLngLAD&usg=AFQjCNFaVc9x-G-H30bungHDGB6U4pONJQ&bvm=bv.56146854,d.dGI&cad=rjt [Date accessed: 14 May 2012]
- 22.Campese V, Romoff M, Levitan D, Lane K, Massry S. Mechanisms of autonomic nervous system dysfunction in uremia. Kidney Int. 1981;20:246–253. doi: 10.1038/ki.1981.127. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt A. Soft-tissue calcification in uremia. Arch Intern Med. 1969;124:544–556. [PubMed] [Google Scholar]
- 24.Dursun I, Poyrazoglu H, Gunduz Z, Ulger H, Yykylmaz A, Dusunsel R, Patyroglu T, Gurgoze M. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2511–2518. doi: 10.1093/ndt/gfp066. [DOI] [PubMed] [Google Scholar]
- 25.Amabile N, Guérin A, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London G, Tedgui A, Boulanger C. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 26.Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566–573. doi: 10.1111/j.1538-7836.2005.01780.x. [DOI] [PubMed] [Google Scholar]
- 27.Rayner H, Pisoni R, Bommer J, Canaud B, Hecking E, Locatelli F, Piera L, Bragg-Gresham J, Feldman H, Goodkin D, Gillespie B, Wolfe R, Held P, Port F. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19:108–120. doi: 10.1093/ndt/gfg483. [DOI] [PubMed] [Google Scholar]
- 28.Weiss M, Scivittaro V, Anderson J. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001;37:970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 29.Moe S. Vascular calcification: hardening of the evidence. Kidney Int. 2006;70:1535–1537. doi: 10.1038/sj.ki.5001892. [DOI] [PubMed] [Google Scholar]
- 30.RP Campos, MM Do Nascimento, DC Chula, DE Do Nascimento, MC Riella. Stenosis in hemodialysis arteriovenous fistula: evaluation and treatment. Hemodial Int. 2006;10:152–161. doi: 10.1111/j.1542-4758.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 31.Hofstra L, Tordoir J, Kitslaar P, Hoeks A, Daemen M. Enhanced cellular proliferation in intact stenotic lesions derived from human arteriovenous fistulas and peripheral bypass grafts. Does it correlate with flow parameters? Circulation. 1996;94:1283–1290. doi: 10.1161/01.cir.94.6.1283. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Chang J, Hwang S, Tsai J, Wang C, Mai H, Lin F, Su H, Chen H. Significant correlation between ankle-brachial index and vascular access failure in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:128–134. doi: 10.2215/CJN.03080608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GM Gagliardi, Rossi S, Condino F, Mancuso D, Greco F, Tenuta R, Savino O, Bonofiglio R, Domma F, Latorre G. Malnutrition, infection and arteriovenous fistula failure: is there a link? J Vasc Access. 2011;12:57–62. doi: 10.5301/jva.2010.5831. [DOI] [PubMed] [Google Scholar]
- 34.Chang C, Ko Y, Ko P, Hsu L, Chen C, Yang C, Hsu T, Pang J. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int. 2005;68:1312–1319. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Rodriguez R, Humphreys M. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant. 2004;19:141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 36.Lilly M, Lynch J, Wish J, Huff E, Chen S, Armistead N, McClellan W. Prevalence of arteriovenous fistulas in incident hemodialysis patients: correlation with patient factors that may be associated with maturation failure. Am J Kidney Dis. 2012;59:541–549. doi: 10.1053/j.ajkd.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 37.SD Marchi, Falleti E, Giacomello R, Stel G, Cecchin E, Sepiacci G, Bortolotti N, Zanello F, Gonano F, Bartoli E. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. J Am Soc Nephrol. 1996;7:1169–1177. doi: 10.1681/ASN.V781169. [DOI] [PubMed] [Google Scholar]
- 38.Miller P, Tolwani A, Luscy C, Deierhoi M, Bailey R, Redden D, Allon M. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int. 1999;56:275–280. doi: 10.1046/j.1523-1755.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y, Choi Y, Kim J, Kim Y, Kim B, Park C, Song H, Yoon S, Ban B. The impact of intima–media thickness of radial artery on early failure of radiocephalic arteriovenous fistula in hemodialysis patients. J Korean Med Sci. 2006;21:284–289. doi: 10.3346/jkms.2006.21.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plumb T, Adelson A, Groggel G, Johanning J, Lynch T, Lund B. Obesity and hemodialysis vascular access failure. Am J Kidney Dis. 2007;50:450–454. doi: 10.1053/j.ajkd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Kats M, Hawxby A, Barker J, Allon M. Impact of obesity on arteriovenous fistula outcomes in dialysis patients. Kidney Int. 2007;71:39–43. doi: 10.1038/sj.ki.5001904. [DOI] [PubMed] [Google Scholar]
- 42.Protack C, Jain A, Vasilas P, Dardik A. The influence of metabolic syndrome on hemodialysis access patency. J Vasc Surg. 2012;56:1656–1662. doi: 10.1016/j.jvs.2012.05.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon B. Weighing in on fistula failure. Kidney Int. 2007;71:12–14. doi: 10.1038/sj.ki.5002021. [DOI] [PubMed] [Google Scholar]
- 44.Rivera J, Choi E, Yoon Y, Chun E, Choi S, Nasir K, Brancati F, Blumenthal R, Chang H. Association between increasing levels of hemoglobin A1c and coronary atherosclerosis in asymptomatic individuals without diabetes mellitus. Coron Artery Dis. 2010;21:157–163. doi: 10.1097/MCA.0b013e328337ff9b. [DOI] [PubMed] [Google Scholar]
- 45.Chatzizisis Y, Giannoglou G, Parcharidis G, Louridas G. Is left coronary system more susceptible to atherosclerosis than right? A pathophysiological insight. Int J Cardiol. 2007;116:7–13. doi: 10.1016/j.ijcard.2006.03.029. [DOI] [PubMed] [Google Scholar]