Abstract
Aging involves progressive loss of cellular function and integrity, presumably caused by accumulated stochastic damage to cells. Alterations in energy metabolism contribute to aging, but how energy metabolism changes with age, how these changes affect aging, and whether they can be modified to modulate aging remain unclear. In locomotory muscle of post-fertile Caenorhabditis elegans, we identified a progressive decrease in cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C), a longevity-associated metabolic enzyme, and a reciprocal increase in glycolytic pyruvate kinase (PK) that were necessary and sufficient to limit lifespan. Decline in PEPCK-C with age also led to loss of cellular function and integrity including muscle activity, and cellular senescence. Genetic and pharmacologic interventions of PEPCK-C, muscle activity, and AMPK signaling demonstrate that declines in PEPCK-C and muscle function with age interacted to limit reproductive life and lifespan via disrupted energy homeostasis. Quantifications of metabolic flux show that reciprocal changes in PEPCK-C and PK with age shunted energy metabolism toward glycolysis, reducing mitochondrial bioenergetics. Last, calorie restriction countered changes in PEPCK-C and PK with age to elicit anti-aging effects via TOR inhibition. Thus, a programmed metabolic event involving PEPCK-C and PK is a determinant of aging that can be modified to modulate aging.
Keywords: aging, energy metabolism, mitochondria, phosphoenolpyruvate carboxykinase, pyruvate kinase, Calorie restriction, physical activity
Introduction
Aging is characterized by the progressive decline in cellular function and integrity that leads to disease vulnerability and eventually death of organisms (1). The leading proposed cause of decline in cellular function and integrity with age is the accumulation of stochastic damage of molecules and organelles by reactive molecules, such as reactive oxygen species (ROS).3 Whether ROS are detrimental to organisms and whether ROS limit lifespan, however, are in debate (2).
Energy metabolism supplies ATP for cellular function and maintenance. Alterations in energy metabolism are linked to the aging process and aging-associated diseases (3). In model organisms, environmental and genetic factors that change energy metabolism, such as calorie restriction (CR) (4), inhibition of target of rapamycin (TOR) (5), and 5′ AMP kinase (AMPK) (6) are determinants of longevity. A large body of aging research has been focusing on the signaling of CR, TOR inhibition, and AMPK in regulating longevity. The exact alterations in energy metabolism that occur with age, how these changes impact aging, and whether they can be modified to modulate aging are understudied and remain poorly understood, largely due to the intrinsic complexity of energy metabolism, and the indirect impact of these longevity paradigms on energy metabolism. This impedes the understanding of aging mechanisms and the development of mechanism-based strategies to modulate aging.
A key regulation of energy metabolism at the cellular level is the reciprocal changes of PK and PEPCK-C (7). Whether this regulation of cellular energy metabolism contributes to organismal aging is currently unknown. Although PK is a rate-limiting glycolytic enzyme, PEPCK-C is well known as the rate-limiting enzyme of gluconeogenesis. PEPCK-C also participates in the synthesis of glyceride-glycerol and serine (8), impacting a wide range of pathophysiology and physiology (9–11). For example, PEPCK-C accelerates the oxidation of amino acids to release ammonia, alleviating renal tubular acidosis in humans (9). We have previously reported that overexpression (OE) of PEPCK-C re-patterns energy metabolism and extends lifespan in mice (12) and Caenorhabditis elegans (13), but the physiological, metabolic, biochemical, and signaling mechanisms of this phenomenon remain unknown. Here, we propose that PEPCK-C is a major energy metabolism adaptor that promotes health and longevity. Because PEPCK-C mRNA is decreased and PK mRNA is increased in aged muscle and liver of mammals (14, 15), we hypothesize that a decline in PEPCK-C with age and a reciprocal increase in PK disrupt energy homeostasis, reduce cellular function and integrity, and promote aging. Because PEPCK-C catalyzes only one chemical reaction, which converts oxaloacetate to phosphoenolpyruvate (PEP), its modification has a direct and traceable impact on energy metabolism. Thus, quantification of metabolic flux related to PEPCK-C with altered PEPCK-C, followed by linkage of those changes with aging traits, would identify key metabolic events of aging and define their physiological contributions. This would help illustrate mechanisms of aging and may suggest strategies to modulate aging. Because PEPCK-C enhances the spontaneous activity in mice (12) and leisure time activity alters metabolism (16) and extends life expectancy in humans (17), it is critical to conduct metabolic quantification in freely behaving animals. To date, such assessment under the aging context has not been reported.
C. elegans has well established genetics and short lifespan (3 weeks), providing certain advantages over mice in testing our hypothesis. Recently, we have established an isotopic tracer method that quantifies metabolic flux in freely behaving worms (13). Here, we longitudinally assessed PK and PEPCK-C. We next altered these enzymes, muscle activity, AMPK signaling, TOR signaling, and food supply, and then examined their effects on lifespan, cellular functions and senescence, metabolic flux, and autophagy. The results have identified a programmed metabolic event that is necessary and sufficient to determine aging, and is used by CR to modulate lifespan.
Experimental Procedures
C. elegans Strains and Preparation
Wild type (WT) (Bristol N2), pck-1 (ok2098), pck-2 (ok2586), rde-1 (ne219), glp-1 (e2141), aak-2 (ok524), aak-2 (rr48), crh-1 (tz2), and DA2123 were from the Caenorhabditis Genetics Center (University of Minnesota) and were out-crossed at least three times. NR350 (Caenorhabditis Genetics Center) and JK701 are rde-1 mutants that have restored rde-1 expression in body wall muscle (BDWM) or intestine, respectively (18). The genes rde-1 (18) and glp-1 (19), respectively, encode an Argonaute protein required for RNAi knockdown (KD) or an Nortch receptor. Transgenic worms over-expressing PEPCK-C or PEPCK-C::rho9 were generated as described (20, 21). Pharynx, BDWM, and intestine-specific promoters are myo-2, myo-3, or ges-1 (13, 22). NR350, akk-2, or crh-1 mutants over-expressing PEPCK-C were generated by genetic cross-facilitated by PCR and endonuclease digestion. PCR primers are 5′-gacactcggagttggtactt-3′, 5′-agggttccacaaagaagtgc-3′, 5′-gctcgatgagcag aacaatg-3′, 5′-gtcatcagtacaccttctga-3′, and 5′-agacttggcacgtgctcatc-3′. In all experiments, PEPCK-C OE animals exhibited a 4.7–10.1-fold increase in PEPCK activity or PEPCK-C abundance. Mutants of glp-1 were maintained at 15 °C and eggs were raised to adults at 25 °C (19). Worms were prepared by standard methods. The pre-fertile period of adulthood was identified as t = 0.
Metabolic Quantification
Measurements were conducted at room temperature. Oxidation of 13C-labeled acetate, glucose, and glutamate (American Radiolabeled Chemicals, St. Louis, MO) (13), PEPCK activity (13), PK activity (23), and AMP/ATP (24) were quantified with intact worms (13) or whole, cytosolic, and mitochondrial worm extracts (25). Oxygen consumption, lactate dehydrogenase (LDH) activity, and lactate production were quantified with 100–2000 worms and 0.4–1.5 ml of Dulbecco's modified Eagle's medium containing 0.2 mm glucose and dead OP50 Escherichia coli. Oxygen consumption was analyzed with a XF24 Analyzer (Seahorse Bioscience, North Billerica, MA) (26). Alternatively, cultures were incubated (3.5 h) and centrifuged. Supernatant (5 μl) or pellets were analyzed for lactate production and LDH activity with MAK064-1KT and MAK066-1KT (Sigma). To simultaneously quantify the conversion of glucose to carbon dioxide and lactate, 2000 worms were cultured in 1.5 ml of Dulbecco's modified Eagle's medium containing dead OP50 bacteria, 2 mm glucose, and 0.5 μCi of [U-14C]glucose for 4 h. Carbon dioxide (13) and lactate (27) were collected or separated, and then quantified.
Immunoblotting, Fluorescent Microscopy, Immunohistochemistry, and Cellular Senescence Analysis
These experiments were performed with antibodies of 1D4 (20), PEPCK-C (13), CKI-1 (28), β-tubulin, and phosphorylated AMPK (pAMPK) (Cell Signaling, Danvers, MA), senescence β-galactosidase staining kit (#9860, Cell Signaling), and a TCS SP2 confocal microscope (Leica Microsystems, Bannockburn, IL). Wavelengths were 340 nm (λex) and 420–440 nm (λem) (autofluorescence) (29), and 488 nm (λex) and 510–530 nm (λem) (others).
Electron Microscopy
Animals were fixed, and samples were prepared and examined with a JEOL 1200 electron microscope (JEOL, Peabody, MA) (30).
RNAi, Q Repressible Binary Expression System, Rapamycin, and Suxamethonium Exposure
The identities of RNAi HT115 bacterial clones (Julie Ahringer library) were verified and bacteria were prepared (13). RNAi KD was initiated at day 1 adults unless stated otherwise. Co-KD of RNase T enzyme encoding eri-1 was used to enhance the efficiency of egl-19 RNAi KD (31). L4440 was used as control. Worms allowing inducible PEPCK-C expression in BDWM were generated with myo-3 promoter and pck-1 genome DNA (32). Quinic acid and rapamycin (Sigma) were used as described (32, 33). For suxamethonium (SUX) (TCI America, Portland, OR) exposure, worms were transferred to SUX containing culturing plates every other day (lifespan and immunoblotting) or 1–2 h before measurements (others).
Analysis of Aging Traits
Lifespan (13), reproduction (34), and locomotion (35) were analyzed at 22 (locomotion) or 20 °C (others). Food in worms was measured (36) using OP50 expressing GFP (Caenorhabditis Genetics Center), a Leica MZ16 fluorescent dissecting microscope mounted with an iXon DV897 camera (Andor Technology, South Windsor, CT) and Visual Assistance 8.5 (National Instruments, Austin, TX). To analyze food consumption, 3000 day 1 rol-6 mutants were transferred onto a 10-cm culturing plate freshly seeded with OP50 bacteria killed with ethanol. After periods, residue food was spotted with a MZ16 microscope. The rol-6 gene encodes a collagen α1 chain precursor-related protein. Pharynx pumping rates were counted with a MZ16 microscope (37). ROS and resistance to paraquat were analyzed with dichlorofluorescein diacetate and paraquat (Sigma) (38).
Statistical Analyses
Error bars represent mean ± S.E. in figures. Log-rank and other statistic tests were analyzed with Stata 12 (StataCorp, College Station, TX).
Results
Aging Involves a Progressive Decline in PEPCK-C and a Reciprocal Increase in PK
We quantified PEPCK and PK activity in whole worm extract over the worm lifecycle. PEPCK activity increased from L4 (the fourth and last larval stage) to adulthood, peaked during the reproductive period, and decreased progressively thereafter (Fig. 1A). In contrast, PK activity continuously increased with age before, during, and after the reproductive stage. Mammals have PEPCK-C and a mitochondrial PEPCK (PEPCK-M) (8). C. elegans has pck-1, pck-2, and pck-3, three genes encoding PEPCK. PCK-1 localizes in cytosol (39) and thus is PEPCK-C. PCK-2 but not PCK-1 or PCK-3 has a predicted mitochondrial targeting sequence (data not shown), thus likely to be PEPCK-M. Global PEPCK-C knock-out (KO) in mice is lethal (40). KO of pck-1, pck-2, and pck-1;pck-2 all survived to adulthood, but had reduced lifespan (data not shown). Because pck-1 KO has >85% reduced PEPCK activity (13) and pck-1;pck-2 KO exhibited no measurable PEPCK activity (data not shown), pck-3 may not encode a functional protein. Using immunoblotting and a PEPCK-C antibody that does not cross-react with PEPCK-M (13), we observed the same lifecycle-dependent dynamics of PEPCK-C enzyme as PEPCK activity (Fig. 1B).
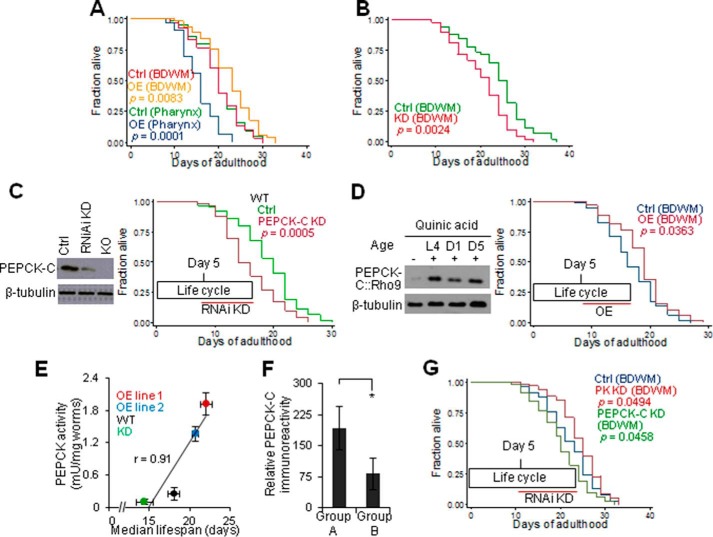
FIGURE 1.

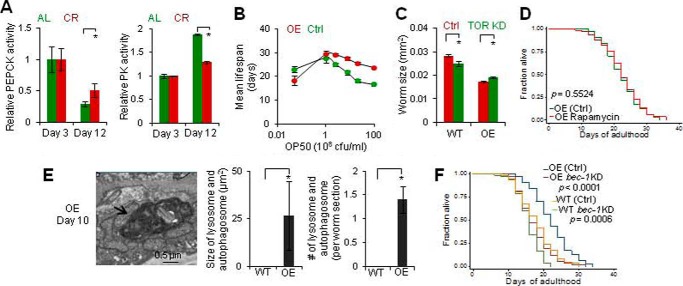
Aging involves a progressive decrease in PEPCK-C and a reciprocal increase in PK. A, PEPCK-C and PK activity of WT (n = 3). The values at L4 (PEPCK) and day 1 (PK) were set as 1 for best data visualization. B, immunoblotting against PEPCK-C in WT. Day 1 PEPCK-C OE and KO samples were also loaded. Upper, sample images. Lower, quantification (n = 3). C, immunohistochemistry against PEPCK-C. Upper, images were cropped differently to show the expression pattern. Lower, quantification (n = 6–12). #, p > 0.05; *, p < 0.05; t test. D, PEPCK activity in WT and glp-1 mutants (n = 3).
To determine the tissue specificity of the decline in PEPCK-C with age, we first examined its expression pattern with immunofluorescent and GFP fluorescent microscopy (Fig. 1C and data not shown). PEPCK-C was most abundant in locomotory BDWM. Significant PEPCK-C was also seen in intestine and pharynx but not in laid or un-laid eggs. From days 1 to 12, PEPCK-C in BDWM and intestine, respectively, decreased by ∼79 and 32%. PEPCK-C immunoreactivity in pharynx was too low to be quantified reliably.
These PEPCK-C expressions suggest that decline in PEPCK activity with age is not due to the lack of metabolically active eggs. To confirm, we quantified PEPCK activity in glp-1 mutants genetically ablated for egg production (19). The increase and peak in PEPCK activity before and during the reproductive stage were attenuated in glp-1 mutants (Fig. 1D), indicating that they are associated with development and egg production. Decline in PEPCK activity after the reproductive peak also occurred in glp-1 mutants, validating that it is not a result of loss of eggs. The reciprocal changes in PEPCK-C and PK with age also cannot be an effect of aging-associated loss of protein homeostasis (41), which predicts declines in both. We conclude that aging involves a programmed metabolic event that includes reciprocal changes in PEPCK-C and PK.
Reciprocal Changes in PEPCK-C and PK with Age Limit Lifespan
Next, we set out to examine if reciprocal changes in PEPCK-C and PK with age is a determinant of aging. We started with lifespan, a key aspect of aging. Because decline in PEPCK-C with age occurred after the reproductive peak and primarily in BDWM, it is important to understand how the tissue and lifecycle specificities of PEPCK-C and PK affect lifespan. In mice, PEPCK-C OE in skeletal muscle extends lifespan but the lifecycle specificity is unknown. Moreover, a secondary increase in endogenous PEPCK-C in liver in these transgenic mice (12) may influence lifespan. In worms, global PEPCK-C OE extends lifespan (13) but both the tissue and lifecycle specificities are unknown. Here, selective PEPCK-C OE in BDWM, pharynx, and intestine extended, reduced, or had no effect on lifespan, respectively (Fig. 2A and supplemental Table S1). Selective PEPCK-C KD in BDWM in NR350 worms and in intestine in JK701 worms (18) reduced or had no effect on lifespan (Fig. 2B and supplemental Table S1), respectively. Thus, PEPCK-C in BDWM is necessary and sufficient to impact lifespan.
FIGURE 2.
Reciprocal changes of PEPCK-C and PK with age limit lifespan. A, survival of control and PEPCK-C OE in BDWM. B, survival of NR350 grown on vector control or pck-1 RNAi bacteria. C, left, day 5 WT worms were subjected to PEPCK-C RNAi KD for 2 days and then immunoblotting. Day 5 PEPCK-C KO worms were included as a control. Right, survival of WT grown on either vector control or pck-1 RNAi bacteria. D, left, the expression of Rho9-tagged PEPCK-C in BDWM was induced by quinic acid at the indicated ages. After 1 day, worms were subjected to immunoblotting. Right, survival of control and induced PEPCK-C OE in BDWM. E, PEPCK activity is correlated with lifespan (n = 3). F, immunohistochemistry of PEPCK-C at day 12. *, p < 0.05; t test. G, survival of NR350 grown on vector control or pck-1/pyk-1 RNAi bacteria.
Next, we used RNAi to reduce PEPCK-C in post-fertile or reproduction active worms, which shortened or had no effect on lifespan, respectively (Fig. 2C and supplemental T a b l e S 1). We then selectively induced PEPCK-C OE in BDWM in post-fertile worms using the Q repressible binary expression system (32), and this extended lifespan (Fig. 2D and supplemental Table S1). Thus, PEPCK-C in the post-fertile stage is necessary and sufficient to impact lifespan.
To determine whether PEPCK-C has a dose effect on lifespan, we generated two transgenic lines over-expressing PEPCK-C. Compared with controls (0.26 milliunits/mg of worms), PEPCK activity in OE line 1 (1.93 milliunits/mg of worms), and OE line 2 (1.36 milliunits/mg of worms) increased 5.2- and 7.4-fold, respectively. We also reduced PEPCK-C activity by ∼40% with RNAi KD. These interventions only affected PEPCK activity in cytosolic but not the mitochondrial portion of the worm extract (data not shown). PEPCK activity was positively correlated with lifespan (Fig. 2E and supplemental Table S1).
To link decline in PEPCK-C in BDWM to lifespan, we quantified PEPCK-C enzyme in two groups of day 12 worms selected from the same culture. In response to a mechanical touch on the tail, animals of group A and group B, respectively, displayed over two or less than one body bend. We and others have reported that worms exhibiting persistent locomotion during aging (group A) live longer than their siblings exhibiting faster decline in locomotion (group B) (35). PEPCK-C enzyme in BDWM was higher in animals from group A than their siblings from group B (Fig. 2F).
Last, we determined if an increase in PK with age affects lifespan. Selective RNAi KD of PEPCK-C or PYK-1, the PK isoform expressed in BDWM (39), in BDWM of post-fertile worms, reduced and extended lifespan, respectively (Fig. 2G and supplemental Table S1). We conclude that reciprocal changes in PEPCK-C and PK with age are necessary and sufficient to limit lifespan.
PEPCK-C Counteracts the Loss of Cellular Function and Integrity, and Cellular Senescence
Loss of function and integrity of virtually all cells and tissues with age is also a key aging component (42). We selected reproduction and resistance to oxidative stress to illustrate if reciprocal changes in PEPCK-C and PK contribute to loss of cellular or tissue function and integrity with age. This is because PEPCK-C peaked during reproduction, and increased oxidative stress is associated with longevity (43). We focused on PEPCK-C because of its well defined linkage with longevity. Similar to mice (12), PEPCK-C OE in BDWM in worms extended the reproductive life without significantly altering the total number of progeny (Fig. 3A). Similar to many long-lived species, PEPCK-C OE worms exhibited increased ROS (Fig. 3B) and enhanced survival against the exposure of oxidative stressor paraquat (38) (Fig. 3C). PEPCK-C KO did not significantly alter ROS (Fig. 3B), but reduced survival against paraquat exposure at low concentrations (Fig. 3D).
FIGURE 3.
PEPCK-C counteracts loss of cellular function and integrity with age and senescence. A, self-fertile reproductive span of control and PEPCK-C OE in BDWM. *, p < 0.05; t test; n = 78–80. B, ROS in day 1 WT (Ctrl) and PEPCK-C KO, rol-6 (Ctrl), and rol-6 with PEPCK-C OE in whole body (n = 3). p < 0.05, t test. C, survival of rol-6 (Ctrl) and rol-6 with PEPCK-C OE in whole body. D, survival of WT and PEPCK-C KO. E-G, deposit of age-pigments (E) and β-galactosidase (F and G) of control and PEPCK-C OE in BDWM (n = 51–97 (E) or 12 (G)). *, p < 0.05; two-way analysis of variance (E) or t test (G). F, β-galactosidase deposit with age in WT. G, quantification of β-galactosidase deposit in day 15 WT and PEPCK-C OE in BDWM. H, immunoblotting against CKI-1 (n = 3). *, p < 0.05; t test. Upper, Sample images. &, nonspecific bands. Lower, immunoreactivity of CKI-1 over β-tubulin in arbitrary unit.
Another key component of aging at the cellular level is senescence (44). PEPCK-C OE in BDWM retarded the aging-associated deposit of age pigments (lipofuscin) (Fig. 3E) and β-galactosidase (Fig. 3, F and G), senescent markers that reflect accumulated molecular wastes in cells (45, 46), mainly in intestine. Moreover, senescent cells are restricted from proliferation (47), marked by increased expression of p27KIP−1 and other cyclin-dependent kinase inhibitors (48). Both day 1 WT and PEPCK-C OE worms did not exhibit significant expression of CKI-1, a worm homolog of p27KIP-1 (28). At day 9, CKI-1 expression was identified in WT but not in PEPCK-C OE worms (Fig. 3H). Thus, PEPCK-C suppresses the increase in CKI-1 expression with age. We conclude that PEPCK-C in BDWM counteracts the loss of cellular function and integrity and senescence in a non-cell-autonomous manner.
Decline in PEPCK-C and Loss of Cellular Function with Age Promote Each Other to Impact Aging
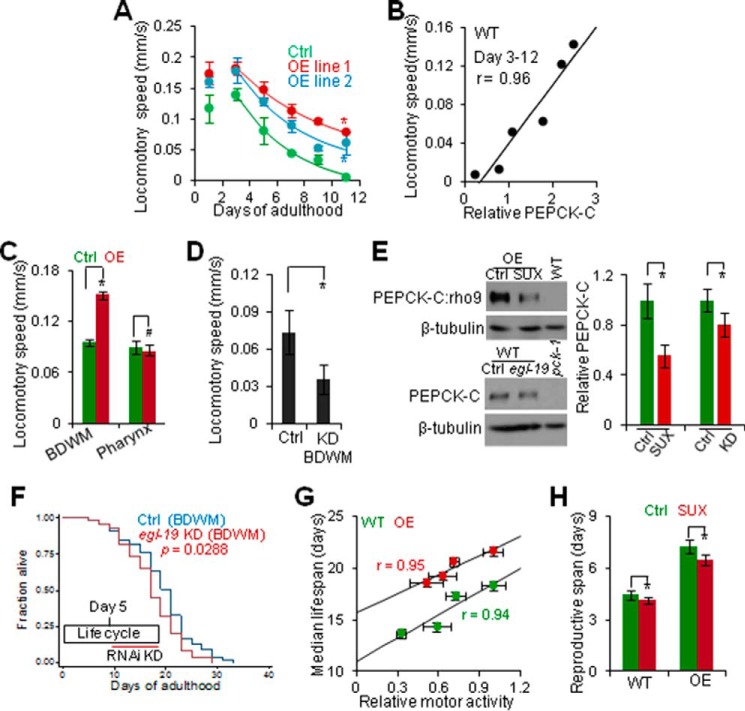
We then set out to determine how reciprocal changes in PEPCK-C and PK with age impact aging. First, we aimed to identify the physiological contribution of decline in PEPCK-C with age to aging by interrogating how this decline is related to loss of cellular function. We focused on motor activity because PEPCK-C OE promotes physical activity in mice (12) and progressive loss of motor activity with age is a negative indicator of health span and lifespan in worms (35). As previously shown (35), motor activity peaked during the reproductive period, and progressively declined thereafter in WT (Fig. 4A). Motor activity of two PEPCK-C OE lines increased at all ages examined. PEPCK-C also retarded decline in motor activity with age in a dose-dependent manner. Specifically, animals of controls, OE line 2 (∼5-fold increase in PEPCK activity) and OE line 1 (∼7-fold increase in PEPCK activity) lost their motor activity at 22%, 20 and 15% every day, respectively. PEPCK-C abundance was also positively correlated with motor activity from days 3 to 12 (Fig. 4B), when PEPCK-C decreased with age (Fig. 1, A and B). Thus, decline in PEPCK-C with age is coupled with loss of motor activity.
FIGURE 4.
Decline in PEPCK-C with age is coupled with loss of muscle activity to impact aging. A, locomotory speed of control and PEPCK-C OE (n = 5–12). *, p < 0.05; two-way analysis of variance. Fitting lines, first order regression. B, correlation of locomotory speed and PEPCK-C abundance in WT. r, Pearson correlation coefficient. C and D, locomotory speed of day 1 PEPCK-C OE in BDWM or pharynx (C) or NR350 grown on vector control or egl-19 RNAi bacteria (D) (n = 9–12). #, p > 0.05. *, p < 0.05; t test. E, immunoblotting against PEPCK-C. Upper left, SUX (200 nm) exposure to WT expressing rho9-tagged PEPCK-C in BDWM was initiated at day 1 and immunoblotting was done at day 3. Lower left, day 3 NR350 grown on control and egl-19 RNAi bacteria. Right, quantification. n = 3. *, p < 0.05; t test. F, survival of NR350 grown on vector control or egl-19 RNAi bacteria. G, correlation of median lifespan and locomotory speed (n = 3 (lifespan) or 10 (motor activity)). r, regression coefficient. H, effect of SUX (200 nm) exposure on reproductive life (n = 200). *, p < 0.05, t test.
In rodents, PEPCK-C in skeletal muscle and physical activity promote each other (12, 49). Thus, we examined if this feedback regulation underlies the coupling of decline in PEPCK-C and loss of motor activity with age. Selective PEPCK-C OE in BDWM but not in pharynx promoted motor activity (Fig. 4C), whereas PEPCK-C KD in BDWM decreased motor activity (Fig. 4D), indicating that PEPCK-C in BDWM is necessary and sufficient to enhance motor activity. To test if muscle activity also impacts PEPCK-C in worms, we exposed worms to SUX, an agonist of nicotinic acetylcholine receptors that specifically induces relaxation of striated muscle (50). SUX exposure reversibly inhibited motor activity in a dose-dependent manner (Fig. 5, A–D). SUX exposure did not affect pharynx pumping, likely because pharynx contains smooth muscle. We also used BDWM-specific KD of egl-19, encoding an L-type voltage-dependent calcium channel expressed in BDWM and required for muscle contraction (51), to reduce motor activity (Fig. 5E). Both pharmacological and genetic inhibition of muscle activity decreased PEPCK-C enzyme (Fig. 4E).
FIGURE 5.
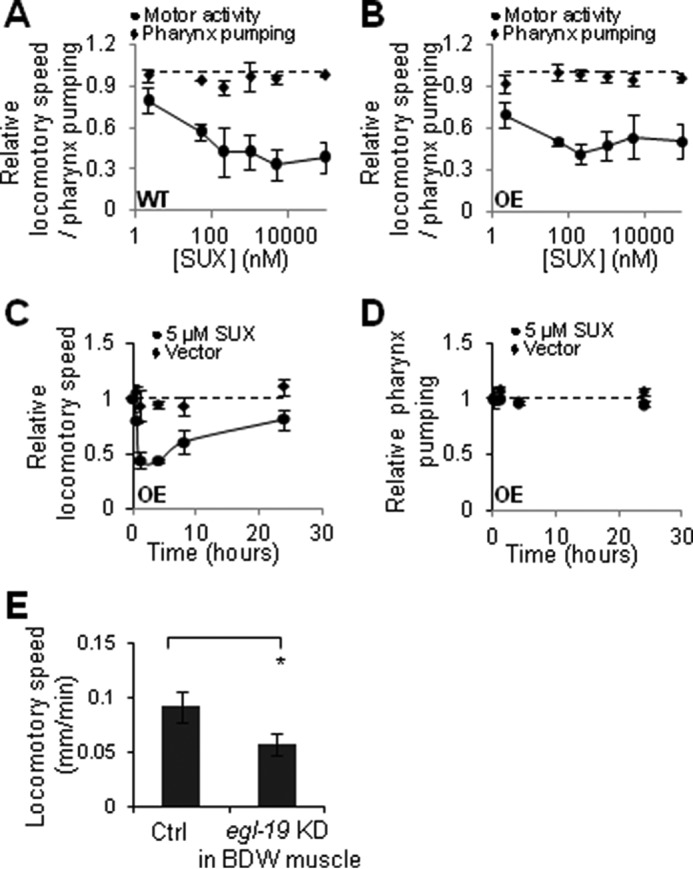
Pharmacological and genetic inhibition of locomotory activity. A and B, titration of SUX. Day 1 WT (A) and PEPCK-C OE in whole body (B) worms were exposed to SUX for 1 h and then analyzed for motor activity or pharynx pumping. C and D, time course of SUX. Day 1 PEPCK-C OE in whole body worms were exposed to SUX (5 μm) for 1 h and then moved to SUX free culturing plates. At indicated time, locomotion (C) or pharynx pumping (D) were analyzed. A–D, n = 9–12. E, NR350 worms were grown on control or egl-19 RNAi bacteria starting L4. Motor activity was analyzed at day 5 (n = 12). p < 0.05; t test.
Last, we examined if decline in PEPCK-C with age interacts with loss of muscle activity to impact aging. BDWM-specific KD of egl-19 and SUX exposure in post-fertile worms was sufficient to reduce lifespan (Fig. 4, F and G, and supplemental Table S1). Importantly, the effect of SUX on lifespan was dose dependent. SUX exposure of animals active in reproduction also shortened reproductive life without impacting lifespan or the total number of progeny (Fig. 4H and supplemental Table S1). We conclude that decline in PEPCK-C with age is coupled with loss of muscle activity to impact multiple aspects of aging, specifically, lifespan and reproductive life.
PEPCK-C Interacts with Muscle Activity to Impact Aging via Altered Energy Homeostasis
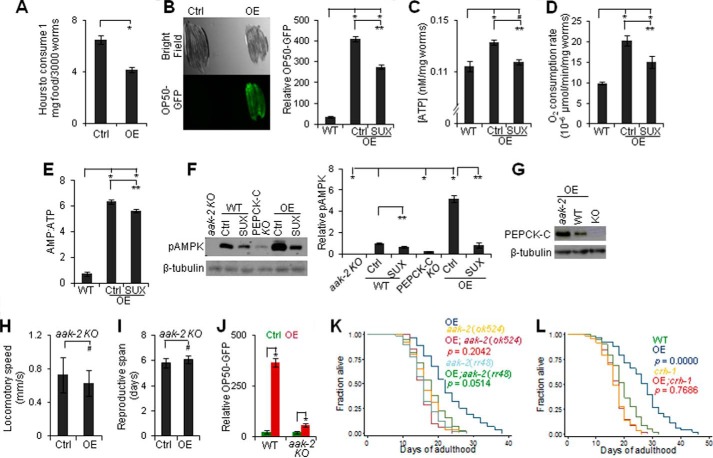
Because muscle activity is a major energy consumer (16), we next examined if PEPCK-C interacts with muscle activity to impact aging via altered energy metabolism. Similar to mice (12), worms globally over-expressing PEPCK-C had smaller body size, greater food consumption, more food in pharynx and gut, and increased fast pharynx pumping that is associated with food swallowing (37) (Fig. 6A and Table 1). PEPCK-C OE in pharynx or intestine also enhanced food intake and pharynx pumping. Although PEPCK-C OE in BDWM did not affect pharynx pumping, these animals showed a marked increase in food intake, as well as increased ATP, oxygen consumption, and a key indicator of cellular energy demand, the AMP:ATP ratio (52) (Fig. 6, B–E). The high AMP:ATP ratio despite high ATP in PEPCK-C OE worms is likely due to that exercise increases both energy demand and the ability to produce ATP (53). To test this idea, we exposed worms to SUX, and this attenuated the enhancements in energy supply and demand associated with PEPCK-C OE. SUX exposure temporally eliminated the effect of PEPCK-C OE on motor activity (Fig. 5), yet it only partially reduced the effect of PEPCK-C OE on food intake, oxygen consumption, and AMP:ATP ratio. Thus, PEPCK-C and muscle activity interact to affect energy homeostasis acutely and chronically.
FIGURE 6.
Decline in PEPCK-C and loss of muscle activity disrupt energy homeostasis to promote aging. A, food consumption of day 1 rol-6 mutants with or without PEPCK-C OE in whole body (n = 3). *, p < 0.05; t test. B–E, food (B), [ATP] (C), oxygen consumption (D), and AMP:ATP (E) in day 1 (B), day 3 (D), or day 9 (C and E) WT and PEPCK-C OE in BDWM with or without SUX (200 nm) exposure (n = 10 (B) or 3 (C-E)). #, p < 0.05; *, p < 0.05; one-way analysis of variance with Dunnett's test. **, p < 0.05; t test. F, immunoblotting against pAMPK in aak-2 (ok524), WT, PEPCK-C KO, and PEPCK-C OE in BDWM. SUX (200 nm) exposure was initiated at day 1 and immunoblotting was done at day 3. Left, sample images. Right, quantification (n = 3). The ratio of immunoreactivity of pAMPK over β-tubulin in WT was set as 1. *, p < 0.05, one-way analysis of variance with Dunnett's test; **, p < 0.05, t test. G, immunoblottings against PEPCK-C in day 1 PEPCK-C KO and WT/aak-2 (ok524) mutant over-expressing PEPCK-C in BDWM. H–L, locomotory speed (H), reproductive life (I), and food intake (day 1, J) of WT or aak-2 (rr48) with and without PEPCK-C OE in BDWM (J) or whole body (H and I) (n = 8–12 (H), 200 (I), or 10 (J)). #, p > 0.05; *, p < 0.05; t test. K and L, survival of WT, aak-2 (K) and crh-1 (L) mutants with or without PEPCK-C OE in whole body.
TABLE 1.
Effects of altered PEPCK-C expression in whole body and each native tissue on food intake, pharynx pumping, locomotion, and lifespan
Note: the symbols represent #, no significant change compared with controls. Food intake was indirectly quantified with accumulation of OP50-GFP. The changes in food intake are, therefore, categorized as *, small increase; **, moderate increase; ***, large increase.
| Tissue | Expression | Food intake | Pharynx pumping | Locomotion | Lifespan |
|---|---|---|---|---|---|
| Whole body | OE | *** | 4% increase | ∼60% increase | ∼30% increase |
| KD | NEa | NE | NE | ∼30% decrease | |
| BDW muscle | OE | ** | # | ∼50% increase | ∼15% increase |
| KD | NE | NE | NE | ∼20% decrease | |
| Pharynx | OE | * | 6% increase | # | ∼15% decrease |
| KD | NE | NE | NE | NE | |
| Intestine | OE | ** | 9% decrease | NE | # |
| KD | NE | NE | NE | # |
a NE, not examined.
AMPK is a major mediator of energy homeostasis activated by the higher AMP:ATP ratio (52). PEPCK-C OE and KO, respectively, increased or decreased a conserved marker for AMPK activation, phosphorylation at Thr-172 (Fig. 6F) (6). SUX exposure reduced pAMPK, indicating that muscle activity contributes to the increased AMPK signaling of these animals. We generated aak-2 and crh-1 mutants over-expressing PEPCK-C (Fig. 6G). The genes aak-2 and crh-1, respectively, encode one of the two worm homologs of the catalytic α subunit of AMPK (6) and the sole worm homolog of the cyclic AMP response element-binding transcription factor family (54). Although aak-2 (ok524) and crh-1 (tz2) are KO mutants, aak-2 (rr48) is a semi-dominant-negative mutation (H208Y) that disrupts the catalytic domain of AAK-2 (55). AMPK promotes physical activity and food intake, links energy state to lifespan, and requires CRH-1 to extend lifespan (6, 54). The effects of PEPCK-C OE on motor activity, reproductive life, food intake, and lifespan were blocked in aak-2 or crh-1 mutants (Fig. 6, H–L, and supplementalTable S 1). We conclude that decline in PEPCK-C with age interacts with loss of cellular function to promote aging via disrupted energy homeostasis.
Reciprocal Changes in PEPCK-C and PK with Age Shunts Energy Metabolism toward Glycolysis
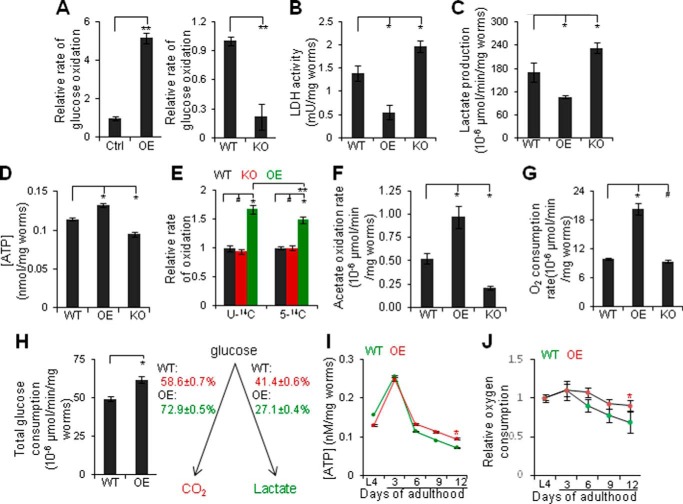
We next determined the mechanisms by which reciprocal changes in PEPCK-C and PK with age disrupt energy homeostasis. In metazoans, ATP is produced in the presence or absence of oxygen (56). For example, glycolysis breaks down glucose to generate pyruvate and 2 ATP. In oxidative metabolism, pyruvate is oxidized to carbon dioxide in mitochondria, producing an additional 28–30 ATP. Alternatively, pyruvate is converted to lactate by LDH in the cytosol (56). PEPCK-C OE promoted glucose oxidation and reduced LDH activity and lactate production; whereas PEPCK-C KO reduced glucose oxidation and increased LDH activity and lactate production (Fig. 7, A–C). In Fig. 7A, rol-6 mutants, whose phenotype is a commonly used genetic marker in aging research (13), were controls. Consistently, PEPCK-C OE and KO increased or reduced ATP, respectively (Fig. 7D).
FIGURE 7.
Reciprocal changes in PEPCK-C and PK shunts energy metabolism toward glycolysis and reduces mitochondrial function. A–G, glucose oxidation (A), LDH activity (B), lactate production (C), [ATP] (D), oxidation of [U-14C]- and [5-14C]glutamate (E), acetate oxidation (F), and oxygen consumption (G) in days 1 (A, E, and F), 3 (G), or 6 (B-D) WT, PEPCK-C OE in BDWM and PEPCK-C KO (n = 3). #, p > 0.05; *, p < 0.05; one-way analysis of variance with Dunnett's test. **, p < 0.05; t test. A, left, the value of rol-6 (Ctrl) (0.68 ± 0.027 × 10−6 units/mg of worms) was set as 1. Right, the value of WT (1.43 ± 0.018 × 10−6 units/mg of worms) was set as 1. E, the values of [U-14C]- and [5-14C]glutamate oxidation (2.3 ± 0.13 ([U-14C]) and 0.63 ± 0.08 ([5-14C]) × 10−6 units/mg of worms)) of WT was set as 1. H, left, total glucose consumption in day 6 WT and PEPCK-C OE. *, p < 0.05; t test. Right, numbers indicate the proportions of the conversion of [U-14C]glucose to carbon dioxide or lactate. I and J, [ATP] (I) and oxygen consumption (J) in WT and PEPCK-C OE in BDWM (n = 3). *, p < 0.05; two-way analysis of variance. The values of L4 (8.98 ± 0.20 (WT) and 18.31 ± 1.09 (OE) × 10−6 units/mg of worms) were set as 1.
We next examined the mechanisms by which PEPCK-C inhibits glycolysis and promotes oxidative metabolism. PEPCK-C OE increased the oxidation of [5-14C]glutamate to carbon dioxide (Fig. 7E), indicating accelerated citric acid cycle flux (57). It also accelerated the oxidation of acetate and [U-14C]glutamate, and oxygen consumption (Fig. 7, E–G), indicating broadly increased oxidative metabolism. The greater increase in oxidation of [U-14C]glutamate than [5-14C]glutamate is consistent with the randomization of isotopic carbons of [U-14C]glutamate in citric acid cycle intermediates. PEPCK-C KO decreased acetate oxidation, although it did not significantly affect glutamate oxidation or oxygen consumption.
We then simultaneously quantified the convert of [U-14C]glucose to carbon dioxide and lactate (Fig. 7H) with liquid worm culture, because the penetration of most released lactate into solid agar medium (data not shown) prevented its absolute quantification with worms on solid agar medium. PEPCK-C OE increased total glucose consumption, i.e. the sum of glucose converted to carbon dioxide and lactate, by ∼25% and the proportion of glucose oxidation by ∼14%. Because glucose oxidation produces ∼15-fold more ATP than anaerobic glycolysis, PEPCK-C OE increased total ATP production by ∼53%.
Last, we examined if PEPCK-C impacts mitochondrial function under the aging context. ATP and oxygen consumption displayed a lifecycle dynamics similar to PEPCK-C, i.e. they increased from L4s to adults, peaked during the reproductive period, and declined thereafter (Fig. 7, I and J). PEPCK-C OE retarded declines in mitochondrial function with age. We conclude that reciprocal changes in PEPCK-C and PK with age shunt energy metabolism toward anaerobic glycolysis, reducing mitochondrial function and bioenergetics.
CR Counteracts Reciprocal Changes in PEPCK-C and PK to Extend Lifespan
Last, we set out to determine whether reciprocal changes in PEPCK-C and PK with age can be modified to modulate aging. We noted that CR, the most robust longevity paradigm, increases PEPCK-C activity and decreases PK activity in mammals (58), and promotes oxidative metabolism in many species (13, 58–61). Moreover, AMPK activation, which was enhanced by PEPCK-C OE, may mimic CR to impact aging (62). Thus, we applied various amounts of OP50 bacteria that model ad libitum (1010 cfu/ml), different degrees of CR, and fasting (5 × 106 cfu/ml) (63). CR retarded reciprocal changes in PEPCK and PK with age (Fig. 8A). CR also extended lifespan in a dose-dependent manner in both WT and PEPCK-C OE worms (Fig. 8B). Critically, the difference in lifespan between WT and PEPCK-C OE worms attenuated progressively with increased degrees of CR, and was eventually eliminated. Under fasting, PEPCK-C OE exhibited a reduced lifespan. Thus, CR interacts with PEPCK-C in lifespan regulation.
FIGURE 8.
Calorie restriction counteracts reciprocal changes in PEPCK-C and PK to retard aging. A, activities of PEPCK and PK in animals of CR (2 × 1010 cfu/ml) and ad libitum. *, p < 0.05; t test; n = 3. The values of ad libitum at day 3 (0.27 ± 0.06 (PEPCK) and 22.1 ± 0.9 (PK) milliunits/mg of worms) were set as 1. B, mean lifespan of WT and PEPCK-C OE in BDWM as a function of food supply. C, body size of control and PEPCK-C OE in BDWM grown on vector control or let-363 RNAi bacteria. *, p < 0.05; t test; n = 9–11. D, survival of PEPCK-C OE in BDWM with and without rapamycin exposure. E, autophagy examined by EM. *, p < 0.05; t test; n = 5. The arrow indicates an EM structure of autophagy. F, survival of control and PEPCK-C OE in BDWM grown on vector control or bec-1 RNAi bacteria. AL, ad libitum.
If CR counteracts reciprocal changes in PEPCK-C and PK to retard aging, one may expect that CR and PEPCK-C OE share downstream mechanisms in eliciting anti-aging effects. AMPK activation inhibits TOR signaling (64). In response to CR, TOR inhibition extends lifespan (65). At the cellular level, TOR inhibition reduces cell size in response to low cellular energy state (66). Interestingly, PEPCK-C OE mice (12) and worms were smaller than controls, and senescent cells (67) and aged worms were enlarged (Fig. 3E). We applied RNAi KD of let-363, which encodes a worm homolog of TOR (68), to inhibit TOR. This reduced worm size in WT but not in PEPCK-C OE worms (Fig. 8C). KD of let-363 also extended the lifespan in WT, but reduced lifespan in PEPCK-C OE worms (supplemental Table S1). Such reduction may result from toxic genetic interaction between let-363 KD and PEPCK-C OE, for example, overly inhibited TOR by RNAi and PEPCK-C OE. Notably, let-363 KD has been shown to shorten the lifespan in genetically modified worms (69). To confirm that TOR and PEPCK-C interact in lifespan regulation, we exposed worms to rapamycin (33). This increased lifespan in WT did not affect the lifespan of PEPCK-C OE worms (Fig. 8D and supplemental Table S1). Thus, TOR inhibition contributes to at least some anti-aging effect of PEPCK-C OE at the cellular and organismal level.
AMPK activation and TOR inhibition promote autophagy (64), which is required by CR to extend lifespan (65, 70). To validate that CR and PEPCK-C OE share downstream pathways in lifespan regulation, we first examined if PEPCK-C impacts autophagy. The number of GFP::LGG-1 (worm ortholog of LC3) puncta in hypodermal seam cells of L3 larvae, a marker of autophagy in worms, however, was reduced by PEPCK-C OE (0.08 ± 0.07, n = 138), compared with WT (0.36 ± 0.1, n = 121). Consistently, electron microscopy (EM) showed that day 1 WT worms exhibited few (0.60 ± 0.27/worm section, n = 5) small (0.03–0.07 μm2) autolysosomes and autophagosomes, which were absent in PEPCK-C OE worms (n = 5). EM also did not detect significant autophagic activity in day 9 WT (n = 5), consistent with a decline in autophagy with age (71). Strikingly, day 9 PEPCK-C OE animals exhibited many autolysosomes and autophagosomes ranging from small to gigantic in size (0.07–113 μm2) (Fig. 8E), indicating that PEPCK-C OE selectively enhanced autophagy in aged worms, likely to clear accumulated molecular and organelle wastes with age. This observation is consistent with the finding that PEPCK-C OE retarded the accumulation of age pigments with age (Fig. 3E). We next applied RNAi KD of bec-1 and atg-7, respectively, encoding the worm ortholog of beclin 1 and Apg7p required for autophagy (72). These interventions moderately reduced or did not affect lifespan in WT, but eliminated or reduced lifespan extension of PEPCK-C OE (Fig. 8F and supplemental Table S1). Thus, PEPCK-C requires autophagy to impact lifespan. We conclude that CR counteracts reciprocal changes in PEPCK-C and PK with age to retard aging via TOR inhibition and autophagy.
Discussion
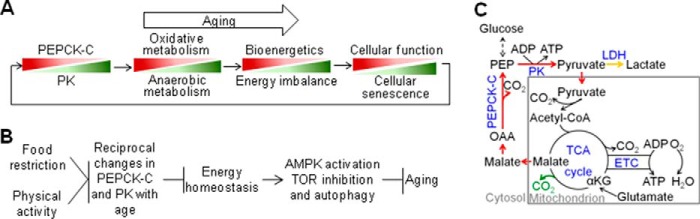
Energy metabolism appears to be optimized for reproduction. After the reproductive peak, a metabolic program involving a progressive decrease in PEPCK-C and a reciprocal increase in PK alters the way organisms produce energy. Specifically, it shunts energy metabolism toward glycolysis, reducing bioenergetics (Fig. 9A). As a result, energy-dependent cellular function and maintenance, such as muscle activity and autophagy, decline due to unmatched energy demand and supply. Decline in cellular function, namely muscle activity, further accelerates this metabolic shift in return, forming a vicious cycle. The causality between changes in energy metabolism and cellular function with age is likely a “the chicken and the egg” dilemma. Thus, in addition to stochastic damage of cells by reactive species, a programmed metabolic event can also be a determinant of loss of cellular function and integrity with age and aging.
FIGURE 9.
Models for aging-associated changes in energy metabolism and aging. A and B, models for the role of reciprocal changes in PEPCK-C and PK in aging. C, a model for the metabolic function of PEPCK-C. Red, pathways of the removal and replenishment of citric acid cycle intermediates by PEPCK-C; yellow, conversion of pyruvate to lactate; green, carbon dioxide generated from the oxidation of C5 of glutamate; αKG, α-ketoglutarate.
Previous and present studies show that CR and physical activity increase PEPCK-C and that CR also reduces PK. These and other evidence suggest that energy balance, achieved by reduced “energy in” from CR, enhanced “energy out” from enhanced physical activity, or their combination, counteracts reciprocal changes in PEPCK-C and PK with age to retard aging via the AMPK and TOR pathways (Fig. 9B). This model is consistent with the notion that both diet and exercise are critical for healthy aging (73). In addition to CR and physical activity, many longevity-associated factors, such as reduced insulin/IGF signaling (74–76), lower ambient temperature (8, 77), and sirtuin (7, 78, 79) also increase or stabilize PEPCK-C and promote oxidative metabolism. Based on these observations, we propose that reciprocal changes in PEPCK-C and PK with age are a lead metabolic event of aging. Environmental and genetic factors that retard it promote healthy aging and longevity.
Interestingly, most cancer cells exhibit higher glycolysis followed by lactate production, rather than lower glycolysis followed by oxidation (80). At the cellular level, this so-called Warburg effect may contribute to the immortality of cancer cells. To organisms, uncontrolled energy expenditure can be destructive. A difference is that cancer cells deprive other cells of glucose and other supplies, whereas glucose supply for aging cells is restricted. Indeed, glucose uptake in many tissues declines with age (81), which may further disrupt energy homeostasis in aged organisms.
PEPCK-C is traditionally recognized as a key gluconeogenic enzyme, but its activity is correlated tightly with the flux of citric acid cycle but not gluconeogenesis (82). It has been proposed that PEPCK-C links the citric acid cycle, core chemical reactions of energy metabolism, with the metabolism of glucose, amino acids, and other metabolites (8) (Fig. 9C). In support of this model, PEPCK-C has been shown to critically regulate the homeostasis of fatty acids, amino acids, and glucose (see Ref. 8 for review). Here, we demonstrate that PEPCK-C accelerated the citric acid cycle and cellular respiration, promoted the oxidation of major fuels, shifted glucose catabolism toward oxidation, and enhanced the efficiency and total bioenergetics. Because mitochondria play a central role in aging (83), these metabolic functions of PEPCK-C likely contribute to aging more significantly than other metabolic functions, although PEPCK-C may impact aging via multiple mechanisms. At the organismal level, PEPCK-C is required for the integration of energy metabolism (84), and participates in diverse pathophysiological and physiological processes. For example, PEPCK-C mutations underlie Smith-Magenis syndrome, a heritable disease marked by hypoglycemia, lactic acidosis, and other metabolic disorders (10). PEPCK-C also is a negative marker of colon cancer (11) and alleviates renal tubular acidosis (9). In model organisms, PEPCK-C supports adaptive response and survival under inflammation (85), osmotic stress (86), and oxidative stress (this study). These metabolic and physiological roles of PEPCK-C indicate that it is a major metabolic adaptor of organisms that promotes health and longevity.
Mechanisms underlying aging-associated changes in PEPCK-C and PK are presently unknown. Enhancing PEPCK-C or reducing PK, however, was sufficient to retard many key aging-associated metabolic and physiological changes, such as loss of mitochondrial function and motor activity, and/or increase lifespan. Because PEPCK-C has conserved metabolic functions, physiological roles, and regulatory mechanisms (8), sustaining PEPCK-C may be a novel strategy to counteract aging in humans.
Author Contributions
Y. Y., N. B., R. H., and Z. F. designed experiments. Y. Y., P. H., C. K., A. K., A. T., A. J., X. H., H. A., E. S., K. X., P. C., Q. S., T. C., and Z. F. performed experiments. A. H., S. E., G. D., R. H., N. B., and Z. F. provided reagents and funds. Y. Y., N. B., R. H., and Z. F. wrote the manuscript.
Supplementary Material
Acknowledgments
We thank Dr. Charles Hopple and Nathan Shock Center, Invertebrate Aging Core (University of Michigan) for technical assistance, Caenorhabditis Genetics Center and Drs. Kang Shen, Edward Kipros, and John Kim for reagents, and Drs. Satish Kalhan, Leslie Webster, Alexander Trubitsyn, Thomas Sundermeier, and Deepak K. Gupta, Johannes Von Lintig, Nelson Hsieh, Malene Hansen, and Joy Alcedo for discussion.
This work was supported by Transdisciplinary Research on Energetics and Cancer Grant U54-CA-116867 (directed by Dr. Nathan A. Berger, Nutrition Obesity Research Center, Case Western Reserve University), and Whitehall Foundation Grant 2013–08-02 (to Z. F.), Ellison Foundation Grant AG-SS-2420-10 (to R. W. H. and N. A. B.), and National Institutes of Health Grants P20-CA-103736 (to R. W. H.), 1R01AG028516 (to A. L. H.) and HL103453, HL60917, and HL081064 (to S. C. E.). The authors indicate no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
In memory of Dr. Richard W. Hanson. It is with sadness but great appreciation that this article is dedicated to Richard for his insight in establishing this line of research and for his generosity of time and guidance that allowed these continued studies on “his enzyme,” phosphoenolpyruvate carboxykinase.

This article contains supplemental Table S1.
- ROS
- reactive oxygen species
- AMPK and pAMPK
- 5′-AMP activated kinase and its phosphorylated form
- BDWM
- body wall muscle
- CR
- calorie restriction
- KD
- knockdown
- KO
- knockout
- LDH
- lactase dehydrogenase
- OE
- overexpression
- OP50
- an E. coli strain
- PEP
- phosphoenolpyruvate
- PEPCK
- PEPCK-C and PEPCK-M, phosphoenolpyruvate carboxykinase and its cytosolic and mitochondrial isoforms
- PK
- pyruvate kinase
- SUX
- suxamethonium
- TOR
- target of rapamycin.
References
- 1.López-Otín C., Blasco M. A., Partridge L., Serrano M., and Kroemer G. (2013) The hallmarks of aging. Cell 153, 1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez V. I., Bokov A., Van Remmen H., Mele J., Ran Q., Ikeno Y., and Richardson A. (2009) Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta 1790, 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzilai N., Huffman D. M., Muzumdar R. H., and Bartke A. (2012) The critical role of metabolic pathways in aging. Diabetes 61, 1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson R. M., and Weindruch R. (2010) Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol. Metab. 21, 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia K., Chen D., and Riddle D. L. (2004) The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131, 3897–3906 [DOI] [PubMed] [Google Scholar]
- 6.Apfeld J., O'Connor G., McDonagh T., DiStefano P. S., and Curtis R. (2004) The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18, 3004–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang W., Wang S., Xiao M., Lin Y., Zhou L., Lei Q., Xiong Y., Guan K. L., and Zhao S. (2011) Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol. Cell 43, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Kalhan S. C., and Hanson R. W. (2009) What is the metabolic role of phosphoenolpyruvate carboxykinase? J. Biol. Chem. 284, 27025–27029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boron W. F., and Boulpaep E. L. (2003) Medical Physiology: A Cellular And Molecular Approach, p. 858, Elsevier/Saunders, Philadelphia, PA [Google Scholar]
- 10.Adams D. R., Yuan H., Holyoak T., Arajs K. H., Hakimi P., Markello T. C., Wolfe L. A., Vilboux T., Burton B. K., Fajardo K. F., Grahame G., Holloman C., Sincan M., Smith A. C., Wells G. A., Huang Y., Vega H., Snyder J. P., Golas G. A., Tifft C. J., Boerkoel C. F., Hanson R. W., Traynelis S. F., Kerr D. S., and Gahl W. A. (2014) Three rare diseases in one Sib pair: RAI1, PCK1, GRIN2B mutations associated with Smith-Magenis Syndrome, cytosolic PEPCK deficiency and NMDA receptor glutamate insensitivity. Mol. Genet. Metab. 113, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blouin J. M., Bortoli S., Nacfer M., Collinet M., Penot G., Laurent-Puig P., and Forest C. (2010) Down-regulation of the phosphoenolpyruvate carboxykinase gene in human colon tumors and induction by ω-3 fatty acids. Biochimie 92, 1772–1777 [DOI] [PubMed] [Google Scholar]
- 12.Hakimi P., Yang J., Casadesus G., Massillon D., Tolentino-Silva F., Nye C. K., Cabrera M. E., Hagen D. R., Utter C. B., Baghdy Y., Johnson D. H., Wilson D. L., Kirwan J. P., Kalhan S. C., and Hanson R. W. (2007) Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J. Biol. Chem. 282, 32844–32855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y., Kadiyala C. S., Ching T. T., Hakimi P., Saha S., Xu H., Yuan C., Mullangi V., Wang L., Fivenson E., Hanson R. W., Ewing R., Hsu A. L., Miyagi M., and Feng Z. (2012) Enhanced energy metabolism contributes to the extended life span of calorie-restricted Caenorhabditis elegans. J. Biol. Chem. 287, 31414–31426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhahbi J. M., Mote P. L., Wingo J., Tillman J. B., Walford R. L., and Spindler S. R. (1999) Calories and aging alter gene expression for gluconeogenic, glycolytic, and nitrogen-metabolizing enzymes. Am. J. Physiol. 277, E352–360 [DOI] [PubMed] [Google Scholar]
- 15.Kil D. Y., Vester Boler B. M., Apanavicius C. J., Schook L. B., and Swanson K. S. (2010) Age and diet affect gene expression profiles in canine liver tissue. PLoS ONE 5, e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coyle E. F. (2000) Physical activity as a metabolic stressor. Am. J. Clin. Nutr. 72, 512S-520S [DOI] [PubMed] [Google Scholar]
- 17.Moore S. C., Patel A. V., Matthews C. E., Berrington de Gonzalez A., Park Y., Katki H. A., Linet M. S., Weiderpass E., Visvanathan K., Helzlsouer K. J., Thun M., Gapstur S. M., Hartge P., and Lee I. M. (2012) Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 9, e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadota H., Inoue M., Hikita T., Köppen M., Hardin J. D., Amano M., Moerman D. G., and Kaibuchi K. (2007) Establishment of a tissue-specific RNAi system in C. elegans. Gene 400, 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yochem J., and Greenwald I. (1989) glp-1 and lin-12, genes implicated in distinct cell-cell interactions in C. elegans, encode similar transmembrane proteins. Cell 58, 553–563 [DOI] [PubMed] [Google Scholar]
- 20.Cao P., Sun W., Kramp K., Zheng M., Salom D., Jastrzebska B., Jin H., Palczewski K., and Feng Z. (2012) Light-sensitive coupling of rhodopsin and melanopsin to Gi/o and Gq signal transduction in Caenorhabditis elegans. FASEB J. 26, 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salom D., Cao P., Sun W., Kramp K., Jastrzebska B., Jin H., Feng Z., and Palczewski K. (2012) Heterologous expression of functional G-protein-coupled receptors in Caenorhabditis elegans. FASEB J. 26, 492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroeher V. L., Kennedy B. P., Millen K. J., Schroeder D. F., Hawkins M. G., Goszczynski B., and McGhee J. D. (1994) DNA-protein interactions in the Caenorhabditis elegans embryo: oocyte and embryonic factors that bind to the promoter of the gut-specific ges-1 gene. Dev. Biol. 163, 367–380 [DOI] [PubMed] [Google Scholar]
- 23.Shimada N., Shinagawa T., and Ishii S. (2008) Modulation of M2-type pyruvate kinase activity by the cytoplasmic PML tumor suppressor protein. Genes Cells 13, 245–254 [DOI] [PubMed] [Google Scholar]
- 24.Boyd-Tressler A., Penuela S., Laird D. W., and Dubyak G. R. (2014) Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J. Biol. Chem. 289, 27246–27263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayser E. B., Morgan P. G., Hoppel C. L., and Sedensky M. M. (2001) Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J. Biol. Chem. 276, 20551–20558 [DOI] [PubMed] [Google Scholar]
- 26.Xu W., Janocha A. J., Leahy R. A., Klatte R., Dudzinski D., Mavrakis L. A., Comhair S. A., Lauer M. E., Cotton C. U., and Erzurum S. C. (2014) A novel method for pulmonary research: assessment of bioenergetic function at the air-liquid interface. Redox Biol. 2, 513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nye C. K., Hanson R. W., and Kalhan S. C. (2008) Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J. Biol. Chem. 283, 27565–27574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng H., Zhong W., Punkosdy G., Gu S., Zhou L., Seabolt E. K., and Kipreos E. T. (1999) CUL-2 is required for the G1-to-S-phase transition and mitotic chromosome condensation in Caenorhabditis elegans. Nat. Cell Biol. 1, 486–492 [DOI] [PubMed] [Google Scholar]
- 29.Gerstbrein B., Stamatas G., Kollias N., and Driscoll M. (2005) In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 4, 127–137 [DOI] [PubMed] [Google Scholar]
- 30.Fujioka H., Tandler B., and Hoppel C. L. (2012) Mitochondrial division in rat cardiomyocytes: an electron microscope study. Anat Rec. (Hoboken) 295, 1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadiya P., and Nazir A. (2014) A pre- and co-knockdown of RNaseT enzyme, Eri-1, enhances the efficiency of RNAi induced gene silencing in Caenorhabditis elegans. PLoS ONE 9, e87635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei X., Potter C. J., Luo L., and Shen K. (2012) Controlling gene expression with the Q repressible binary expression system in Caenorhabditis elegans. Nat. Methods 9, 391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robida-Stubbs S., Glover-Cutter K., Lamming D. W., Mizunuma M., Narasimhan S. D., Neumann-Haefelin E., Sabatini D. M., and Blackwell T. K. (2012) TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15, 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes S. E., Evason K., Xiong C., and Kornfeld K. (2007) Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 3, e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu A. L., Feng Z., Hsieh M. Y., and Xu X. Z. (2009) Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging 30, 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mair W., Panowski S. H., Shaw R. J., and Dillin A. (2009) Optimizing dietary restriction for genetic epistasis analysis and gene discovery in C. elegans. PLoS ONE 4, e4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C., Xiong C., and Kornfeld K. (2004) Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 101, 8084–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz T. J., Zarse K., Voigt A., Urban N., Birringer M., and Ristow M. (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 6, 280–293 [DOI] [PubMed] [Google Scholar]
- 39.Meissner B., Rogalski T., Viveiros R., Warner A., Plastino L., Lorch A., Granger L., Segalat L., and Moerman D. G. (2011) Determining the sub-cellular localization of proteins within Caenorhabditis elegans body wall muscle. PLoS ONE 6, e19937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakimi P., Johnson M. T., Yang J., Lepage D. F., Conlon R. A., Kalhan S. C., Reshef L., Tilghman S. M., and Hanson R. W. (2005) Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr. Metab. (Lond.) 2, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vukoti K., Yu X., Sheng Q., Saha S., Feng Z., Hsu A. L., and Miyagi M. (2015) Monitoring newly synthesized proteins over the adult life span of Caenorhabditis elegans. J. Proteome Res. 14, 1483–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murshid A., Eguchi T., and Calderwood S. K. (2013) Stress proteins in aging and life span. Int. J. Hyperthermia 29, 442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui H., Kong Y., and Zhang H. (2012) Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker D. J., Wijshake T., Tchkonia T., LeBrasseur N. K., Childs B. G., van de Sluis B., Kirkland J. L., and van Deursen J. M. (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung T., Höhn A., and Grune T. (2010) Lipofuscin: detection and quantification by microscopic techniques. Methods Mol. Biol. 594, 173–193 [DOI] [PubMed] [Google Scholar]
- 46.Lee B. Y., Han J. A., Im J. S., Morrone A., Johung K., Goodwin E. C., Kleijer W. J., DiMaio D., and Hwang E. S. (2006) Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187–195 [DOI] [PubMed] [Google Scholar]
- 47.Tan W., Gu Z., Shen B., Jiang J., Meng Y., Da Z., Liu H., Tao T., and Cheng C. (2015) PTEN/Akt-p27(kip1) Signaling promote the BM-MSCs senescence and apoptosis in SLE patients. J. Cell. Biochem. 116, 1583–1594 [DOI] [PubMed] [Google Scholar]
- 48.Chkhotua A. B., Gabusi E., Altimari A., D'Errico A., Yakubovich M., Vienken J., Stefoni S., Chieco P., Yussim A., and Grigioni W. F. (2003) Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am. J. Kidney Dis. 41, 1303–1313 [DOI] [PubMed] [Google Scholar]
- 49.Novak C. M., Escande C., Gerber S. M., Chini E. N., Zhang M., Britton S. L., Koch L. G., and Levine J. A. (2009) Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS ONE 4, e5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall C. G., Ogden D. C., and Colquhoun D. (1990) The actions of suxamethonium (succinyldicholine) as an agonist and channel blocker at the nicotinic receptor of frog muscle. J. Physiol. 428, 155–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jospin M., Jacquemond V., Mariol M. C., Ségalat L., and Allard B. (2002) The L-type voltage-dependent Ca2+ channel EGL-19 controls body wall muscle function in Caenorhabditis elegans. J. Cell Biol. 159, 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardie D. G. (2004) AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med. Sci. Sports Exerc. 36, 28–34 [DOI] [PubMed] [Google Scholar]
- 53.Egan B., and Zierath J. R. (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17, 162–184 [DOI] [PubMed] [Google Scholar]
- 54.Mair W., Morantte I., Rodrigues A. P., Manning G., Montminy M., Shaw R. J., and Dillin A. (2011) Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470, 404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narbonne P., and Roy R. (2006) Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1, and AMPK signalling. Development 133, 611–619 [DOI] [PubMed] [Google Scholar]
- 56.Voet D., and Voet J. (1995) Biochemistry, John Wiley & Sons, Inc., New York: [DOI] [PubMed] [Google Scholar]
- 57.Salon C., Raymond P., and Pradet A. (1988) Quantification of carbon fluxes through the tricarboxylic acid cycle in early germinating lettuce embryos. J. Biol. Chem. 263, 12278–12287 [PubMed] [Google Scholar]
- 58.Feuers R. J., Duffy P. H., Leakey J. A., Turturro A., Mittelstaedt R. A., and Hart R. W. (1989) Effect of chronic caloric restriction on hepatic enzymes of intermediary metabolism in the male Fischer 344 rat. Mech. Ageing Dev. 48, 179–189 [DOI] [PubMed] [Google Scholar]
- 59.Lin S. J., Kaeberlein M., Andalis A. A., Sturtz L. A., Defossez P. A., Culotta V. C., Fink G. R., and Guarente L. (2002) Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348 [DOI] [PubMed] [Google Scholar]
- 60.Moroz N., Carmona J. J., Anderson E., Hart A. C., Sinclair D. A., and Blackwell T. K. (2014) Dietary restriction involves NAD+-dependent mechanisms and a shift toward oxidative metabolism. Aging Cell 13, 1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.López-Lluch G., Hunt N., Jones B., Zhu M., Jamieson H., Hilmer S., Cascajo M. V., Allard J., Ingram D. K., Navas P., and de Cabo R. (2006) Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. U.S.A. 103, 1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canto C., and Auwerx J. (2011) Calorie restriction: is AMPK a key sensor and effector? Physiology (Bethesda) 26, 214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ching T. T., Paal A. B., Mehta A., Zhong L., and Hsu A. L. (2010) drr-2 encodes an eIF4H that acts downstream of TOR in diet-restriction-induced longevity of C. elegans. Aging Cell 9, 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J., Kundu M., Viollet B., and Guan K. L. (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen M., Chandra A., Mitic L. L., Onken B., Driscoll M., and Kenyon C. (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 4, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fingar D. C., Salama S., Tsou C., Harlow E., and Blenis J. (2002) Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16, 1472–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayflick L. (1965) The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 [DOI] [PubMed] [Google Scholar]
- 68.Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A. L., Orosz L., and Muller F. (2003) Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620. [DOI] [PubMed] [Google Scholar]
- 69.Pan K. Z., Palter J. E., Rogers A. N., Olsen A., Chen D., Lithgow G. J., and Kapahi P. (2007) Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell 6, 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R., Asara J. M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., and Shaw R. J. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cuervo A. M. (2008) Autophagy and aging: keeping that old broom working. Trends Genet 24, 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hars E. S., Qi H., Ryazanov A. G., Jin S., Cai L., Hu C., and Liu L. F. (2007) Autophagy regulates ageing in C. elegans. Autophagy 3, 93–95 [DOI] [PubMed] [Google Scholar]
- 73.Evans W. J. (1995) Exercise, nutrition, and aging. Clin. Geriatr Med. 11, 725–734 [PubMed] [Google Scholar]
- 74.Dong M. Q., Venable J. D., Au N., Xu T., Park S. K., Cociorva D., Johnson J. R., Dillin A., and Yates J. R. 3rd. (2007) Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science 317, 660–663 [DOI] [PubMed] [Google Scholar]
- 75.Depuydt G., Xie F., Petyuk V. A., Smolders A., Brewer H. M., Camp D. G. 2nd, Smith R. D., and Braeckman B. P. (2014) LC-MS proteomics analysis of the insulin/IGF-1-deficient Caenorhabditis elegans daf-2(e1370) mutant reveals extensive restructuring of intermediary metabolism. J. Proteome Res. 13, 1938–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zarse K., Schmeisser S., Groth M., Priebe S., Beuster G., Kuhlow D., Guthke R., Platzer M., Kahn C. R., and Ristow M. (2012) Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 15, 451–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao R., Zhang B., Dong Y., Gong J., Xu T., Liu J., and Xu X. Z. (2013) A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152, 806–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mouchiroud L., Houtkooper R. H., Moullan N., Katsyuba E., Ryu D., Cantó C., Mottis A., Jo Y. S., Viswanathan M., Schoonjans K., Guarente L., and Auwerx J. (2013) The NAD+/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sack M. N., and Finkel T. (2012) Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb. Perspect. Biol. 4, a013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warburg O. (1956) On the origin of cancer cells. Science 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 81.dos Santos J. M., Benite-Ribeiro S. A., Queiroz G., and Duarte J. A. (2012) The effect of age on glucose uptake and GLUT1 and GLUT4 expression in rat skeletal muscle. Cell Biochem. Funct. 30, 191–197 [DOI] [PubMed] [Google Scholar]
- 82.Burgess S. C., He T., Yan Z., Lindner J., Sherry A. D., Malloy C. R., Browning J. D., and Magnuson M. A. (2007) Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 5, 313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gouspillou G., Bourdel-Marchasson I., Rouland R., Calmettes G., Biran M., Deschodt-Arsac V., Miraux S., Thiaudiere E., Pasdois P., Detaille D., Franconi J. M., Babot M., Trézéguet V., Arsac L., and Diolez P. (2014) Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 13, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.She P., Shiota M., Shelton K. D., Chalkley R., Postic C., and Magnuson M. A. (2000) Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol. Cell. Biol. 20, 6508–6517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sadasivam M., Ramatchandirin B., Balakrishnan S., Selvaraj K., and Prahalathan C. (2014) The role of phosphoenolpyruvate carboxykinase in neuronal steroidogenesis under acute inflammation. Gene 552, 249–254 [DOI] [PubMed] [Google Scholar]
- 86.Frazier H. N. 3rd, and Roth M. B. (2009) Adaptive sugar provisioning controls survival of C. elegans embryos in adverse environments. Curr. Biol. 19, 859–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.