Abstract
In humans and mice, megakaryocytes/platelets and endothelial cells constitutively synthesize P-selectin and mobilize it to the plasma membrane to mediate leukocyte rolling during inflammation. TNF-α, interleukin 1β, and LPS markedly increase P-selectin mRNA in mice but decrease P-selectin mRNA in humans. Transgenic mice bearing the entire human SELP gene recapitulate basal and inducible expression of human P-selectin and reveal human-specific differences in P-selectin function. Differences in the human SELP and murine Selp promoters account for divergent expression in vitro, but their significance in vivo is not known. Here we generated knockin mice that replace the 1.4-kb proximal Selp promoter with the corresponding SELP sequence (SelpKI). SelpKI/KI mice constitutively expressed more P-selectin on platelets and more P-selectin mRNA in tissues but only slightly increased P-selectin mRNA after injection of TNF-α or LPS. Consistent with higher basal expression, leukocytes rolled more slowly on P-selectin in trauma-stimulated venules of SelpKI/KI mice. However, TNF-α did not further reduce P-selectin-dependent rolling velocities. Blunted up-regulation of P-selectin mRNA during contact hypersensitivity reduced P-selectin-dependent inflammation in SelpKI/− mice. Higher basal P-selectin in SelpKI/KI mice compensated for this defect. Therefore, divergent sequences in a short promoter mediate most of the functionally significant differences in expression of human and murine P-selectin in vivo.
Keywords: cell adhesion, endothelial cell, gene regulation, inflammation, leukocyte, platelet, selectin
Introduction
Neutrophils roll on P- and E-selectin expressed on venular endothelial cells in the first step of the inflammatory response (1–3). Endothelial cells in skin and bone marrow constitutively express E-selectin in humans and mice (4–6). In other tissues, TNF-α, IL-1β, or LPS translocates NF-κB, activating transcription factor 2 (ATF-2), and other transcription factors to the nucleus (7). These proteins activate the human SELE and murine Sele genes by binding to conserved promoter elements.
In humans and mice, megakaryocytes/platelets and endothelial cells constitutively express P-selectin, which is stored in secretory granules (1–3). Resident peritoneal macrophages also express P-selectin (8). Thrombin or histamine rapidly mobilizes the basal stores of P-selectin to the plasma membrane (1–3). TNF-α, IL-1β, or LPS further up-regulates mRNA for P-selectin in mice (9, 10) and other mammals (11–13) but not in humans and other primates. TNF-α decreases mRNA for P-selectin in cultured human endothelial cells (14–16). Baboons infused with Escherichia coli shed LPS and express TNF-α, which increase mRNA for E-selectin but decrease mRNA for P-selectin in many organs (16). Transgenic mice bearing the entire human SELP gene constitutively express human P-selectin in megakaryocytes/platelets, endothelial cells, and resident peritoneal macrophages (17). TNF-α or LPS infused into transgenic mice that retain the endogenous Selp gene markedly increases mRNA for murine P-selectin but decreases mRNA for human P-selectin in many organs (17). Therefore, the basal and inducible expression of the SELP transgene recapitulates that of the native gene in humans.
In vitro studies suggest that distinct elements in the proximal 1.4-kb promoters of the SELP and Selp genes account, at least in part, for divergent basal and inducible expression of P-selectin in humans and mice. The Selp gene has canonical binding sites for NF-κB (p50/p52 heterodimers) and ATF-2 like those in the SELE and Sele genes (18). TNF-α increases the expression of a reporter gene driven by the Selp promoter in transfected endothelial cells (19). Mutation of the NF-κB and ATF-2 sites abrogates TNF-α-inducible expression (18). The SELP promoter lacks these sites, and TNF-α does not augment the expression of the reporter gene driven by the SELP promoter (19). Instead, the SELP promoter has a non-canonical binding site for NF-κB (p50 or p52 homodimers) (20, 21). Mutation of this site reduces constitutive expression of the reporter gene (21). It is not known whether these distinct elements account for the divergent expression of human and murine P-selectin in vivo. In this study, we generated knockin mice that replace the 1.4-kb proximal promoter of Selp with the corresponding sequence from SELP. We compared the basal and inducible expression of murine P-selectin in knockin and WT mice.
Experimental Procedures
Mice
A targeting vector was constructed containing a portion of the murine Selp allele (19, 22) in which the 1.4-kb sequence immediately before the translation start site was replaced with the corresponding 1.4-kb sequence from the human SELP allele (19). A loxP-flanked hygromycin cassette (a gift from Dr. David S. Milstone, Harvard Medical School, Boston, MA) was inserted into intron 1 for selection of transfected embryonic stem cells with hygromycin B. A thymidine kinase (tk) cassette was placed just outside the 3′-flanking homologous sequence to further select targeted clones with ganciclovir. The fidelity of the targeting construct was confirmed by DNA sequencing. The linearized targeting vector was electroporated into CJ7 murine embryonic stem cells (23). After drug selection, the loxP-flanked hygromycin cassette was removed by transient in vitro expression of Cre recombinase. Targeted clones were confirmed by Southern blot (17). After confirming a normal karyotype, embryonic stem cells from one of the targeted clones were injected into C57BL/6J blastocysts, and the blastocysts were implanted into pseudopregnant mice. Chimeric offspring were bred with C57BL/6J mice for germline transmission. Progeny homozygous for the knockin allele (SelpKI/KI) were backcrossed over 10 generations into the C57BL/6J background. Some mice were bred with Selp−/− mice (22) (C57BL/6J background, The Jackson Laboratory) to generate heterozygous SelpKI/− mice. C57BL/6J mice were used as WT controls. All mice were housed in a specific pathogen-free facility. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Peripheral Blood Counts
Peripheral blood counts were measured with a Hemavet 950 veterinary hematology analyzer (Drew Scientific, Inc.).
Flow Cytometry
Whole blood collected by retro-orbital bleeding from anesthetized mice was transferred directly into a tube coated with EDTA (Microvette® 200 potassium EDTA). To study platelets, 2 μl of blood was diluted 1:100 in Hanks' balanced salt solution (HBSS)3 containing 5 mm EDTA (HBSS/EDTA). In some experiments, diluted blood was incubated with 1 unit/ml human thrombin (EMD Millipore) for 15 min at 37 °C to activate platelets. Resident peritoneal cells were collected by flushing the peritoneal cavity with 6 ml of HBSS/EDTA. All antibody incubations were performed on ice. Cells were preincubated with 5 μg/ml murine Fc Block (anti-murine CD16/CD32, BD Biosciences) for 15 min. Cells were then incubated for 30 min with 10 μg/ml of FITC-labeled RB40.34 (rat IgG1, anti-murine P-selectin, BD Biosciences), phycoerythrin-labeled MWReg30 (rat IgG1, anti-murine CD41, BioLegend), phycoerythrin-labeled BM8 (rat IgG2a, anti-murine F4/80, macrophage marker, BioLegend), FITC-labeled 1A8 (rat IgG2a, anti-murine Ly6G, BioLegend), or labeled isotype control mAbs. The cells were then washed, resuspended in HBSS containing 0.1% human serum albumin and 5 mm EDTA, and analyzed on a FACScan instrument using CellQuest software (BD Biosciences). Platelet, macrophage, and neutrophil subpopulations were distinguished by expression of CD41, F4/80, and Ly6G, respectively.
Quantitative RT-PCR (qRT-PCR)
Total RNA from murine lung, heart, liver, and ear was isolated using a RNeasy fibrous tissue mini kit (Qiagen) according to the instructions of the manufacturer. RNA integrity was verified by ethidium bromide staining after electrophoresis and quantified by optical density at 260 nm. 1 μg of RNA in a 20-μl reaction volume was reverse-transcribed using SsoAdvancedTM Universal SYBR® Green Supermix (Bio-Rad) as specified by the manufacturer. The RT reaction volume was diluted to 100 μl with double-distilled H2O, 2 μl of which was used as template for qRT-PCR in a 96-well plate with 0.2 μm of each primer and SYBR® Green PCR Master Mix (Life Technologies). qRT-PCR was performed on a CFX96TM real-time system (Bio-Rad), and amplification was performed according to the protocol of the manufacturer. Relative gene expression was analyzed with CFX ManagerTM software (Bio-Rad) using gapdh as an internal control. qRT-PCR assays were conducted in triplicate for each sample. The sequences of gapdh primers were 5′-GAAGGTGAAGGTCGGAGTC-3′ (sense) and 5′-GAAGATGGTGATGGGATTTC-3′ (antisense). The sequences of murine Selp primers were 5′-GGTATCCGAAAGATCAACAATAAGTGG-3′ (sense) and 5′-TTACTCTTGATGTAGATCTCCACACA-3′ (antisense).
Immunofluorescence
Murine tissues were fixed in 4% paraformaldehyde overnight at 4 °C, transferred into 20% sucrose overnight at 4 °C, embedded in Tissue-Tek O.C.T. compound (Triangle Biomedical Sciences, Inc.), and processed into 5-μm sections. After fixation and permeabilization in acetone at −20 °C for 2 min, cryosections were rinsed with PBS containing 0.01% saponin, incubated with serum-free protein block (Dako) at room temperature for 60 min, and then incubated with goat anti-murine P-selectin polyclonal antibody with 0.01% saponin overnight at 4 °C. The tissue sections were stained with Alexa Fluor 555-conjugated donkey anti-goat IgG antibody with 0.01% saponin at room temperature for 1 h. After washing, mounting medium was added to the slides. The images in the slides were visualized on a Zeiss Axiovert 200 m (Carl Zeiss, LLC) microscope at ×63 magnification and captured by a Carl Zeiss AxioCam MRm Rev. 3.0 camera using the acquisition software Carl Zeiss AxioVision V. 4.8.
Intravital Microscopy
Intravital video microscopy of anesthetized mice was performed as described previously (17, 23–25). The cremaster muscle was isolated and superfused with thermo-controlled (37 °C) HBSS. Microscopy was performed immediately after isolation or 2 h after intrascrotal injection of murine TNF-α (R&D Systems, 0.5 μg/mouse in 0.3 ml of sterile saline). A blocking anti-murine E-selectin mAb, 9A9 (26) (30 μg in 100 μl of saline), was injected intravenously 3–5 min before TNF-α injection. Microvessel diameters, lengths, and centerline velocities were comparable in mice from all genotypes. Leukocyte rolling velocities were measured as described previously (17, 23–25).
Thioglycollate-induced Peritonitis
Mice were injected intraperitoneally with 1.5 ml of 0.9% saline or 4% thioglycollate. Some mice received 30 μg of anti-murine P-selectin mAb 5H1 (27) intravenously immediately before administration of thioglycollate. After 4 h, mice were sacrificed, and the peritoneal cavity was lavaged with 6 ml of PBS containing 5 mm EDTA. The recovered cells were analyzed by flow cytometry. Neutrophils were counted on the basis of scatter properties and high expression of Ly6G.
Oxazolone-induced Contact Hypersensitivity
On day 0, mice were sensitized by topical application of 100 μl of 2% oxazolone (4-ethoxymethylene-2-oxazolin-5-one, Sigma-Aldrich) in acetone/olive oil (4:1) to shaved abdominal skin and of 5 μl of the same mixture to each paw. On day 7, mice were challenged by painting the right ear with 1% oxazolone (10 μl on each side). The left ear was painted with acetone/olive oil as a control. In some mice, mAbs (100 μg in 100 μl saline) to murine P-selectin (5H1) or murine E-selectin (9A9) were injected intravenously immediately before the challenge. Control mice were injected with saline. Ear thickness 24 h after challenge was measured with an electronic digital micrometer (Marathon Watch). Ear thickness was expressed as the absolute increase in micrometers and calculated as treated ear thickness − control ear thickness. For flow cytometry, ear tissue collected 24 h after challenge was chopped into small pieces and digested with a mixture containing type I and II collagenase, DNase, and RNase (Roche). After a 3-h digestion at room temperature, the lysate was passed through a 100-μm strainer and stained with anti-Ly6G and anti-F4/80 antibodies to identify neutrophils and monocyte/macrophages, respectively. To study gene expression, treated and control ears were removed 8 h after challenge, frozen in dry ice, and homogenized in lysis buffer (Qiagen) without thawing. Total RNA was purified using the RNeasy fibrous tissue mini kit (Qiagen). qRT-PCR was performed as described above.
Statistics
Statistical analysis was performed using Student's t test for unpaired samples. Results were considered significant at p < 0.05.
Results
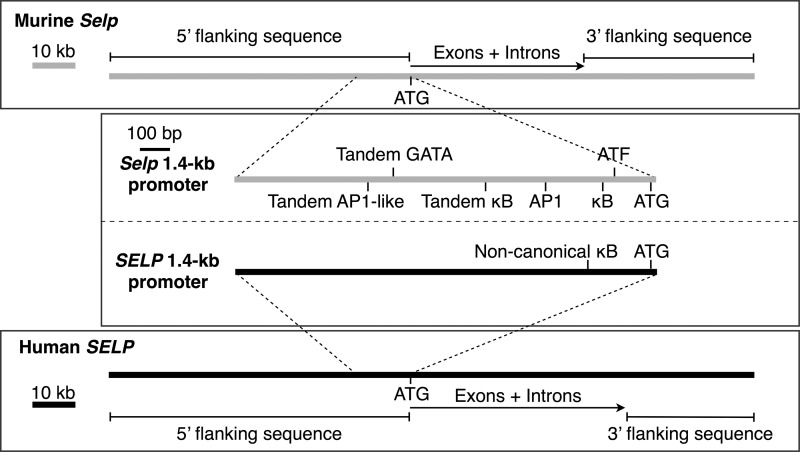
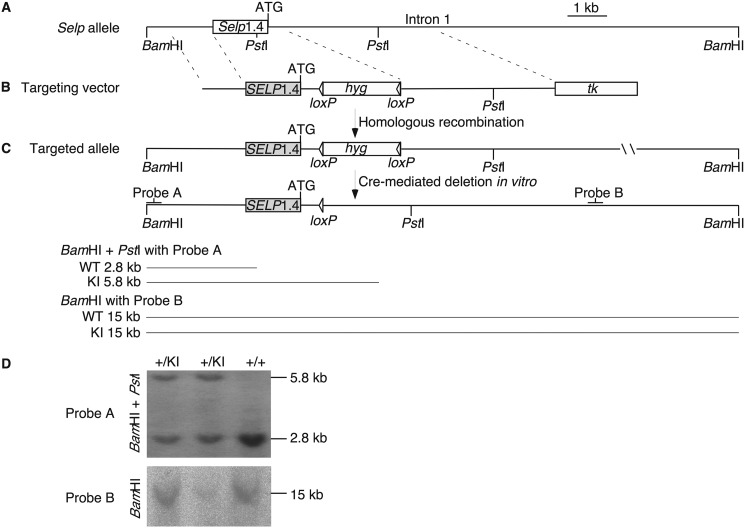
Human SELP and murine Selp have similar exon/intron organizations (28, 29) (Fig. 1). A SELP transgene encompassing all exons and introns plus 70 kb of 5′ flanking sequence and 29 kb of 3′ flanking sequence drives the basal and inducible expression of human P-selectin in mice as the native gene does in humans (17) (Fig. 1). In vitro, distinct elements in the proximal 1.4-kb promoters of SELP and Selp mediate species-specific differences in the basal and inducible expression of reporter genes (18–21) (Fig. 1). To determine whether divergence of these short promoters is sufficient to confer species-specific expression in vivo, we made knockin mice that replace the 1.4-kb promoter sequence of Selp with the corresponding sequence from SELP (Fig. 2, A–C). Southern blots of genomic DNA confirmed the correct integration of the targeted allele (Fig. 2D). Some mice homozygous for the knock in allele (SelpKI/KI) were bred with Selp−/− mice to generate heterozygous SelpKI/− mice. Both homozygous and heterozygous knockin mice were healthy with normal blood counts.4
FIGURE 1.
Schematics of the murine Selp and human SELP genes. Exon 1 of each gene ends in an ATG codon for the translation start site. Unique elements in the proximal 1.4-kb promoter sequence of each gene are shown. The lengths of the 5′ and 3′ flanking regions of SELP correspond to those incorporated in a SELP transgene that recapitulates basal and inducible expression of human P-selectin in mice (17).
FIGURE 2.
Strategy for generating the SelpKI allele. A, partial restriction map of the murine Selp allele. B, diagram of the targeting vector. C, diagram of the targeted (knockin) allele before and after Cre-mediated deletion of the hygromycin (hyg) selection cassette, the probes used for Southern blots, and the predicted sizes of the restriction fragments for the WT and knockin alleles. D, Southern blot of genomic DNA isolated from tails of WT (+/+) mice and of mice expressing one WT and one knockin allele (+/KI).
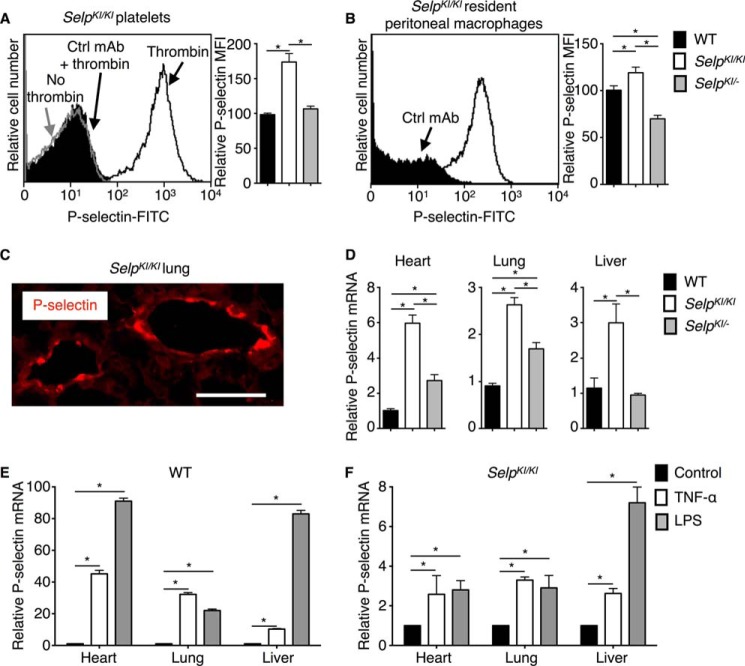
Anti-murine P-selectin mAb bound to thrombin-activated but not resting SelpKI/KI platelets, consistent with redistribution of P-selectin from α-granules to the plasma membrane (2, 3) (Fig. 3A). Activated SelpKI/KI platelets expressed ∼1.5-fold more P-selectin than activated WT or SelpKI/− platelets. This is consistent with ∼1.5-fold higher levels of human P-selectin on activated platelets from homozygous SELP transgenic mice than of murine P-selectin on activated platelets from WT mice (17). SelpKI/KI peritoneal macrophages also expressed more P-selectin (Fig. 3B). Immunofluorescence revealed staining for P-selectin in venular endothelial cells of the lung (Fig. 3C) and other organs.4 Basal P-selectin mRNA levels in the heart, lung, and liver were 2- to 6-fold higher in SelpKI/KI mice, with intermediate elevations in SelpKI/− mice (Fig. 3D). Therefore, SelpKI/KI mice express higher basal levels of P-selectin than WT mice. As noted previously (17), WT mice injected intravenously with TNF-α or LPS up-regulated P-selectin mRNA by 10- to 100-fold (Fig. 3E). SelpKI/KI mice only up-regulated P-selectin mRNA 2- to 7-fold (Fig. 3F), although the absolute level was greater because of higher basal mRNA expression. TNF-α or LPS decreases P-selectin mRNA in SELP transgenic mice (17). Therefore, substituting the SELP 1.4-kb promoter sequence eliminates most, but not all, of the responsiveness of Selp to TNF-α or LPS.
FIGURE 3.
Basal and inducible expression of P-selectin in SelpKI/KI and SelpKI/− mice. A, left panel, flow cytometric analysis of resting (no thrombin) or thrombin-activated platelets from SelpKI/KI mice stained with FITC-conjugated anti-murine P-selectin mAb or FITC-conjugated control mAb (Ctrl). Right panel, mean fluorescence intensity (MFI) for P-selectin on thrombin-activated platelets from WT, SelpKI/KI, or SelpKI/− mice (n = 5–10 mice/group). B, left panel, flow cytometric analysis of resident peritoneal macrophages from SelpKI/KI mice stained with FITC-conjugated anti-murine P-selectin mAb or FITC-conjugated control mAb. Right panel, MFI for P-selectin on resident peritoneal macrophages from WT, SelpKI/KI, or SelpKI/− mice (n = 5–10 mice/group). C, immunofluorescence of lungs from SelpKI/KI mice. Cryosections were incubated with goat anti-P-selectin polyclonal antibody followed by Alexa Fluor 555-conjugated donkey anti-goat antibody and observed under a fluorescence microscope. Red fluorescence indicates the distribution of P-selectin. Scale bar = 50 μm. D, total RNA was isolated from tissues of WT, SelpKI/KI, or SelpKI/− mice. P-selectin mRNA was quantified by real-time PCR. Expression was normalized to mRNA for GAPDH. -Fold changes were normalized to mRNA of WT tissues (n = 10–15 mice/group). E, quantification of P-selectin mRNA 3 h after intravenous injection of control albumin, TNF-α, or LPS in WT mice normalized to control-treated mice (n = 10–15 mice/group). F, quantification of P-selectin mRNA 3 h after intravenous injection of control albumin, TNF-α, or LPS in SelpKI/KI mice normalized to control-treated mice (n = 10–15 mice/group). Error bars are mean ± S.E. *, p < 0.05.
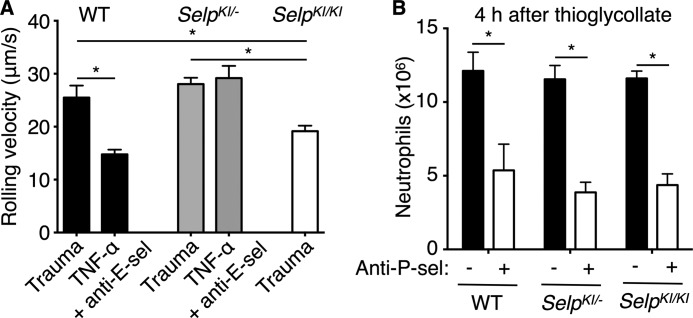
Trauma during surgical exposure of the cremaster muscle mobilizes P-selectin to the venular surface (17). Neutrophils rolled more slowly in trauma-stimulated venules of SelpKI/KI mice, consistent with higher basal expression of P-selectin, whereas neutrophils rolled with similar velocities in venules of SelpKI/− and WT mice (Fig. 4A). Intrascrotal injection of TNF-α increases synthesis of P- and E-selectin in WT mice (17, 30). We injected blocking anti-E-selectin mAb intravenously to isolate P-selectin-dependent rolling. Compared with velocities in trauma-stimulated venules, TNF-α reduced rolling velocities in WT mice, consistent with up-regulated synthesis of P-selectin. TNF-α did not alter velocities in SelpKI/− mice (Fig. 4A). Injecting blocking anti-P-selectin mAb eliminated rolling.4 These results support increased basal expression but dampened inducible expression of P-selectin in knockin mice.
FIGURE 4.
P-selectin-dependent neutrophil rolling and migration in SelpKI/KI and SelpKI/− mice. A, mean leukocyte rolling velocities in trauma-stimulated venules (n = 150–200 leukocytes from 15–20 venules/group) or in TNF-α-stimulated venules of mice injected with blocking anti-E-selectin mAb (anti-E-sel) (n = 320–330 leukocytes from 32–33 venules/group). B, WT, SelpKI/KI,or SelpKI/− mice were injected intraperitoneally with thioglycollate. Some mice were injected intravenously with blocking anti-P-selectin mAb (anti-P-sel) at the time of thioglycollate challenge. After 4 h, peritoneal cells were lavaged, and neutrophils were quantified by flow cytometry (n = 7–10 mice/group). *, p < 0.05.
Similar numbers of neutrophils migrated into the peritoneum of WT, SelpKI/−, and SelpSelpKI/KI mice 4 h after challenge with thioglycollate (Fig. 4B). Anti-P-selectin mAb reduced migration equivalently. Therefore, differences in basal or inducible expression of P-selectin did not alter neutrophil migration in this model, which may rely more on local macrophage release of chemokines like CXCL1 than of cytokines like TNF-α (31).
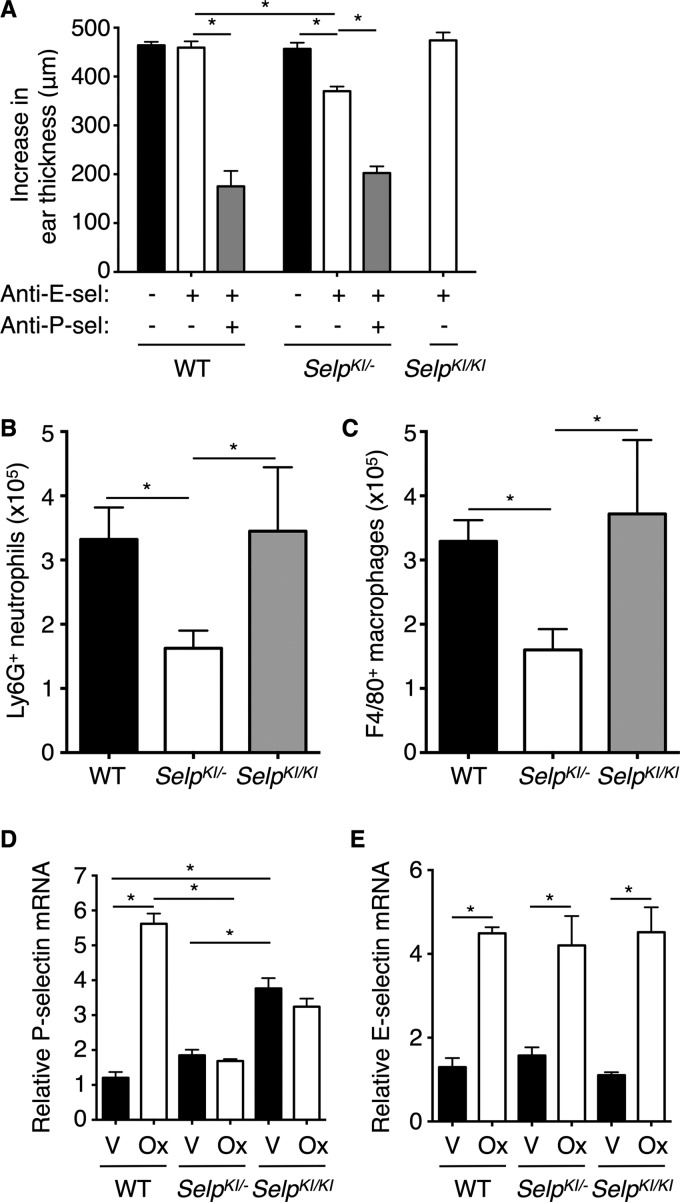
Both P- and E-selectin contribute to oxazolone-induced contact hypersensitivity in the ears of WT mice (17, 32–36). As observed previously (17), ear swelling in WT mice was reduced by injecting anti-P-selectin and anti-E-selectin mAbs but not by anti-E-selectin mAb alone (Fig. 5A). Anti-E-selectin mAb alone decreased swelling in SelpSelpKI/− mice but not in SelpKI/KI mice. Fewer Ly6G-positive neutrophils or F4/80-positive monocytes/macrophages entered the inflamed ears of SelpKI/− mice (Fig. 5, B and C). These data suggest that higher basal expression of P-selectin in SelpKI/KI mice compensates for its blunted inducible expression. In the ears of WT mice, mRNA for P- and E-selectin peaks 8 h after oxazolone challenge (17). At this time point, we confirmed higher basal expression of P-selectin mRNA in the vehicle-challenged ears of SelpKI/KI mice (Fig. 5D). Oxazolone-challenged ears up-regulated P-selectin mRNA only in WT mice (Fig. 5D), whereas they up-regulated E-selectin mRNA in all mice (Fig. 5E). In SELP transgenic mice, P-selectin contributes much less to contact hypersensitivity, in part because of lower basal expression in dermal venules (17), as also observed in human skin (37). Therefore, substituting the SELP 1.4-kb promoter sequence confers human-like basal expression of P-selectin in most tissues but less so in skin.
FIGURE 5.
P-selectin-dependent contact hypersensitivity in SelpKI/KI and SelpKI/− mice. A, WT, SelpKI/KI, or SelpKI/− mice were sensitized with 2% oxazolone on the abdomen and paws. After 7 days, the mice were challenged with 1% oxazolone on the right ear and with vehicle only on the left ear. Immediately before the challenge, some mice were injected intravenously with anti-E-selectin mAb (anti-E-sel), anti-P-selectin mAb (anti-P-sel), or both anti-E-selectin and anti-P-selectin mAbs. Control mice received no treatment or were injected with saline, which yielded identical results. The net increase in ear thickness was measured 24 h after challenge (n = 6–25 mice/group). B and C, after measuring ear thickness, oxazolone-challenged ears of anti-E-selectin treated groups were digested for 3 h. The numbers of Ly6G+ or F4/80+ cells were quantified by flow cytometry (n = 4–8 mice/group). D and E, ears of WT, SelpKI/KI, or SelpKI/− mice were homogenized 8 h after challenge with vehicle (V) or oxazolone (Ox). P- or E-selectin mRNA was quantified and normalized to vehicle-treated ears of WT mice (n = 4–10 mice/group). Error bars are mean ± S.E. *, p < 0.05.
Discussion
Remarkably, our results demonstrate that sequence variations limited to the 1.4-kb proximal promoter account for most of the differences in expression of the murine Selp and human SELP genes. Our data are derived from mice expressing a chimeric Selp that retains its entire genetic and epigenetic architecture except for the substituted proximal promoter from SELP. This approach permits comparative analysis of promoter function in vivo in a physiologically relevant environment. Previous studies of species-specific differences in gene expression used short promoter/enhancer sequences that drive reporter genes in transfected cells or in transgenic mice (38–44). Whether the identified regulatory elements function in the context of the native genes was not determined.
Our data for murine Selp and human SELP likely extend to the corresponding genes in other mammals. Genomic databases indicate that the canonical binding sites for NF-κB and ATF-2 in the murine Selp promoter are conserved in Selp promoters of other mammals but not in SELP promoters of primates. The loss of these sites in primate promoters (19) probably explains the blunted up-regulation of P-selectin mRNA by TNF-α and other mediators. Cis elements outside of the 1.4-kb region may enable these mediators to further down-regulate mRNA in primates and may influence basal expression of P-selectin in skin and other tissues. Our results provide mechanistic insights into the differences in Selp/SELP expression that must be considered when extrapolating data from animal models to humans. Why regulatory sequences have diverged in primate SELP genes is an interesting issue for further study.
Author Contributions
Z. L., N. Z., B. S., and S. R. P. performed the research. Z. L., N. Z., J. F., and R. P. M. analyzed the data. Z. L., N. Z., and R. P. M. designed the research. N. Z. and R. P. M. wrote the paper with final approval from all authors.
This work was supported by National Institutes of Health Grants HL034363 and HL085607. R. P. M. has interests in Selexys and Tetherex. The other authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Z. Liu, N. Zhang, B. Shao, S. R. Panicker, J. Fu, and R. P. McEver, unpublished data.
- HBSS
- Hanks' balanced salt solution
- qRT-PCR
- quantitative RT-PCR
- KI
- knockin.
References
- 1.Vestweber D., and Blanks J. E. (1999) Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 79, 181–213 [DOI] [PubMed] [Google Scholar]
- 2.McEver R. P., and Zhu C. (2010) Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 26, 363–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEver R. P. (2015) Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 107, 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schweitzer K. M., Dräger A. M., van der Valk P., Thijsen S. F., Zevenbergen A., Theijsmeijer A. P., van der Schoot C. E., and Langenhuijsen M. M. (1996) Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am. J. Pathol. 148, 165–175 [PMC free article] [PubMed] [Google Scholar]
- 5.Weninger W., Ulfman L. H., Cheng G., Souchkova N., Quackenbush E. J., Lowe J. B., and von Andrian U. H. (2000) Specialized contributions by α(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity 12, 665–676 [DOI] [PubMed] [Google Scholar]
- 6.Chong B. F., Murphy J. E., Kupper T. S., and Fuhlbrigge R. C. (2004) E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: a foundation for a cutaneous immunosurveillance system. J. Immunol. 172, 1575–1581 [DOI] [PubMed] [Google Scholar]
- 7.Collins T., Read M. A., Neish A. S., Whitley M. Z., Thanos D., and Maniatis T. (1995) Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J. 9, 899–909 [PubMed] [Google Scholar]
- 8.Tchernychev B., Furie B., and Furie B. C. (2003) Peritoneal macrophages express both P-selectin and PSGL-1. J. Cell Biol. 163, 1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders W. E., Wilson R. W., Ballantyne C. M., and Beaudet A. L. (1992) Molecular cloning and analysis of in vivo expression of murine P-selectin. Blood 80, 795–800 [PubMed] [Google Scholar]
- 10.Weller A., Isenmann S., and Vestweber D. (1992) Cloning of the mouse endothelial selectins: expression of both E- and P-selectin is inducible by tumor necrosis factor. J. Biol. Chem. 267, 15176–15183 [PubMed] [Google Scholar]
- 11.Auchampach J. A., Oliver M. G., Anderson D. C., and Manning A. M. (1994) Cloning, sequence comparison and in vivo expression of the gene encoding rat P-selectin. Gene 145, 251–255 [DOI] [PubMed] [Google Scholar]
- 12.Bischoff J., and Brasel C. (1995) Regulation of P-selectin by tumor necrosis factor-α. Biochem. Biophys. Res. Commun. 210, 174–180 [DOI] [PubMed] [Google Scholar]
- 13.Doré M., and Sirois J. (1996) Regulation of P-selectin expression by inflammatory mediators in canine jugular endothelial cells. Vet. Pathol. 33, 662–671 [DOI] [PubMed] [Google Scholar]
- 14.Burns S. A., DeGuzman B. J., Newburger J. W., Mayer J. E. Jr., Neufeld E. J., and Briscoe D. M. (1995) P-selectin expression in myocardium of children undergoing cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 110, 924–933 [DOI] [PubMed] [Google Scholar]
- 15.Yao L., Pan J., Setiadi H., Patel K. D., and McEver R. P. (1996) Interleukin 4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J. Exp. Med. 184, 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao L., Setiadi H., Xia L., Laszik Z., Taylor F. B., and McEver R. P. (1999) Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood 94, 3820–3828 [PubMed] [Google Scholar]
- 17.Liu Z., Miner J. J., Yago T., Yao L., Lupu F., Xia L., and McEver R. P. (2010) Differential regulation of human and murine P-selectin expression and function in vivo. J. Exp. Med. 207, 2975–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan J., Xia L., Yao L., and McEver R. P. (1998) Tumor necrosis factor-α- or lipopolysaccharide-induced expression of the murine P-selectin gene in endothelial cells involves novel kB sites and a variant ATF/CRE element. J. Biol. Chem. 273, 10068–10077 [DOI] [PubMed] [Google Scholar]
- 19.Pan J., Xia L., and McEver R. P. (1998) Comparison of promoters for the murine and human P-selectin genes suggests species-specific and conserved mechanisms for transcriptional regulation in endothelial cells. J. Biol. Chem. 273, 10058–10067 [DOI] [PubMed] [Google Scholar]
- 20.Pan J., and McEver R. P. (1993) Characterization of the promoter for the human P-selectin gene. J. Biol. Chem. 268, 22600–22608 [PubMed] [Google Scholar]
- 21.Pan J., and McEver R. P. (1995) Regulation of the human P-selectin promoter by Bcl-3 and specific homodimeric members of the NF-κB/Rel family. J. Biol. Chem. 270, 23077–23083 [DOI] [PubMed] [Google Scholar]
- 22.Bullard D. C., Qin L., Lorenzo I., Quinlin W. M., Doyle N. A., Bosse R., Vestweber D., Doerschuk C. M., and Beaudet A. L. (1995) P-selectin/ICAM-1 double mutant mice: acute emigration of neutrophils into the peritoneum is completely absent but is normal into pulmonary alveoli. J. Clin. Invest. 95, 1782–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia L., Sperandio M., Yago T., McDaniel J. M., Cummings R. D., Pearson-White S., Ley K., and McEver R. P. (2002) P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J. Clin. Invest. 109, 939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miner J. J., Xia L., Yago T., Kappelmayer J., Liu Z., Klopocki A. G., Shao B., McDaniel J. M., Setiadi H., Schmidtke D. W., and McEver R. P. (2008) Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood 112, 2035–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yago T., Petrich B. G., Zhang N., Liu Z., Shao B., Ginsberg M. H., and McEver R. P. (2015) Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J. Exp. Med. 212, 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkel E. J., and Ley K. (1996) Distinct phenotype of E-selectin-deficient mice: E-selectin is required for slow leukocyte rolling in vivo. Circ. Res. 79, 1196–1204 [DOI] [PubMed] [Google Scholar]
- 27.Ramos C. L., Kunkel E. J., Lawrence M. B., Jung U., Vestweber D., Bosse R., McIntyre K. W., Gillooly K. M., Norton C. R., Wolitzky B. A., and Ley K. (1997) Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood 89, 3009–3018 [PubMed] [Google Scholar]
- 28.Johnston G. I., Bliss G. A., Newman P. J., and McEver R. P. (1990) Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J. Biol. Chem. 265, 21381–21385 [PubMed] [Google Scholar]
- 29.Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., and Wagner D. D. (1993) Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 74, 541–554 [DOI] [PubMed] [Google Scholar]
- 30.Kunkel E. J., Jung U., and Ley K. (1997) TNF-α induces selectin-mediated leukocyte rolling in mouse cremaster muscle arterioles. Am. J. Physiol. Heart Circ. Physiol. 272, H1391–H1400 [DOI] [PubMed] [Google Scholar]
- 31.Cailhier J. F., Partolina M., Vuthoori S., Wu S., Ko K., Watson S., Savill J., Hughes J., and Lang R. A. (2005) Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J. Immunol. 174, 2336–2342 [DOI] [PubMed] [Google Scholar]
- 32.Staite N. D., Justen J. M., Sly L. M., Beaudet A. L., and Bullard D. C. (1996) Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood 88, 2973–2979 [PubMed] [Google Scholar]
- 33.Catalina M. D., Estess P., and Siegelman M. H. (1999) Selective requirements for leukocyte adhesion molecules in models of acute and chronic inflammation: participation of E- and P- but not L-selectin. Blood 93, 580–589 [PubMed] [Google Scholar]
- 34.Hwang J. M., Yamanouchi J., Santamaria P., and Kubes P. (2004) A critical temporal window for selectin-dependent CD4+ lymphocyte homing and initiation of late-phase inflammation in contact sensitivity. J. Exp. Med. 199, 1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Leary J. G., Goodarzi M., Drayton D. L., and von Andrian U. H. (2006) T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 [DOI] [PubMed] [Google Scholar]
- 36.Labow M. A., Norton C. R., Rumberger J. M., Lombard-Gillooly K. M., Shuster D. J., Hubbard J., Bertko R., Knaack P. A., Terry R. W., and Harbison M. L. (1994) Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity 1, 709–720 [DOI] [PubMed] [Google Scholar]
- 37.Silber A., Newman W., Reimann K. A., Hendricks E., Walsh D., and Ringler D. J. (1994) Kinetic expression of endothelial adhesion molecules and relationship to leukocyte recruitment in two cutaneous models of inflammation. Lab. Invest. 70, 163–175 [PubMed] [Google Scholar]
- 38.Rose S. D., and MacDonald R. J. (1997) Evolutionary silencing of the human elastase I gene (ELA1). Hum. Mol. Genet. 6, 897–903 [DOI] [PubMed] [Google Scholar]
- 39.Thomas G. P., Bourne A., Eisman J. A., and Gardiner E. M. (2000) Species-divergent regulation of human and mouse osteocalcin genes by calciotropic hormones. Exp. Cell Res. 258, 395–402 [DOI] [PubMed] [Google Scholar]
- 40.Spitsin S. V., Koprowski H., and Michaels F. H. (1996) Characterization and functional analysis of the human inducible nitric oxide synthase gene promoter. Mol. Med. 2, 226–235 [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X., Laubach V. E., Alley E. W., Edwards K. A., Sherman P. A., Russell S. W., and Murphy W. J. (1996) Transcriptional basis for hyporesponsiveness of the human inducible nitric oxide synthase gene to lipopolysaccharide/interferon-γ. J. Leukocyte Biol. 59, 575–585 [DOI] [PubMed] [Google Scholar]
- 42.Chu S. C., Marks-Konczalik J., Wu H. P., Banks T. C., and Moss J. (1998) Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters. Biochem. Biophys. Res. Commun. 248, 871–878 [DOI] [PubMed] [Google Scholar]
- 43.Zhang N., Weber A., Li B., Lyons R., Contag P. R., Purchio A. F., and West D. B. (2003) An inducible nitric oxide synthase-luciferase reporter system for in vivo testing of anti-inflammatory compounds in transgenic mice. J. Immunol. 170, 6307–6319 [DOI] [PubMed] [Google Scholar]
- 44.Yu Z., Xia X., and Kone B. C. (2005) Expression profile of a human inducible nitric oxide synthase promoter reporter in transgenic mice during endotoxemia. Am. J. Physiol. Renal Physiol. 288, F214–220 [DOI] [PubMed] [Google Scholar]