Abstract
Muscle weakness and myopathy are observed in vitamin D deficiency and chronic renal failure, where concentrations of the active vitamin D3 metabolite, 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), are low. To evaluate the mechanism of action of 1α,25(OH)2D3 in skeletal muscle, we examined mitochondrial oxygen consumption, dynamics, and biogenesis and changes in expression of nuclear genes encoding mitochondrial proteins in human skeletal muscle cells following treatment with 1α,25(OH)2D3. The mitochondrial oxygen consumption rate (OCR) increased in 1α,25(OH)2D3-treated cells. Vitamin D3 metabolites lacking a 1α-hydroxyl group (vitamin D3, 25-hydroxyvitamin D3, and 24R,25-dihydroxyvitamin D3) decreased or failed to increase OCR. 1α-Hydroxyvitamin D3 did not increase OCR. In 1α,25(OH)2D3-treated cells, mitochondrial volume and branching and expression of the pro-fusion protein OPA1 (optic atrophy 1) increased, whereas expression of the pro-fission proteins Fis1 (fission 1) and Drp1 (dynamin 1-like) decreased. Phosphorylated pyruvate dehydrogenase (PDH) (Ser-293) and PDH kinase 4 (PDK4) decreased in 1α,25(OH)2D3-treated cells. There was a trend to increased PDH activity in 1α,25(OH)2D3-treated cells (p = 0.09). 83 nuclear mRNAs encoding mitochondrial proteins were changed following 1α,25(OH)2D3 treatment; notably, PDK4 mRNA decreased, and PDP2 mRNA increased. MYC, MAPK13, and EPAS1 mRNAs, which encode proteins that regulate mitochondrial biogenesis, were increased following 1α,25(OH)2D3 treatment. Vitamin D receptor-dependent changes in the expression of 1947 mRNAs encoding proteins involved in muscle contraction, focal adhesion, integrin, JAK/STAT, MAPK, growth factor, and p53 signaling pathways were observed following 1α,25(OH)2D3 treatment. Five micro-RNAs were induced or repressed by 1α,25(OH)2D3. 1α,25(OH)2D3 regulates mitochondrial function, dynamics, and enzyme function, which are likely to influence muscle strength.
Keywords: cell signaling; gene expression; mitochondria; skeletal muscle; vitamin D; 1,25-dihydroxyvitamin D3; RNA-seq; WTSS; oxygen consumption
Introduction
Vitamin D3, through the activity of its active metabolite, 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3),2 is essential for normal calcium and phosphorus balance and the maintenance of skeletal health (1–4). Vitamin D3 and its metabolites also have important clinical effects on muscle function, the biochemical basis of which is not well understood. Skeletal muscle weakness and myopathy are prominent in humans with vitamin D deficiency, who rapidly respond to treatment with vitamin D3 (5, 6). Sarcopenia is common in chronic and end stage kidney disease (7, 8), where concentrations of 1α,25(OH)2D3 are low. The administration of vitamin D3 has been reported to improve muscle strength in humans (9). Reduced serum 1α,25(OH)2D3 concentrations have been linked to falls in humans (10, 11), and clinical trials have demonstrated salutary effects of αcalcidol on fall prevention (12). Improvement in muscle fiber size has been documented in elderly subjects treated with 1α,25(OH)2D3 (13).
Previous reports have shown that in avian and rodent isolated skeletal muscle cells and cultured myoblast cell lines, vitamin D3 metabolites, such as 25-hydroxyvitamin D3 (25(OH)D3) and 1α,25(OH)2D3, influence cellular calcium and phosphorus uptake, cellular growth, differentiation, and the expression of a limited number of genes (14–19). Many reports suggest that the vitamin D receptor (VDR) is expressed in skeletal muscle (20–24), and VDR deletion in mice results in alterations in muscle function and strength (25, 26). Treatment of vitamin D-deficient humans with cholecalciferol improves muscle phosphocreatine recovery after exercise (27), suggesting that vitamin D3 or its metabolites alter skeletal muscle oxidative capacity.
To assess the mechanism of action of the active metabolite of vitamin D3, 1α,25(OH)2D3, in human skeletal muscle cells, we examined changes in mitochondrial oxygen consumption (OCR), mitochondrial dynamics, mitochondrial OXPHOS proteins, pyruvate dehydrogenase phosphorylation, and nuclear gene expression using whole transcriptome shotgun sequencing (WTSS, RNA-seq) of messenger RNAs and micro-RNAs following treatment of cells with 1α,25(OH)2D3. The dependence of the observed changes upon the presence of the VDR was assessed. Our results show extensive 1α,25(OH)2D3-mediated changes in skeletal muscle mitochondrial OCR, dynamics, pyruvate dehydrogenase phosphorylation, and expression of nuclear genes encoding mitochondrial proteins. These changes suggest important effects on skeletal muscle that are likely to alter skeletal muscle performance.
Experimental Procedures
General
All human studies were by approved by the institutional review board of the Mayo Clinic. 25-Hydroxyvitamin D3, 24(R),25-dihydroxyvitamin D3, 1α-hydroxyvitamin D3, and lα,25-dihydroxyvitamin D3 were gifts from Dr. Milan Uskokovic (Hoffman-La Roche, Nutley, NJ).
Muscle Biopsies and Mitochondrial Isolation
Muscle biopsies were performed, and mitochondria were isolated as described previously (28, 29).
Cell Culture
Primary human skeletal muscle cells (hSkMCs)were purchased from Lonza (CC-2561) and grown at 37 °C in 5% CO2 in SkM medium (CC-3161). Cells were subcultured at a seeding density of 3500 cells/cm2, using Lonza reagents (CC-5034).
Measurement of Oxygen Uptake by Cells
An XF24 extracellular flux analyzer (Seahorse Biosciences) was utilized to measure OCR and glycolytic activity (30). Cells were seeded in a microplate at a density of 3500 cells/well. At ∼80% confluence, cells were treated with vitamin D analogs (final concentration, 10−8 m) or ethanol, added 48 h, 24 h, and immediately prior to OCR measurements. OCR was measured following the sequential addition of oligomycin (0.5 μg/ml), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (1 μm), and rotenone (0.5 μm)/antimycin A (1 μm). Baseline respiration rate (basal OCR − rotenone/antimycin A OCR), coupled respiration rate (basal respiration rate − oligomycin OCR), maximal respiration rate (carbonyl cyanide p-trifluoromethoxyphenylhydrazone OCR − rotenone/antimycin A OCR), and oxidative reserve (carbonyl cyanide p-trifluoromethoxyphenylhydrazone OCR − basal respiration rate) were calculated (31). Oxygen uptake in isolated mitochondria was determined using an Oroboros Oxygraph (Innsbruck, Austria) as described previously (28).
Immunohistochemistry/Microscopy
Skeletal muscle cells were grown on collagen-treated glass plates (MatTek Corp.) and were fixed and treated with VDR antibody as described earlier (32). Skeletal muscle sections (American MasterTech Scientific) were stained with VDR antibody or preadsorbed VDR antibody, as described (32).
Construction of VDR pEGFP Expression Vectors and Transfection into Human Skeletal Muscle Cells
Human VDR cDNA was amplified by PCR with primers with a KpnI site in the 5′ primer and BamHI site in the 3′ primer. The product was ligated into pEGFP-C1 or pEGFP-N1 (Clontech). HSkMCs were transfected using Lipofectamine 2000 (Life Technologies).
Knockdown of VDR with Silencing RNA
VDR in skeletal muscle cells was knocked down using two Silencer® Select siRNAs to the human VDR (siRNA ID s14777 and s14779, Life Technologies). Silencer® Select negative control 1 nonspecific siRNA was used in matching cells in equimolar amounts. Lipofectamine (Life Technologies) siRNA complex in Opti-MEM medium was used for transfection. RNA was isolated as detailed below and reverse-transcribed using Superscript III and an oligo(dT) or random hexamer primer, and percentage knockdown of the VDR RNA was determined following quantitative PCR relative to the RPL13A reference gene.
Assessment of Mitochondrial Volume and Fragmentation
To observe dynamic changes in mitochondrial structure, cultured hSkMCs (treated with either vehicle or 1α,25(OH)2D3 for 48 h) were plated on 8-well glass bottom plates (LabTek) and incubated with 100 nm MitoTracker Deep Red (Life Technologies; excitation 638 nm/emission 700 nm) for 15 min at room temperature. Following thorough rinsing with dye-free solution, cells were imaged using a Nikon A1R confocal system (Nikon Instruments Inc.). Images were acquired at 2048 × 2048 pixels and 12-bit depth using a Plan-Apo ×60/1.4 numerical aperture oil objective. Images were corrected for background intensity variations using ImageJ software (National Institutes of Health).
For mitochondrial volume measurement, we performed optical sectioning with a 0.5-μm step. For each cell, mitochondria volume was determined using Nikon Elements software.
Mitochondrial morphometric analysis was performed using procedures developed by Koopman et al. (33–35). To assess the extent of filamentous versus fragmented morphometry, the form factor (an index of mitochondrial branching) and aspect ratio (an index of mitochondrial branch length) were calculated for each cell using a custom-written MATLAB (The MathWorks)-based program (36, 37). A decrease in aspect ratio and/or form factor indicates mitochondrial fragmentation (33–37).
RNA Preparation for RNA-seq, miRNA-seq, and qPCR
RNA was prepared using RNA/protein spin columns (Clontech).
Digital PCR and Quantitative PCR
Human mRNAs (VDR, CYP24A1, MSTN, PDK4) were quantitated by real-time digital PCR with the QuantStudio® three-dimensional digital PCR system using FAM-labeled TaqMan assays. RPL13A was used as a reference gene. Data were analyzed on-line using QuantStudio® 3D AnalysisSuiteTM Cloud Software. qPCR for determination of VDR following siRNA knockdown was carried out using a Roche LightCycler 480 qPCR apparatus (Roche Applied Science) with SYBR Green master mix, Universal RT mix (Roche Applied Science), and an intron-spanning qPCR primer pair for the VDR (Roche Applied Science).
Assessment of Mitochondrial Protein Expression Using Western Blotting with Specific Antibodies
We assessed changes in mitochondrial protein expression using antibodies or antibody mixtures (all from Abcam unless otherwise noted) directed against the following mitochondrial proteins or complexes: VDAC1 or porin (ab15895), pyruvate dehydrogenase Western blot antibody mixture (ab110416), pyruvate dehydrogenase E1-α subunit (phosphorylation at position 293) (ab177461), pyruvate dehydrogenase phosphatase 2 (ab133982), pyruvate dehydrogenase kinase 4 (ab71240), total OXPHOS human Western blot mixture (ab110411), mitofusin 1 (ab57602), mitofusin 2 (ab56889), OPA1 (ab42364), Drp-1 (ab56788), and Fis1 (sc-98900, Santa Cruz Biotechnology, Inc.). Human skeletal muscle cells were plated in T175 flasks and treated with vehicle or 1α,25(OH)2D3 (10−8 m) for 48 h. Homogenates of cells were prepared in extraction buffer (ab193970). Cellular protein was quantitated. 15 μg of cellular protein was treated with SDS-loading buffer containing 20 mm dithiothreitol and loaded on 10% bis-tris polyacrylamide gels. Proteins were separated by electrophoresis and transferred onto PVDF membranes. The membranes were probed with the appropriate primary antibodies at concentrations recommended by the manufacturer. Peroxidase-labeled secondary antibodies were used to generate a chemiluminescent signal that was detected on x-ray film. The intensity of the bands was quantitated using ImageJ software. Equivalence of mitochondrial protein loading was assured by assessing porin intensity.
Measurement of Pyruvate Dehydrogenase in Cell Homogenates
hSkMCs were treated with vehicle (n = 5) or 1α,25(OH)2D3, 10−8 m (n = 6), for 48 h. Pyruvate dehydrogenase (PDH) activity was measured in 96-well plates with a pyruvate dehydrogenase activity colorimetric assay (BioVision, Milpitas, CA). The increase in absorbance at 450 nm with time was measured. Values for PDH activity were obtained using a standard curve of increasing NADH concentrations.
Assessment of Mitochondrial and Nuclear DNA
Total DNA was prepared from hSkMCs grown in 6-well plates and treated with vehicle (ethanol, n = 9) or 10−8 m 1α,25(OH)2D3 (n = 9) for 48 h. Confluent cells were lifted using 0.25% trypsin-EDTA, pelleted at 300 × g in a microcentrifuge, and then resuspended in 200 μl of Dulbecco's phosphate-buffered saline. DNA was prepared using QIAamp DNA Mini Kit spin columns. DNA was treated with RNase, and purified DNA was eluted from columns into 200 μl of water. Purified DNA was used to measure the human mitochondrial genes ND1 and ND6 and nuclear genes BECN1 and NEB using a NovaQUANTTM human mitochondrial to nuclear DNA ratio kit (EMD Millipore). Quantitative PCR was performed on 2 ng of DNA using specific PCR primers and a Roche LightCycler 480 qPCR apparatus with SYBR Green master I mix (Roche Applied Science). The ratios of mitochondrial genes ND1 and ND6 to nuclear genes BECN1 and NEB in cells treated with 10−8 m 1α,25(OH)2D3 or vehicle (ethanol) were determined using crossing points and a standard curve generated with 0.02–20 ng of human DNA.
Preparation of Libraries
mRNA-seq libraries were prepared as described previously (38). RNA libraries were prepared with a TruSeq RNA Sample Prep Kit version 2 (Illumina). Reverse transcription and adaptor ligation steps were performed manually. Poly(A) mRNA was purified from total RNA using oligo(dT) magnetic beads. RNA was treated with RNase-free DNase during preparation of RNAs using the Nucleospin RNA/Protein kit (Clontech). Purified mRNA was fragmented at 95 °C for 8 min, eluted from the beads, and primed for first strand cDNA synthesis. The RNA fragments were then copied into first strand cDNA using SuperScript III reverse transcriptase and random primers (Invitrogen). Second strand cDNA synthesis was performed using DNA polymerase I and RNase H. The double-stranded cDNA was purified using a single AMPure XP bead (Agencourt) clean-up step. The cDNA ends were repaired and phosphorylated using Klenow, T4 polymerase, and T4 polynucleotide kinase followed by AMPure XP bead clean-up. The blunt-ended cDNAs were modified to include a single 3′-adenylate (A) residue using Klenow exo− (3′–5′ exo minus). Paired end DNA adaptors (Illumina) with a single “T” base overhang at the 3′-end were immediately ligated to the “A-tailed” cDNA population. Unique indexes, included in the standard TruSeq Kits (12-Set A and 12-Set B) were incorporated at the adaptor ligation step for multiplex sample loading on the flow cells. The adaptor-modified DNA fragments were enriched by 12 cycles of PCR using primers included in the Illumina Sample Prep Kit. The concentration and size distribution of the libraries were determined on an Agilent Bioanalyzer DNA 1000 chip (Santa Clara, CA).
mRNA libraries were loaded onto Illumina TruSeq version 3 paired end flow cells at concentrations of 9 pm to generate cluster densities of 600,000–800,000/mm2 following Illumina's standard protocol using the Illumina cBot and TruSeq Rapid Paired End Cluster Kit version 3. The flow cells were sequenced as 51 × 2 paired end reads on an Illumina HiSeq 2000 using the TruSeq SBS Sequencing Kit version 3 and HiSeq data collection version 2.0.12.0 software. Base-calling was performed using Illumina's RTA version 1.17.21.3.
miRNA-seq libraries from total RNA samples were synthesized with a NEBNext® multiplex small RNA kit (New England Biolabs) as described previously (39). Adaptors were ligated to the 3′-ends of the small non-coding RNAs present in 500 ng of total RNA. A complementary primer was annealed to the 3′-adaptor sequences, followed by ligation of a 5′ RNA adaptor. A cDNA library was created by reverse transcriptase (Superscript III, Invitrogen) treatment of adaptor-ligated and annealed small RNAs. The library was enriched by 15 cycles of PCR employing a common 5′ primer and a 3′ primer containing one of eight index primers (3′-adaptor complement; index sequences equivalent to Illumina TruSeq Small RNA sequences). The PCR products were purified. The libraries were assessed for miRNA products by Agilent Bioanalyzer DNA 1000 (Santa Clara, CA) analysis. The 130–160-bp region was quantitated to determine equimolar amounts of vehicle and 1α,25(OH)2D3-treated sample libraries to pool. The pooled small RNA libraries were fractionated to extract an miRNA-enriched sample via 3% Pippin Prep (Sage Science) gel cassettes. Pooled miRNA fractions were assessed by a second Agilent DNA 1000 assay. A predominant peak at 140–150 bp indicated that the miRNA modification and size selection steps were performed as expected. Libraries were loaded onto single end flow cells at concentrations of 8–10 pm to generate cluster densities of 700,000/mm2 following Illumina's standard protocol using the Illumina cBot cluster kit version 3. The flow cells were sequenced as two reads: 51 cycles using the small RNA sequencing primer (read 1) and an index read to demultiplex the samples. Libraries were sequenced on an Illumina HiSeq 2000 using TruSeq SBS sequencing kit version 3 and SCS version 1.4.8 data collection software. Base calling was performed using Illumina's RTA version 1.12.4.2.
mRNA-seq Data Analysis
mRNA-seq data were processed by the Mayo Bioinformatics Core Facility to identify genes with differential expressions between vehicle- and 1α,25(OH)2D3-treated groups. The processing of the mRNA data was performed using MAP-RSeq (40) workflow (version 1.2.1.3). MAP-RSeq consists of the following steps: alignment, quality control, obtaining genomic features per sample, and finally summarizing the data across samples. The pipeline provides detailed quality control data across genes using the RSeQC (41) software (version 2.3.2). Paired end reads are aligned by TopHat (42) (version 2.0.6) against the hg19 genome build using the bowtie1aligner (43). Gene counts were generated using HTSeq (44) software (version 0.5.3p9), and the gene annotation files were obtained from Illumina. Differential expression analysis was performed with edgeR version 2.6.2 (45) to identify genes with altered expression by 1α,25(OH)2D3 treatment. A cut-off for false discovery rate-adjusted p value was set at 0.01 to determine the genes with significant expression change between conditions.
miRNA-seq Data Analysis
miRNA-seq data were processed with a microRNA deep sequencing analysis workflow called CAP-miRSeq version 1.1 (46). This workflow provides comprehensive analysis of miRNA sequencing data, including read preprocessing, alignment, mature/precursor/novel miRNA quantification, and variant detection in the miRNA coding region. In particular, CAP-miRSeq utilizes miRDeep2 (47) to classify and quantify the expressions of known and novel miRNAs, based on the annotations from miRBase version 19 (48). Normalization and differential analysis were performed with edgeR version 2.6.2 (45) to identify known miRNAs with altered expression by 1α,25(OH)2D3 treatment.
miRNA Target Identification
A two-step approach was used to identify potential target genes for the miRNAs differentially expressed between 1α,25(OH)2D3 treatment and control. First, predicted targets were collected from TargetScan version 6.2 (49) for each miRNA. Second, the correlations between differentially expressed miRNAs and mRNAs were evaluated with the Spearman correlation coefficient and its associated statistical significance. The miRNA-mRNA pairs with statistically significant correlation (p ≤ 0.05) were used to identify target genes. Finally, the common target genes identified by both steps were treated as high confidence targets for each miRNA.
Pathway Analysis
DAVID (version 6.7) (50, 51) was used to perform pathway enrichment analysis for differentially expressed genes or miRNA-targeted genes. DAVID provides pathways from databases, including the Kyoto Encyclopedia of Genes and Genomes (52–54), Panther (55), BioCarta, and Reactome (56). A cut-off p value was set at 0.01 to determine the significantly enriched pathways. The results were further confirmed with Ingenuity Pathway Analysis (Ingenuity Systems).
Analysis of Genes That Encode Mitochondrial Proteins
We specifically examined the differential expression of genes that encode mitochondrial proteins. Mitochondrial proteins were identified based on a compendium from MitoCarta (57). The collection of 1158 nuclear and mitochondrial DNA genes encoding proteins with strong support of mitochondrial localization was searched for differentially expressed genes from 1α,25(OH)2D3- or vehicle-treated cells.
Statistical Methods
Statistical differences between samples were analyzed using Student's two-tailed t test, assuming equal variance. A p value of <0.05 was regarded as statistically significant.
Data Sharing
All of the sequencing data that were analyzed in this report have been deposited in the Gene Expression Omnibus (GSE70934).
Results
The Vitamin D Receptor Is Expressed in Human Muscle Cells and Skeletal Muscle Homogenates
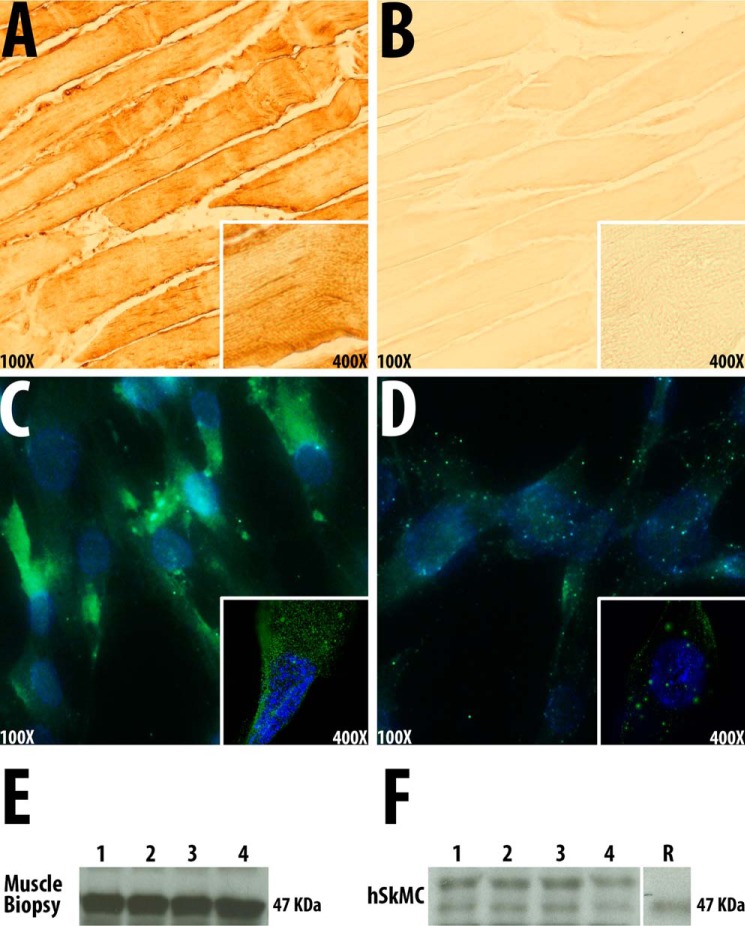
We confirmed the presence of the VDR in human muscle tissue and hSkMCs by immunohistochemistry. VDR was detected in cellular cytoplasm but not in mitochondria (Fig. 1, A and C). Immunostaining was reduced when VDR preadsorbed antibody was used (Figs. 1, B and D). Western blot analysis with a VDR antibody demonstrated bands of the appropriate mobility (Mr ∼48,000) that co-migrated with recombinant VDR in homogenates of human skeletal muscle biopsies and hSkMC (Fig. 1, E and F). The absence of localization of the VDR in mitochondria was corroborated in cells transfected with a VDR-eGFP expression plasmid, where no mitochondrial localization of fluorescence was noted. We confirmed the presence of the VDR mRNA in muscle cells by isolating mRNA, synthesizing cognate cDNA, and sequencing the VDR mRNA.
FIGURE 1.
The vitamin D receptor is expressed in human muscle and muscle cells. A, localization of VDR in sections of normal human skeletal muscle using a polyclonal VDR antiserum (magnification, ×100; inset magnification, ×400). B, localization of VDR in sections of normal human skeletal muscle using a polyclonal VDR antiserum, preadsorbed with VDR (magnification, ×100; inset magnification, ×400). C, localization of VDR in primary cultures of human skeletal muscle cells using a polyclonal VDR antiserum (magnification, ×100; inset magnification, ×630). D, localization of VDR in primary cultures of human skeletal muscle cells using a polyclonal VDR antiserum preadsorbed with VDR (magnification, ×100; inset magnification, ×630). E, Western blot of homogenates of normal human skeletal muscle tissue obtained by biopsy using a polyclonal VDR antibody. F, Western blot of homogenates of primary cultures of human skeletal muscle cells using a polyclonal VDR antibody. R, recombinant VDR.
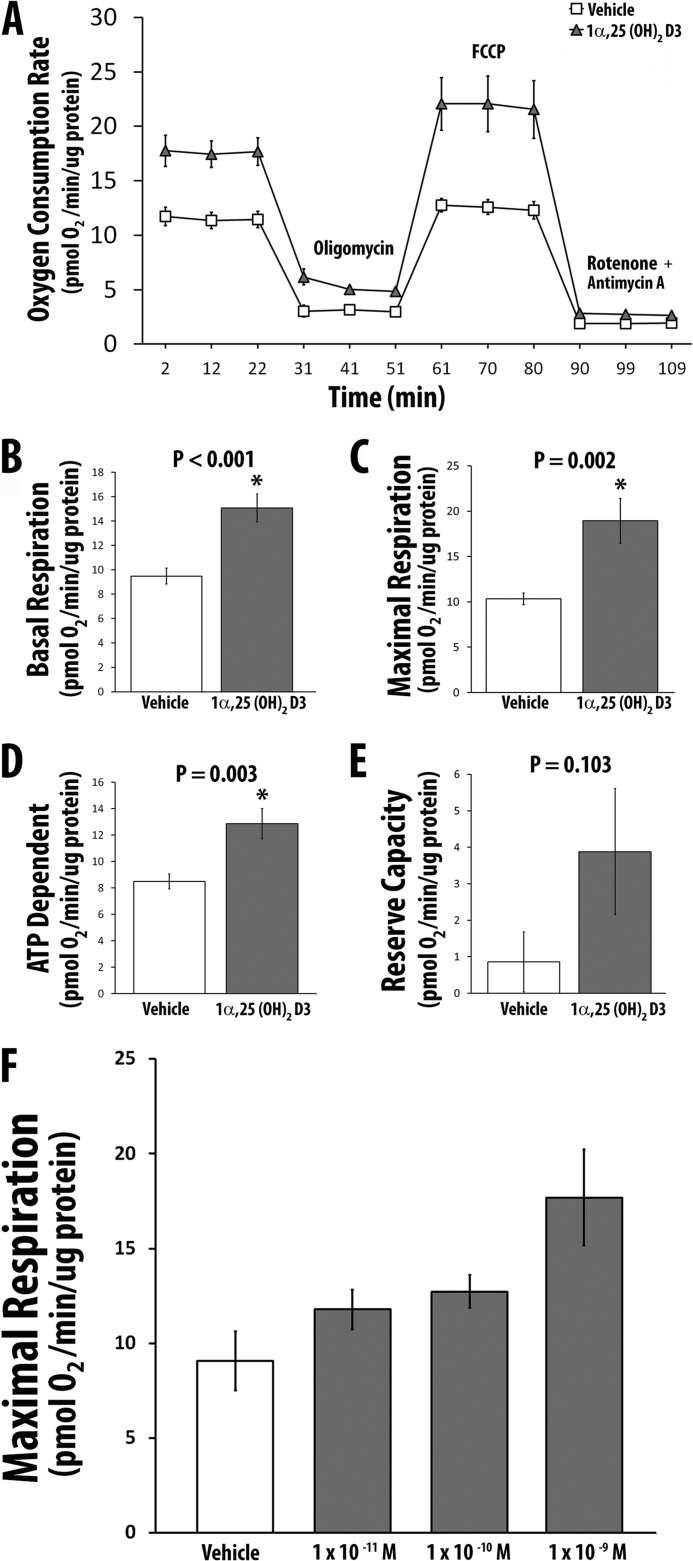
1α,25(OH)2D3 Increases Mitochondrial Oxygen Consumption in Adult Human Skeletal Muscle Cells
We measured cellular/mitochondrial OCR following treatment of hSkMCs with 10−8 m 1α,25(OH)2D3 or vehicle for 48 h. 1α,25(OH)2D3 increased basal (standard), coupled (associated with the generation of ATP), and maximal (uncoupled respiration) OCR compared with vehicle (Fig. 2, A–D). Non-mitochondrial OCR was not changed (Fig. 2E). There was a dose-dependent increase in mitochondrial OCR following the addition of increasing amounts of 1α,25(OH)2D3 to cultures of skeletal muscle cells (Fig. 2F). Simultaneous measurement of OCR and glycolytic activity showed no change in the rate of glycolysis (1α,25(OH)2D3-treated cells = 0.943 ± 0.13 milli-pH units/min/μg protein versus vehicle-treated cells = 0.763 ± 0.06 milli-pH units/min/μg protein, p = 0.213).
FIGURE 2.
1α,25(OH)2D3 increases cellular OCR in primary cultures of the human skeletal muscle cells. A, OCR in the presence of 1α,25(OH)2D3 (n = 5) or vehicle (n = 8) and various inhibitors. B, effect of 1α,25(OH)2D3 or vehicle on basal respiration. C, effect of 1α,25(OH)2D3 or vehicle on maximal respiration. D, effect of 1α,25(OH)2D3 or vehicle on coupled respiration. E, effect of 1α,25(OH)2D3 or vehicle on reserve capacity. F, effect of increasing concentrations of 1α,25(OH)2D3 on maximal respiration (vehicle (n = 3), 1 × 10−11 m (n = 4), 1 × 10−10 m (n = 4), 1 × 10−9 m (n = 3). p values (compared with vehicle) for each concentration of 1α,25(OH)2D3 are as follows: 1 × 10−11 m, p = 0.19, 1 × 10−10 m, p = 0.08, 1 × 10−9 m, p = 0.04). All values in A–F are expressed as mean ± S.E. (error bars).
The Increase in Mitochondrial Oxygen Consumption Rate by 1α,25(OH)2D3 Is VDR-dependent
We treated hSkMCs with either a VDR-specific antisense silencing RNA (siRNA) or a scrambled/control siRNA. Following treatment of cells with VDR siRNA, cellular VDR mRNA decreased by >80%; treatment of cells with control siRNA did change VDR mRNA. Human skeletal muscle cells with reduced VDR expression had decreased basal OCR (p = 0.002), maximal respiration (p = 0.003), coupled OCR (p < 0.001), and respiratory reserve OCR (p = 0.02) following 1α,25(OH)2D3 treatment compared with cells with normal VDR expression. The direct addition of 1α,25(OH)2D3 to isolated mitochondria failed to increase OCR, suggesting that the effects of 1α,25(OH)2D3 on OCR are dependent on extramitochondrial biochemical events.
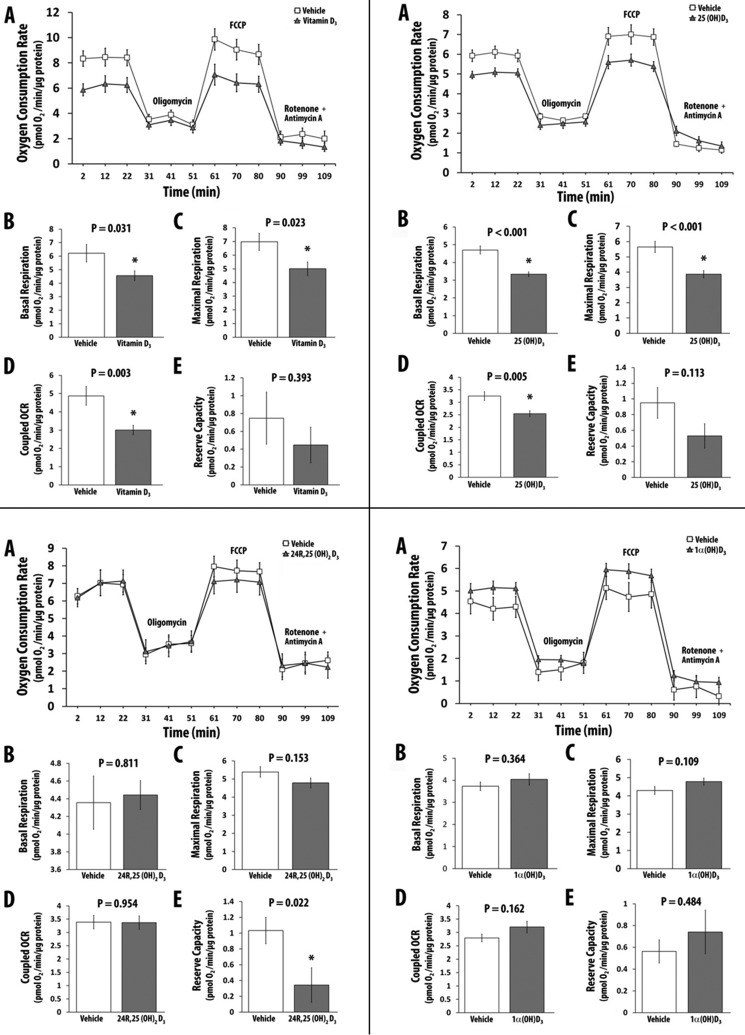
C-1 and C-25 Hydroxyl Groups Are Required for Vitamin D3 Analogs to Increase OCR
Both C-1 and C-25 hydroxyl groups are required for optimal binding of vitamin D3 analogs to the VDR (58). To determine the influence of C-1 or C-25 hydroxyl groups or both in vitamin D analogs or metabolites on OCR, we treated hSkMCs with 10−8 m vitamin D3, 25(OH)D3, 24R,25(OH)2D3, or 1α(OH)D3 for 48 h, following which OCR was determined. Treatment of cells with vitamin D3 (Fig. 3, top left) and 25(OH)D3 (Fig. 3, top right) decreased maximal OCR; treatment with 24R,25(OH)2D3 did not change maximal OCR (Fig. 3, bottom left), and treatment with 1α(OH)D3, a 1α-hydroxylated vitamin D analog, showed a small but statistically insignificant change in OCR (Fig. 3, bottom right). The data demonstrate that only 1α,25(OH)2D3 that has both C-1 and C-25 hydroxyl groups and that binds to the VDR with high affinity has a significant effect on OCR in skeletal muscle cells.
FIGURE 3.
The effect of various vitamin D metabolites/analogs on cellular OCR in primary cultures of the human skeletal muscle cells. A, OCR in the presence or absence of vitamin D metabolite and various inhibitors. B, effect of vitamin D metabolite or vehicle on basal respiration. C, effect of vitamin D metabolite or vehicle on maximal respiration. D, effect of vitamin D metabolite or vehicle on coupled respiration. E, effect of vitamin D metabolite or vehicle on reserve capacity. Top left, vitamin D3; top right, 25(OH)D3; bottom left, 24R,25(OH)2D3; bottom right, 1α(OH)D3. All values in A–E are expressed as mean ± S.E. (error bars). All replicates had a minimum value of n = 7. FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone. *, p ≤ 0.05.
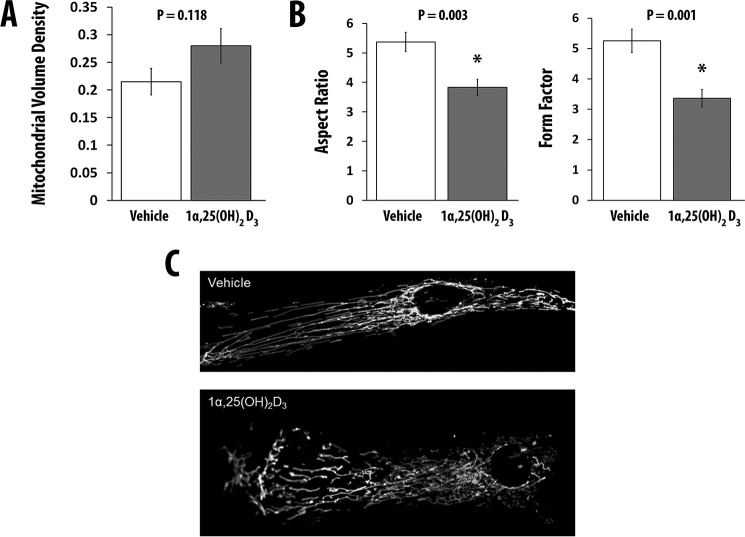
1α,25(OH)2D3 Increases Mitochondrial Volume and Branchingin Primary Human Skeletal Muscle Cells
We assessed whether 1α,25(OH)2D3 alters mitochondrial volume and branching or fragmentation. We treated hSkMCs with 1α,25(OH)2D3 for 48 h. The volume fraction of mitochondria (Fig. 4A) and mitochondrial branching increased in cells treated with 1α,25(OH)2D3 (Fig. 4B). As shown in Fig. 4C, 1α,25(OH)2D3 treatment of hSkMCs resulted in morphological changes in mitochondria and a redistribution of mitochondria within the cell.
FIGURE 4.
1α,25(OH)2D3 increases mitochondrial volume and fragmentation. A and B, effect of 1α,25(OH)2D3 or vehicle on mitochondrial volume density (mitochondrial volume normalized to total cell volume) (A) and mitochondrial morphometry (aspect ratio and form factor) (B). C, effect of 1α,25(OH)2D3 or vehicle on mitochondrial ultrastructure. For all measurements, the number of replicates was 10. Values are expressed as mean ± S.E. (error bars). *, p ≤ 0.05.
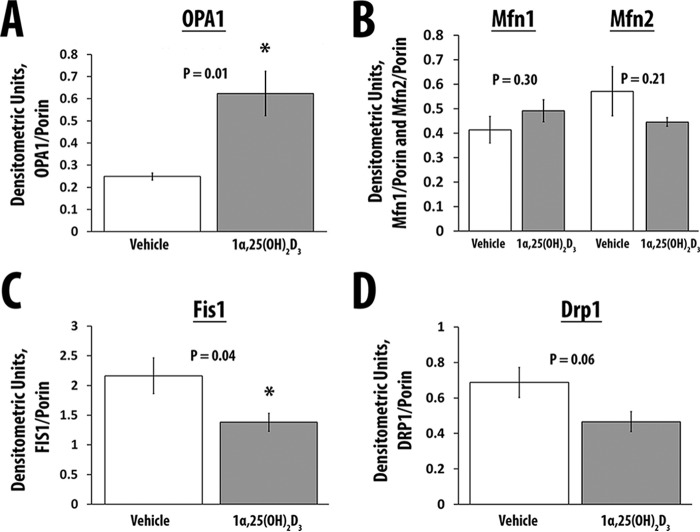
1α,25(OH)2D3 Increases the Expression of Mitochondrial Proteins That Alter Mitochondrial Fusion and Decreases the Expression of Proteins Associated with Mitochondrial Fission
Treatment of hSkMCs with 1α,25(OH)2D3 increased the expression of OPA1 (p = 0.01), a GTPase responsible for fusion of the inner mitochondrial membrane (59–61) (Table 1 and Fig. 5A). Mfn1 and Mfn2, proteins that mediate the fusion of the outer mitochondrial membrane, did not change (p = 0.30 and p = 0.21, respectively) (Table 1 and Fig. 5B) (59, 60, 62). Concomitantly, 1α,25(OH)2D3 treatment of hSkMCs reduced the expression of Fis1 and DRP-1, proteins that increase mitochondrial fission (p = 0.04 and 0.06, respectively) (Table 1 and Fig. 5, C and D) (59, 60, 62). There were no changes in the mRNAs for these proteins on WTSS (see supplemental Table 1).
TABLE 1.
Quantitation of OPA1, Mfn1, Mfn2, Fis1, and Drp1 proteins
All values are corrected for the expression of porin. Data are expressed as means ± S.E.
| OPA1 | Mfn1 | Mfn2 | Fis1 | Drp1 | |
|---|---|---|---|---|---|
| Vehicle, n = 4 | 0.25 ± .02 | 0.41 ± 0.06 | 0.57 ± 0.1 | 2.16 ± 0.3 | 0.69 ± 0.08 |
| 1,25(OH)2D3, 10−8 m, n = 5 | 0.62 ± 0.1 | 0.49 ± 0.05 | 0.45 ± 0.02 | 1.38 ± 0.15 | 0.47 ± 0.06 |
| p | 0.01 | 0.3 | 0.2 | 0.04 | 0.06 |
FIGURE 5.
The effect of 1α,25(OH)2D3 on the mediators of mitochondrial fusion (OPA1, Mfn1, and Mfn2) and fission (Fis1 and Drp1). A, expression of OPA1 corrected for the expression of Vdac1/porin in cells treated with vehicle (n = 4) or 1α,25(OH)2D3 (n = 5). B, expression of Mfn1 and Mfn2 corrected for the expression of Vdac1/porin in cells treated with vehicle (n = 4) or 1α,25(OH)2D3 (n = 5). C, expression of Fis1 corrected for the expression of Vdac1/porin in cells treated with vehicle (n = 4) or 1α,25(OH)2D3 (n = 5). D, expression of Drp1 corrected for the expression of Vdac1/porin in cells treated with vehicle (n = 4) or 1α,25(OH)2D3 (n = 5). Values are expressed as mean ± S.E. (error bars). *, p ≤ 0.05.
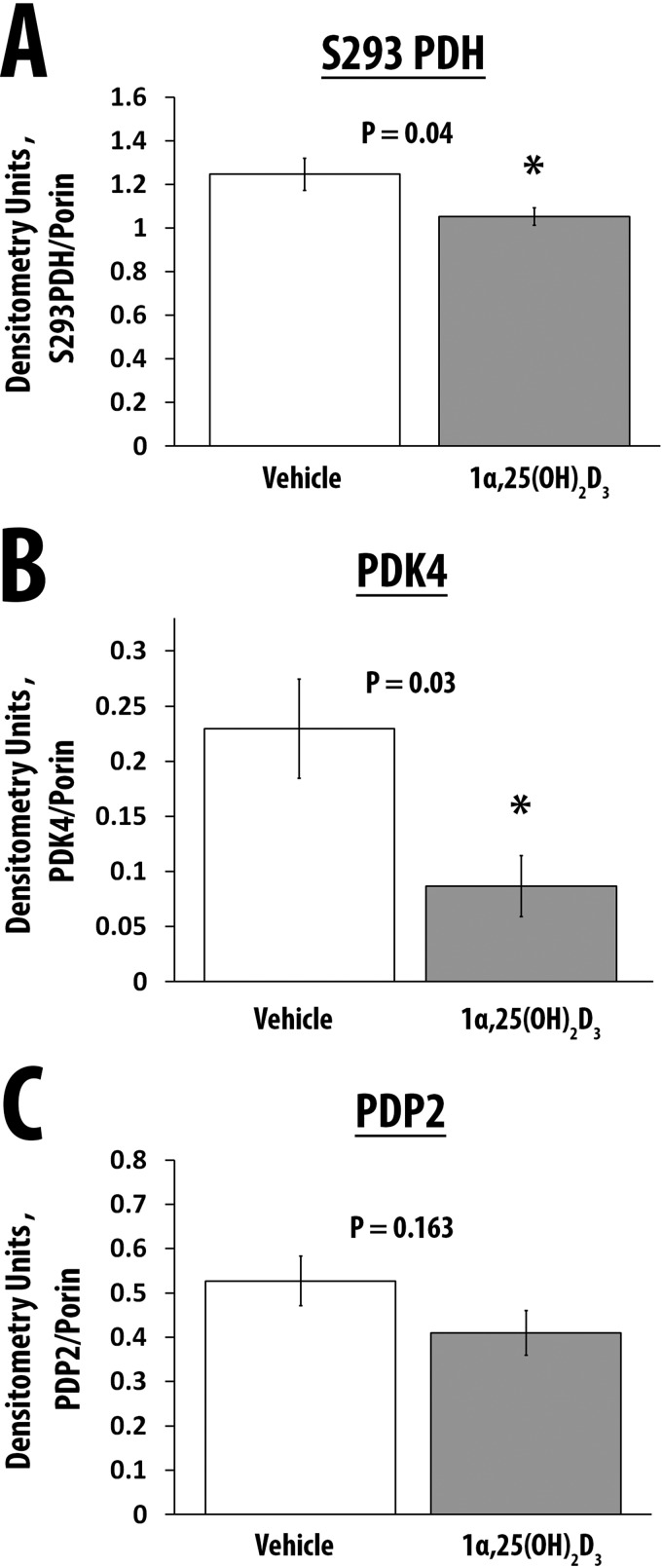
1α,25(OH)2D3 Decreases the Expression of Phosphorylated Pyruvate Dehydrogenase and Pyruvate Dehydrogenase Kinase 4
The activity of the PDH complex, located in the outer mitochondrial matrix, regulates the formation of acetyl-CoA from pyruvate and is altered by the phosphorylation status of its E1 component through the activity of pyruvate dehydrogenase kinases 1–4 (PDK1–4) and pyruvate dehydrogenase phosphatases 1 and 2 (PDP1 and -2) (63–67). Non-phosphorylated PDH is catalytically more active compared with the phosphorylated form of PDH. We observed a decrease in the expression of phosphorylated PDH and PDK4 following treatment of hSkMCs with 1α,25(OH)2D3 (p = 0.04 and p = 0.03, respectively) (Table 2 and Fig. 6, A and B). Treatment of cells with 1α,25(OH)2D3 was associated with a trend toward an increase (∼13.4%) in PDH activity (vehicle, 20.58 ± 0.19 nmol of NADH/min/mg of protein (n = 5); 1α,25(OH)2D3, 23.34 ± 2.84 nmol of NADH/minute/mg (n = 6); p = 0.09). On WTSS, PDK4 mRNA expression decreased 0.55-fold (p = 7.21E−10). At the protein level, the expression of the PDH phosphatase, PDP2, tended to increase, but the changes did not reach statistical significance (Table 2 and Fig. 6C, p = 0.16). Of note, on WTSS, PDP2 mRNA expression increased 1.2-fold (p = 2.35E−07).
TABLE 2.
Quantitation of phospho-PDH E1 (Ser-293), PDK4, and PDP2 proteins
All values are corrected for the expression of porin. Data are expressed as means ± S.E.
| Phospho-PDH E1 (Ser-293) | PDK4 | PDP2 | |
|---|---|---|---|
| Vehicle, n = 4 | 1.25 ± .07 | 0.23 ± 0.05 | 0.53 ± 0.06 |
| 1,25(OH)2D3, 10−8 m, n = 5 | 1.05 ± 0.04 | 0.09 ± 0.03 | 0.41 ± 0.05 |
| p | 0.04 | 0.03 | 0.16 |
FIGURE 6.
The effect of 1α,25(OH)2D3 on the expression of phospho-PDH, PDK4, and PDP2. A, expression of phospho-PDH corrected for the expression of Vdac1/porin in cells treated with vehicle (n = 4) or 1α,25(OH)2D3 (n = 5). B, expression of PDK4 corrected for the expression of Vdac1/porin in cells treated with vehicle (n = 4) or 1α,25(OH)2D3 (n = 5). C, expression of PDP2 corrected for the expression of Vdac1/porin in cells treated with vehicle (n = 4) or 1α,25(OH)2D3 (n = 5). Values are expressed as mean ± S.E. (error bars).
Effect of 1α,25(OH)2D3 on Mitochondrial DNA/Nuclear DNA Ratios
Treatment of hSkMCs with 1α,25(OH)2D3 did not change the amount of mitochondrial DNA relative to nuclear DNA (mean of ND1 and ND6: 1α,25(OH)2D3 = 2.8 ± 0.78 ng (mean ± S.D.) versus vehicle = 2.6 ± 0.31 ng (n = 9) (p = 0.508)); mean of BECN1 and NEB: 1,25-dihydroxyvitamin D3 = 4.9 ± 1.39 ng versus vehicle = 3.8 ± 0.99 ng (n = 9) (p = 0.068).
1α,25(OH)2D3 Does Not Significantly Alter the Expression of Proteins in the Mitochondrial Respiratory Chain
Treatment of hSkMCs with 1α,25(OH)2D3 did not significantly change proteins in complexes I–V in the mitochondrial respiratory chain (Table 3).
TABLE 3.
Quantitation of proteins in respiratory chain complexes I–V
All values are corrected for the expression of porin. Data are expressed as means ± S.E. NDUFB8, NADH dehydrogenase (ubiquinone) 1 β subcomplex 8, 19 kDa; SDHB, succinate dehydrogenase complex, subunit B, iron sulfur; UQCR2, ubiquinol-cytochrome c reductase core protein II; COX2, cytochrome c oxidase II; ATP5A, ATP synthase, H+-transporting, mitochondrial F1 complex α.
| NDUFB8, Complex 1 | SDHB, Complex 2 | UQCR2, Complex III | COX2, Complex IV | ATP5A, Complex V | |
|---|---|---|---|---|---|
| Vehicle, n = 4 | 1.18 ± .09 | 1.09 ± 0.12 | 0.65 ± 0.14 | 0.49 ± 0.06 | 0.73 ± 0.07 |
| 1,25(OH)2D3, 10−8 m, n = 5 | 1.1 ± 0.04 | 0.83 ± 0.05 | 0.47 ± 0.07 | 0.45 ± 0.02 | 0.6 ± 0.03 |
| p | 0.4 | 0.06 | 0.25 | 0.49 | 0.09 |
1α,25(OH)2D3 Alters the Expression of Genes Encoding Mitochondrial Proteins, and Genes Encoding Cellular Signaling and Growth-regulatory Pathways in Adult Human Skeletal Muscle Cells
Primary cultures of hSkMCs expressing normal or reduced amounts of the VDR were treated with 1α,25(OH)2D3 or vehicle prior to analysis of gene expression by WTSS. The mRNA-seq data obtained from control or antisense VDR silencing RNA-treated cells, which were subsequently treated with either 1α,25(OH)2D3 or vehicle, showed ∼400 million total reads with a high degree of correlation of expression profiles (Pearson correlation coefficient = 1); >95% mRNAs were successfully mapped to known genes (Table 4). Differential gene expression in response to 1α,25(OH)2D3 treatment compared with vehicle with a false discovery rate (or p value adjusted for multiple comparisons) of <0.01 in cells expressing normal VDR concentrations is shown in supplemental Table 1.
TABLE 4.
Overview of mRNA-seq results
| Normal VDR expression |
Inhibited VDR expression |
|||
|---|---|---|---|---|
| Vehicle | 1α,25(OH)2D3 | Vehicle | 1α,25(OH)2D3 | |
| No. of total reads | 420,947,554 ± 31,279,048 | 393,213,995 ± 42,385,160 | 387,829,949 ± 64,716,956 | 355,215,074 ± 43,827,913 |
| No. of mapped reads | 408,977,138 ± 31,442,664 | 381,511,528 ± 41,785,455 | 376,167,940 ± 63,253,459 | 340,370,655 ± 40,479,111 |
| Percentage of reads mapped | 97.14 ± 0.26 | 97.01 ± 0.23 | 96.98 ± 0.11 | 95.87 ± 0.77 |
853 nuclear mRNAs encoding mitochondrial proteins were detected in primary cultures of human muscle cells. Table 5 lists significantly up-regulated mRNAs, and Table 6 lists significantly down-regulated nuclear mRNAs encoding mitochondrial proteins following treatment with 1α,25(OH)2D3. A 25,000-fold increase in CYP24A1 mRNA expression was seen. Note the decreases in mitochondrial PDK4 (pyruvate dehydrogenase kinase 4) and increases in PDP2 (encoding pyruvate dehydrogenase phosphatase 2) expression. The protein products regulate the activity of pyruvate dehydrogenase that provides acetyl-CoA for the tricarboxylic acid cycle.
TABLE 5.
Significantly induced mRNAs encoding mitochondrial proteins following treatment of primary human skeletal muscle cells expressing the VDR with 1α,25(OH)2D3
| Gene name | Change | p |
|---|---|---|
| -fold | ||
| CYP24A1, cytochrome P450, 24 A1 | 25,598 | 0.00E+00 |
| C15orf48, Chr 15 Orf 48 | 3.5 | 1.66E−03 |
| ADHFE1, alcohol dehydrogenase | 1.4 | 9.17E−03 |
| SDSL, serine dehydratase-like | 1.4 | 1.30E−03 |
| SQRDL, sulfide:quinone oxidoreductase | 1.4 | 1.11E−20 |
| HSPB7, heat shock 27 kDa 7 | 1.4 | 3.48E−06 |
| SARDH, sarcosine dehydrogenase | 1.4 | 8.55E−04 |
| NTHL1, Nth endonuclease III-like 1 | 1.3 | 2.26E−03 |
| OXR1, oxidation resistance protein 1 | 1.3 | 2.39E−20 |
| PDP2, pyruvate dehydrogenase phosphatase subunit 2 | 1.2 | 2.35E−07 |
| BCAT1, branched chain amino acid transaminase 1 | 1.2 | 3.86E−18 |
| AGPAT5, 1-acylglycerol-3-phosphate O-acyltransferase 5 | 1.2 | 3.08E−11 |
| HSPA9, heat shock 70-kDa protein 9 (mortalin) | 1.19 | 2.10E−13 |
| GUF1, GTPase homolog | 1.19 | 1.08E−05 |
| SLC25A36, solute carrier family 25, member 36 | 1.19 | 1.30E−10 |
| PDK3, pyruvate dehydrogenase kinase, isozyme 3 | 1.19 | 3.65E−03 |
| TRIT1, tRNA isopentenyltransferase 1 | 1.19 | 2.79E−03 |
| IDH2, isocitrate dehydrogenase 2 (NADP+) | 1.14 | 3.18E−07 |
| SLC16A1, solute carrier family 16 (monocarboxylate transporter), member 1 | 1.139 | 1.47E−05 |
| ACADSB, acyl-CoA dehydrogenase, short/branched chain | 1.136 | 9.44E−03 |
| GRPEL2, GrpE-like 2, mitochondrial | 1.130 | 8.47E−03 |
| PTCD3, pentatricopeptide repeat domain 3 | 1.129 | 1.31E−04 |
| PDPR, pyruvate dehydrogenase phosphatase regulatory subunit | 1.120 | 2.44E−03 |
| PISD, phosphatidylserine decarboxylase | 1.120 | 8.97E−03 |
| ATAD1, ATPase family, AAA domain-containing 1 | 1.119 | 4.90E−04 |
| LRPPRC, leucine-rich pentatricopeptide repeat-containing | 1.117 | 2.92E−05 |
| PPA2, pyrophosphatase (inorganic) 2 | 1.115 | 1.27E−03 |
| COQ9, coenzyme Q9 | 1.114 | 4.72E−03 |
| MRPS6, mitochondrial ribosomal protein S6 | 1.114 | 9.52E−03 |
| PNPT1, polyribonucleotide nucleotidyltransferase 1 | 1.106 | 4.50E−03 |
| HSPD1, heat shock 60-kDa protein 1 (chaperonin) | 1.098 | 5.54E−04 |
| ATIC, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase | 1.094 | 2.91E−03 |
| HK2, hexokinase 2 | 1.080 | 9.42E−03 |
TABLE 6.
Significantly repressed mRNAs encoding mitochondrial proteins following treatment of primary human skeletal muscle cells expressing the VDR with 1α,25(OH)2D3
| Gene name | Change | p |
|---|---|---|
| -fold | ||
| PDK4, pyruvate dehydrogenase kinase 4 | 0.55 | 7.21E−10 |
| TMEM160, transmembrane protein 160 | 0.67 | 6.26E−04 |
| MAOA, monamine oxidase A | 0.79 | 3.46E−12 |
| ELN, elastin | 0.79 | 1.63E−04 |
| PC, pyruvic carboxylase | 0.80 | 1.35E−09 |
| CPT1A, carnitine palmitoyltransferase 1A | 0.81 | 2.67E−05 |
| SNPH, syntaphilin | 0.82 | 1.48E−03 |
| COX17, cytochrome c oxidase copper chaperone | 0.82 | 2.18E−03 |
| RAB32 | 0.82 | 1.90E−10 |
| HAGH, hydroxyacylglutathione hydrolase | 0.82 | 9.31E−04 |
| ECH1 | 0.830 | 8.20E−09 |
| SACSL1 | 0.831 | 1.26E−10 |
| ALDH4A1, aldehyde dehydrogenase 4 family, member A1 | 0.835 | 7.09E−03 |
| GLRX, glutaredoxin (thioltransferase). | 0.847 | 4.04E−08 |
| PYCR1, pyrroline-5-carboxylate reductase 1 | 0.847 | 1.42E−09 |
| DDAH1, dimethylarginine dimethylaminohydrolase 1 | 0.848 | 3.53E−09 |
| MRPL41, mitochondrial ribosomal protein L41 | 0.849 | 2.68E−03 |
| ATP5D, ATP synthase, H+ transporting, mitochondrial F1 complex, δ subunit | 0.853 | 2.15E−04 |
| ATP10D, ATPase, Class V, Type 10D | 0.855 | 2.70E−06 |
| FASN, fatty acid synthase | 0.856 | 1.23E−05 |
| ACAT2, acetyl-CoA acetyltransferase 2 | 0.861 | 1.06E−04 |
| ALKBH7, AlkB, alkylation repair homolog 7 | 0.861 | 9.57E−04 |
| TSPO, translocator protein (18 kDa) | 0.863 | 9.13E−05 |
| TAP1, transporter 1, ATP-binding cassette, subfamily B | 0.866 | 3.01E−03 |
| GSR, glutathione reductase | 0.874 | 2.56E−04 |
| AURKAIP1, aurora kinase A-interacting protein 1 | 0.874 | 1.87E−04 |
| CYB5R1, cytochrome B5 reductase 1 | 0.874 | 6.79E−03 |
| ISOC2, isochorismatase domain-containing 2 | 0.880 | 8.18E−03 |
| TSHZ3, teashirt zinc finger homeobox 3 | 0.882 | 6.66E−04 |
| FDPS, farnesyl diphosphate synthase | 0.884 | 2.01E−06 |
| RAB1B, member RAS oncogene family | 0.888 | 1.20E−04 |
| PRDX5, peroxiredoxin 5 | 0.888 | 6.22E−05 |
| ETFB, electron transfer flavoprotein, β polypeptide | 0.891 | 9.14E−05 |
| NDUFB2, NADH dehydrogenase ubiquinone 1 β subcomplex 2 | 0.893 | 1.73E−04 |
| RAB35, member RAS oncogene family | 0.894 | 1.31E−03 |
| PINK1, PTEN-induced putative kinase 1 | 0.895 | 1.51E−03 |
| SLC25A1, solute carrier family 25, member 1 | 0.895 | 5.36E−04 |
| NDUFA4, NADH dehydrogenase ubiquinone 1α subcomplex 4 | 0.898 | 2.27E−03 |
| OXCT1, 3-oxoacid CoA transferase 1 | 0.898 | 3.75E−03 |
| BAX, BCL2-associated X protein | 0.900 | 6.61E−04 |
| PPIF, peptidylprolyl isomerase F | 0.902 | 8.62E−04 |
| FASTK, Fas-activated serine/threonine kinase | 0.903 | 1.26E−03 |
| NME4, NME/NM23 nucleoside diphosphate kinase 4 | 0.909 | 1.25E−03 |
| GLUD1, glutamate dehydrogenase 1 | 0.910 | 1.22E−03 |
| SLC25A6, solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 6 | 0.912 | 1.96E−03 |
| UXS1, UDP-glucuronate decarboxylase 1 | 0.913 | 8.50E−03 |
| COMT, catechol-O-methyltransferase | 0.914 | 9.85E−03 |
| GPX4, glutathione peroxidase 4 | 0.916 | 5.34E−03 |
| OAT, ornithine aminotransferase | 0.922 | 7.79E−03 |
| MTCH1, mitochondrial carrier 1 | 0.925 | 6.07E−03 |
We examined RNA-seq data to determine whether the expression of mRNAs known to be involved in mitochondrial biogenesis was altered. Messenger RNAs for MYC, MAPK13, and EPAS1, which encode proteins known to increase mitochondrial biogenesis, were increased following treatment of skeletal muscle cells with 1α,25(OH)2D3. Messenger RNAs for other factors known to alter mitochondrial biogenesis, such as PGC-1α, TFAM, AMPK, FOXO3, SIRT1, SIRT3, NRF-1, NRF-2, LKB1, ADRB3, CAV2, CEBPA, COX10, CRYAA, CXADR, DNAJA3, FXN, GNAS, HTT, LETM1, MAN2A1, P2RX7, PRDX3, PTCD2, RAB3A, and SHARPIN were unchanged.
1947 mRNAs (10.2% of ∼19,000 known human protein-coding genes (68)) are differentially expressed following treatment of muscle cells expressing the VDR with 1α,25(OH)2D3. The most significantly increased mRNA, CYP24A1 (1α,25(OH)2D3/25(OH)2D3-24-hydroxylase; >25,000-fold change, p = 0), encodes a mitochondrial cytochrome P450 responsible for the metabolism of 1α,25(OH)2D3 to 1α,24,25(OH)3D3 and conversion of 25(OH)2D3 to 24R,25(OH)2D3. Messenger RNAs encoding proteins involved in muscle relaxation (PVALB, parvalbumin, 21.7-fold change, p = 1.92E−22), protein synthesis (IGFN1, Ig-like, fibronectin type III domain-containing 1, 17.5-fold change, p = 3.22E−08), cytoskeletal dynamics (ARHGEF16, ρ guanine nucleotide exchange factor, 10.5-fold change, p = 1.53E−06), RNA and nucleotide binding (TDRD1, tudor domain-containing protein 10, 19.7-fold change, p = 7.56E−18), cellular energy metabolism (IGFL3, insulin growth factor-like 3, 9.5-fold change, p = 1.59E−10), apoptosis (FAIM2, Fas apoptotic inhibitory molecule 2, 6.5-fold change, p = 5.83E−22), and nucleosome function (HIST1H3J, histone cluster 1, H3j, 4.8-fold, p = 4.16E−05) are significantly up-regulated. The most significantly repressed mRNAs encode a calcium-sensitive cysteine protease (CAPN11, calpain 11, 0.10-fold change, p = 3.92E−04) and proteins involved in cell migration (PODN, podocan, 0.29-fold change, p = 7.82E−04) and cellular proliferation (WFDC1, WAP four-disulfide core domain 1, 0.48-fold change, p = 2.64E−0.05). We confirmed changes in the expression of select mRNAs seen on RNA-seq by measuring select mRNAs by digital PCR or qPCR (Table 7).
TABLE 7.
Quantitation of mRNA by digital or quantitative PCR
Data are expressed as mean ± S.D.
| CYP24A1/RPL13A | VDR/RPL13A | MSTN/RPL13A | PDK4/RPL13A | PVALB/RPL13A | |
|---|---|---|---|---|---|
| Vehicle, n = 4 | 0.69 ± 0.26 | 0.62 ± 0.27 | 1.05 ± 0.04 | 1.09 ± 0.23 | 0.57 ± 0.43 |
| 1,25(OH)2D3, 10−8 m, n = 4 | 34303.4 ± 2950.21 | 0.83 ± 1.02 | 0.72 ± 0.17 | 0.58 ± 0.07 | 594.3 ± 68.1 |
| p | 3.59E−05 | 0.71 | 0.02 | 0.007 | 0.0001 |
Table 8 shows the most significantly changed cellular pathways analyzed with the DAVID (Database for Annotation, Visualization, and Integrated Discovery) program. 1α,25-Dihydroxyvitamin D3-regulated mRNAs encoded cellular proteins involved in focal adhesion, muscle contraction, and signaling through extracellular matrix receptor, integrin, JAK/STAT, MAPK, growth factor, and p53 pathways.
TABLE 8.
Pathways significantly altered by 1α,25(OH)2D3 treatment of primary human skeletal muscle cells using DAVID analysis and associated databases
| Pathway term (gene count) | p | Database |
|---|---|---|
| Focal adhesion (61) | 7.52E−12 | KEGG |
| ECM-receptor interaction (35) | 3.64E−11 | KEGG |
| Integrin signaling (68) | 3.15E−10 | Panther |
| Integrin signaling (24) | 2.72E−05 | Reactome |
| Gap junction (23) | 7.79E−04 | KEGG |
| Axon guidance (30) | 6.74E−04 | KEGG |
| Signaling by PDGF (20) | 7.06E−05 | Reactome |
| JAK/STAT signaling (10) | 0.003 | Panther |
| Pathways in cancer (57) | 0.005 | KEGG |
| Signaling by NGF (33) | 0.006 | Reactome |
| Regulation of actin cytoskeleton (40) | 0.006 | KEGG |
| Metabolism of nucleotides (17) | 0.014 | Reactome |
| p53 pathway (16) | 0.014 | Panther |
| Interferon-signaling (11) | 0.017 | Panther |
| Muscle contraction (9) | 0.022 | Reactome |
| Terpenoid backbone biosynthesis (6) | 0.028 | KEGG |
| Cell adhesion molecules (24) | 0.048 | KEGG |
In hSkMCs in which VDR expression was inhibited by anti-VDR siRNA (>80% inhibition observed), only 15 mRNAs changed following treatment of cells with 1α,25(OH)2D3, compared with 1947 mRNAs altered following treatment of cells with 1α,25(OH)2D3 when the VDR expression is normal. The mRNAs whose expression changed are identical to those altered by 1α,25(OH)2D3 in cells expressing normal amounts of the VDR, but the changes are quantitatively reduced. Knockdown of the VDR in cells resulted in changes in gene expression that were in the opposite direction of those observed following 1α,25(OH)2D3 treatment of VDR-expressing cells (supplemental Table 2).
1α,25(OH)2D3 Alters the Expression of miRNAs in Human Skeletal Muscle Cells
1α,25(OH)2D3 alters the expression of nine miRNAs; of these, five (hsa-miR-143-3p, hsa-miR-133a, hsa-miR-17-5p, hsa-miR-129-5p, and hsa-miR-125b-1-3p) have binding targets in mRNAs that are differentially expressed as a result of 1α,25(OH)2D3 treatment (Table 9). Several signaling pathways (growth factor, MAPK, integrin) are modulated by these miRNAs through their target mRNAs (Table 10).
TABLE 9.
MicroRNAs that are induced or repressed in muscle cells following treatment with 1α,25(OH)2D3
| miRNA name | Log2 (-fold change) | p | Differentially expressed target mRNAs | No. of differentially expressed target mRNAs |
|---|---|---|---|---|
| hsa-miR-143-3p | −1.974 | 0.010 | Yes | 13 |
| hsa-miR-133a | −0.987 | 0.012 | Yes | 8 |
| hsa-miR-17-5p | −1.258 | 0.013 | Yes | 15 |
| hsa-miR-129-5p | 0.815 | 0.015 | Yes | 279 |
| hsa-miR-532-3p | −0.926 | 0.017 | No | |
| hsa-miR-501-5p | −0.968 | 0.025 | No | |
| hsa-miR-618 | −1.019 | 0.037 | No | |
| hsa-miR-1268a | 0.719 | 0.040 | No | |
| hsa-miR-125b-1-3p | 0.493 | 0.046 | Yes | 72 |
TABLE 10.
Pathways/GO terms significantly enriched in the targets (differentially expressed mRNAs) of miRNAs induced or repressed by 1α,25(OH)2D3
| miRNAs | Pathways/gene ontology terms | No. of differentially expressed target genes | p | Database |
|---|---|---|---|---|
| miR-125b | Golgi stack | 3 | 1.44E−02 | GOTERM_CC |
| miR-125b | Regulation of apoptosis | 9 | 1.53E−02 | GOTERM_BP |
| miR-125b | Regulation of programmed cell death | 9 | 1.62E−02 | GOTERM_BP |
| miR-125b | Regulation of cell death | 9 | 1.65E−02 | GOTERM_BP |
| miR-125b | Nerve growth factor binding | 2 | 2.02E−02 | GOTERM_MF |
| miR-125b | Neurotrophin binding | 2 | 3.22E−02 | GOTERM_MF |
| miR-125b | Positive regulation of membrane protein ectodomain proteolysis | 2 | 3.60E−02 | GOTERM_BP |
| miR-125b | MAPKKK cascade | 4 | 3.88E−02 | GOTERM_BP |
| miR-125b | Identical protein binding | 7 | 4.52E−02 | GOTERM_MF |
| miR-125b | Protein homodimerization activity | 5 | 4.70E−02 | GOTERM_MF |
| miR-125b | Regulation of hydrolase activity | 5 | 4.78E−02 | GOTERM_BP |
| miR-125b | Intrinsic to plasma membrane | 10 | 4.91E−02 | GOTERM_CC |
| miR-125b | Endoplasmic reticulum part | 5 | 4.92E−02 | GOTERM_CC |
| miR-133a | Transmembrane transport | 3 | 3.22E−02 | GOTERM_BP |
| miR-133a | Plasma membrane part | 4 | 3.88E−02 | GOTERM_CC |
| miR-143 | Cytoskeletal part | 5 | 6.60E−03 | GOTERM_CC |
| miR-143 | Protein domain-specific binding | 3 | 2.07E−02 | GOTERM_MF |
| miR-143 | Cytoskeleton | 5 | 2.40E−02 | GOTERM_CC |
| miR-143 | Signaling by NGF | 3 | 2.41E−02 | REACTOME |
| miR-143 | Positive regulation of DNA binding | 2 | 4.56E−02 | GOTERM_BP |
| miR-129-5p | Integrin signaling pathway | 15 | 4.70E−04 | PANTHER |
| miR-129-5p | ECM-receptor interaction | 8 | 5.62E−04 | KEGG |
| miR-129-5p | Focal adhesion | 12 | 6.19E−04 | KEGG |
| miR-129-5p | Signaling by PDGF | 5 | 6.88E−03 | REACTOME |
| miR-129-5p | Axon guidance | 4 | 2.05E−02 | REACTOME |
| miR-129-5p | Regulation of actin cytoskeleton | 9 | 3.16E−02 | KEGG |
| miR-129-5p | Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 5 | 4.19E−02 | KEGG |
Discussion
The salient findings of our report are that 1α,25(OH)2D3 has important effects on mitochondrial physiology, morphology, and expression of key mitochondrial proteins. Mitochondrial OCR increases in skeletal muscle cells treated with 1α,25(OH)2D3. In particular, respiration coupled to the generation of ATP is increased, which suggests that the hormone increases energy production in muscle. The increase in mitochondrial OCR is specific for 1α,25(OH)2D3 that has both C-1 and C-25 hydroxyl groups and does not occur with other vitamin D3 analogs that lack either one of both C-1 and C-25 hydroxyls, such as 25(OH)D3, 25R,25(OH)2D3, 1α-(OH)D3, and vitamin D3. The findings are consistent with the high binding affinity of 1α,25(OH)2D3 for the VDR relative to the lower affinities of the other tested analogs (58).
Several mechanisms might account for the increases in mitochondrial OCR. First, increases in mitochondrial volume fraction and branching consistent with mitochondrial fusion and biogenesis (69–72) occur following treatment of muscle cells with 1α,25(OH)2D3. Indeed, there are appropriate increases and decreases in mediators of mitochondrial fusion (OPA1) and fission (Fis1 and Drp1) that are consistent with the observed changes in mitochondrial morphology (59, 60, 62). Increases in the mRNA for mediators of increased mitochondrial biogenesis, such as MYC, MAPK13, and EPAS1, are likely to play a role in increasing the numbers of mitochondrial. An increase in mitochondrial volume could account for the increase in mitochondrial OCR (59–62, 73).
Second, we observe decreases in the amount of inactive, phosphorylated pyruvate dehydrogenase following treatment of skeletal muscle cells with 1α,25(OH)2D3. The alterations in the phosphorylation state of PDH are associated with a concomitant decrease in the expression of the PDH kinase, PDK4. There is a trend toward an increase in the expression of the PDH phosphatase, PDP2. The changes in protein expression are supported by changes in mRNA expression for PDK4 and PDP2. Treatment of cells with 1α,25(OH)2D3 was associated with a trend to an increase in PDH activity (p = 0.09). The small increase in PDH complex (PDC) activity could potentially increase the amount of acetyl-CoA entering the tricarboxylic acid cycle and thus might account for an increase in mitochondrial OCR. Insulin is known to decrease PDK4 expression, whereas glucocorticoids have the opposite effect (63, 67, 74–77). In aggregate, the data suggest that 1α,25(OH)2D3 might regulate carbohydrate and fatty acid metabolism in muscle cells. Confirmation of such effects in vivo would be of great interest.
The change in expression of ∼2000 nuclear mRNAs, 83 of which encode proteins known to localize in mitochondria following treatment of skeletal muscle cells with 1α,25(OH)2D3, is of interest. In addition to the quantitatively large up-regulation of the mitochondrial CYP24A1, mRNAs for other mitochondrial proteins that play a role in carbohydrate and fatty acid metabolism were noted to be either induced or repressed. Of note, a significantly down-regulated mRNA, PDK4, encodes the pyruvate dehydrogenase kinase, isoenzyme 4, a serine/threonine kinase that phosphorylates the pyruvate dehydrogenase subunits PDHA1 and PDHA2, and regulates metabolite flux through the tricarboxylic acid cycle (63, 67, 76–78). Conversely, the mRNA for PDP2, which encodes pyruvate dehydrogenase phosphatase catalytic subunit 2, is significantly increased. PDP2 catalyzes the dephosphorylation and concomitant reactivation of the α subunit of the E1 component of the pyruvate dehydrogenase complex (78–80). The changes in the expression of mRNAs for PDP2 and PDK4 and documented alterations in the expression of the encoded proteins would function together to increase PDC activity.
The altered mRNAs represent 10.2% of known protein coding genes in the human genome (68). The differentially expressed mRNAs encode proteins that play significant roles in multiple biochemical pathways involved in muscle contraction and relaxation; extracellular matrix-cell receptor interactions; actin cytoskeleton remodeling; and JAK-STAT, insulin-like growth factor, and p53 signaling. The most profoundly up-regulated mRNA following 1α,25(OH)2D3 treatment of muscle cells is the CYP24A1 (>25,000-fold increase) that encodes the cytochrome P450 for the 1α,25(OH)2D3/25(OH)D3-24-hydroxylase, which catalyzes the transformation of 1α,25(OH)2D3 and 25(OH)D3 to less active metabolites 1α,24,25(OH)3D3 and 24R,25(OH)2D3. Of note, the mRNA for the calcium-binding protein, parvalbumin, that regulates muscle relaxation is significantly induced by 1α,25(OH)2D3. In addition, the expression of mRNAs encoding several growth factors related to the insulin-like growth factor family and nerve growth factor are up-regulated following treatment of cells with 1α,25(OH)2D3. Pathway analysis indicates the regulation of several factors involved in muscle cellular signaling, apoptosis, and growth. The massive change in mRNA expression following treatment of cells with 1α,25(OH)2D3 is consistent with our earlier findings showing similar large scale, 1α,25(OH)2D3-mediated changes in mRNA and miRNA expression in Danio rerio embryos (38, 39).
The effects of 1α,25(OH)2D3 on mitochondrial OCR may help to explain the observation that reduced serum 1α,25(OH)2D3 concentrations in humans have been linked to falls through reduced muscle strength (10, 11) and that clinical trials have demonstrated salutary effects of 1α(OH)D3, a 1α-hydroxylated vitamin D analog that is rapidly metabolized to 1α,25(OH)2D3 in vivo, on fall prevention (12). The data also suggest that vitamin D3 and 25(OH)D3 will not be useful in the treatment of muscle weakness unless they are metabolized to 1α,25(OH)2D3, a circumstance that is operative in the context of vitamin D deficiency, where high parathyroid hormone levels drive the rapid metabolism of 25(OH)D3 to 1α,25(OH)2D3. Our findings of increased OCR following treatment of skeletal muscle cells with 1α,25(OH)2D3 are consistent with the report of Sinha et al. (27), which showed that treatment of vitamin D-deficient humans with cholecalciferol improves muscle phosphocreatine recovery after exercise, suggesting an effect of 1α,25(OH)2D3 on the formation of high energy phosphorylated intermediates and mitochondrial function. The increase in the numbers of mitochondria could also account for the increase in cellular OCR.
In conclusion, 1α,25(OH)2D3 alters mitochondrial OCR, mitochondrial biogenesis, and PDC activity, demonstrating unique effects of the sterol hormone on muscle biochemistry. In addition, there is a profound change in the expression of several hundred nuclear mRNAs, several of which encode mitochondrial proteins and proteins involved in cell signaling and cell growth. The observed effects could explain the myopathy of vitamin D deficiency that is seen in patients with impaired intake of vitamin D and the myopathy of chronic renal failure, where the production of 1α,25(OH)2D3 is impaired.
Author Contributions
Z. C. R., T. A. C., C. D. F., X. W., I. R. L., N. S. S., and J. L. S. performed the experiments and analyzed the data; K. S. N., A. T., G. C. S., and R. K. designed the experiments and analyzed the data; R. K. wrote the manuscript.
Supplementary Material
This work was supported by National Institutes of Health Grants HL126451 (to G. C. S.), HL126451 (to C. D. L. F.), DK066013 (to R. K.), and AG009531 (to K. S. N.), Mayo Clinic Grant CCATS UL1TR000135, the Leducq Foundation, and the Mayo Clinic Center for Regenerative Medicine. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Tables 1 and 2.
- 1α,25(OH)2D3
- 1α,25-dihydroxyvitamin D3
- 25(OH)D3
- 25-hydroxyvitamin D3
- VDR
- vitamin D receptor
- OCR
- oxygen consumption rate
- WTSS
- whole transcriptome shotgun sequencing
- miRNA
- microRNA
- RNA-seq
- mRNA-seq, and miRNA-seq, RNA-, mRNA-, and miRNA-sequencing, respectively
- hSkMC
- human skeletal muscle cell
- qPCR
- quantitative PCR
- bis-tris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- PDH
- phosphorylated pyruvate dehydrogenase
- PDK
- PDH kinase
- 1α,24,25(OH)3D3
- 1α,24R,25-trihydroxyvitamin D3
- 24R,25(OH)2D3
- 24R,25-dihydroxyvitamin D3
- 1α(OH)D3
- 1α-hydroxylated vitamin D.
References
- 1.DeLuca H. F. (2004) Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 80, 1689S–1696S [DOI] [PubMed] [Google Scholar]
- 2.Haussler M. R., Haussler C. A., Bartik L., Whitfield G. K., Hsieh J. C., Slater S., and Jurutka P. W. (2008) Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 66, S98–S112 [DOI] [PubMed] [Google Scholar]
- 3.Norman A. W. (2006) Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147, 5542–5548 [DOI] [PubMed] [Google Scholar]
- 4.Wasserman R. H., Smith C. A., Brindak M. E., De Talamoni N., Fullmer C. S., Penniston J. T., and Kumar R. (1992) Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology 102, 886–894 [DOI] [PubMed] [Google Scholar]
- 5.Schott G. D., and Wills M. R. (1976) Muscle weakness in osteomalacia. Lancet 1, 626–629 [DOI] [PubMed] [Google Scholar]
- 6.Prineas J. W., Mason A. S., and Henson R. A. (1965) Myopathy in metabolic bone disease. Br. Med. J. 1, 1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley R. N., Wang C., Ishani A., Collins A. J., and Murray A. M. (2007) Kidney function and sarcopenia in the United States general population: NHANES III. Am. J. Nephrol. 27, 279–286 [DOI] [PubMed] [Google Scholar]
- 8.Domański M., and Ciechanowski K. (2012) Sarcopenia: a major challenge in elderly patients with end-stage renal disease. J. Aging Res. 2012, 754739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murad M. H., Elamin K. B., Abu Elnour N. O., Elamin M. B., Alkatib A. A., Fatourechi M. M., Almandoz J. P., Mullan R. J., Lane M. A., Liu H., Erwin P. J., Hensrud D. D., and Montori V. M. (2011) Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J. Clin. Endocrinol. 96, 2997–3006 [DOI] [PubMed] [Google Scholar]
- 10.Gallagher J. C., Rapuri P., and Smith L. (2007) Falls are associated with decreased renal function and insufficient calcitriol production by the kidney. J. Steroid Biochem. Mol. Biol. 103, 610–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher J. C. (2004) The effects of calcitriol on falls and fractures and physical performance tests. J. Steroid Biochem. Mol. Biol. 89, 497–501 [DOI] [PubMed] [Google Scholar]
- 12.Dukas L., Bischoff H. A., Lindpaintner L. S., Schacht E., Birkner-Binder D., Damm T. N., Thalmann B., and Stähelin H. B. (2004) Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily. J. Am. Geriatr Soc. 52, 230–236 [DOI] [PubMed] [Google Scholar]
- 13.Ceglia L., Niramitmahapanya S., da Silva Morais M., Rivas D. A., Harris S. S., Bischoff-Ferrari H., Fielding R. A., and Dawson-Hughes B. (2013) A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J. Clin. Endocrinol. Metab. 98, E1927–E1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Boland A. R., Gallego S., and Boland R. (1983) Effects of vitamin D-3 on phosphate and calcium transport across and composition of skeletal muscle plasma cell membranes. Biochim. Biophys. Acta 733, 264–273 [DOI] [PubMed] [Google Scholar]
- 15.de Boland A. R., and Boland R. L. (1984) Effects of vitamin D3 on in vivo labelling of chick skeletal muscle proteins with [3H]leucine. Z. Naturforsch. C 39, 1015–1016 [DOI] [PubMed] [Google Scholar]
- 16.Buitrago C. G., Ronda A. C., de Boland A. R., and Boland R. (2006) MAP kinases p38 and JNK are activated by the steroid hormone 1α,25(OH)2-vitamin D3 in the C2C12 muscle cell line. J. Cell. Biochem. 97, 698–708 [DOI] [PubMed] [Google Scholar]
- 17.Buitrago C. G., Arango N. S., and Boland R. L. (2012) 1α,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J. Cell. Biochem. 113, 1170–1181 [DOI] [PubMed] [Google Scholar]
- 18.Girgis C. M., Clifton-Bligh R. J., Mokbel N., Cheng K., and Gunton J. E. (2014) Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 155, 347–357 [DOI] [PubMed] [Google Scholar]
- 19.Birge S. J., and Haddad J. G. (1975) 25-Hydroxycholecalciferol stimulation of muscle metabolism. J. Clin. Invest. 56, 1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka M., Kishimoto K. N., Okuno H., Saito H., and Itoi E. (2014) Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 49, 700–708 [DOI] [PubMed] [Google Scholar]
- 21.Srikuea R., Zhang X., Park-Sarge O. K., and Esser K. A. (2012) VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am. J. Physiol. Cell Physiol. 303, C396–C405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girgis C. M., Mokbel N., Cha K. M., Houweling P. J., Abboud M., Fraser D. R., Mason R. S., Clifton-Bligh R. J., and Gunton J. E. (2014) The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 155, 3227–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischoff-Ferrari H. A., Borchers M., Gudat F., Dürmüller U., Stähelin H. B., and Dick W. (2004) Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 19, 265–269 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., and DeLuca H. F. (2011) Is the vitamin D receptor found in muscle? Endocrinology 152, 354–363 [DOI] [PubMed] [Google Scholar]
- 25.Endo I., Inoue D., Mitsui T., Umaki Y., Akaike M., Yoshizawa T., Kato S., and Matsumoto T. (2003) Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144, 5138–5144 [DOI] [PubMed] [Google Scholar]
- 26.Sakai S., Suzuki M., Tashiro Y., Tanaka K., Takeda S., Aizawa K., Hirata M., Yogo K., and Endo K. (2015) Vitamin D receptor signaling enhances locomotive ability in mice. J. Bone Miner. Res. 30, 128–136 [DOI] [PubMed] [Google Scholar]
- 27.Sinha A., Hollingsworth K. G., Ball S., and Cheetham T. (2013) Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 98, E509–E513 [DOI] [PubMed] [Google Scholar]
- 28.Lanza I. R., and Nair K. S. (2009) Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 457, 349–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanza I. R., Bhagra S., Nair K. S., and Port J. D. (2011) Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J. Magn. Reson. Imaging 34, 1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folmes C. D., Arrell D. K., Zlatkovic-Lindor J., Martinez-Fernandez A., Perez-Terzic C., Nelson T. J., and Terzic A. (2013) Metabolome and metaboproteome remodeling in nuclear reprogramming. Cell Cycle 12, 2355–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folmes C. D., Martinez-Fernandez A., Perales-Clemente E., Li X., McDonald A., Oglesbee D., Hrstka S. C., Perez-Terzic C., Terzic A., and Nelson T. J. (2013) Disease-causing mitochondrial heteroplasmy segregated within induced pluripotent stem cell clones derived from a patient with MELAS. Stem Cells 31, 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar R., Schaefer J., Grande J. P., and Roche P. C. (1994) Immunolocalization of calcitriol receptor, 24-hydroxylase cytochrome P-450, and calbindin D28k in human kidney. Am. J. Physiol. 266, F477–F485 [DOI] [PubMed] [Google Scholar]
- 33.Koopman W. J., Distelmaier F., Esseling J. J., Smeitink J. A., and Willems P. H. (2008) Computer-assisted live cell analysis of mitochondrial membrane potential, morphology and calcium handling. Methods 46, 304–311 [DOI] [PubMed] [Google Scholar]
- 34.Koopman W. J., Visch H. J., Smeitink J. A., and Willems P. H. (2006) Simultaneous quantitative measurement and automated analysis of mitochondrial morphology, mass, potential, and motility in living human skin fibroblasts. Cytometry A 69, 1–12 [DOI] [PubMed] [Google Scholar]
- 35.Koopman W. J., Visch H. J., Verkaart S., van den Heuvel L. W., Smeitink J. A., and Willems P. H. (2005) Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am. J. Physiol. Cell Physiol. 289, C881–C890 [DOI] [PubMed] [Google Scholar]
- 36.Aravamudan B., Kiel A., Freeman M., Delmotte P., Thompson M., Vassallo R., Sieck G. C., Pabelick C. M., and Prakash Y. S. (2014) Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 306, L840–L854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delmotte P., and Sieck G. C. (2015) Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca2+ regulation in airway smooth muscle (ASM). Can. J. Physiol. Pharmacol. 93, 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craig T. A., Zhang Y., McNulty M. S., Middha S., Ketha H., Singh R. J., Magis A. T., Funk C., Price N. D., Ekker S. C., and Kumar R. (2012) Research resource: whole transcriptome RNA sequencing detects multiple 1α,25-dihydroxyvitamin D3-sensitive metabolic pathways in developing zebrafish. Mol. Endocrinol. 26, 1630–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craig T. A., Zhang Y., Magis A. T., Funk C. C., Price N. D., Ekker S. C., and Kumar R. (2014) Detection of 1α,25-dihydroxyvitamin D-regulated miRNAs in zebrafish by whole transcriptome sequencing. Zebrafish 11, 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalari K. R., Nair A. A., Bhavsar J. D., O'Brien D. R., Davila J. I., Bockol M. A., Nie J., Tang X., Baheti S., Doughty J. B., Middha S., Sicotte H., Thompson A. E., Asmann Y. W., and Kocher J. P. (2014) MAP-RSeq: Mayo analysis pipeline for RNA sequencing. BMC Bioinformatics 15, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., Wang S., and Li W. (2012) RSeQC: quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 [DOI] [PubMed] [Google Scholar]
- 42.Trapnell C., Pachter L., and Salzberg S. L. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langmead B., Trapnell C., Pop M., and Salzberg S. L. (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anders S., Pyl P. T., and Huber W. (2015) HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson M. D., McCarthy D. J., and Smyth G. K. (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Z., Evans J., Bhagwate A., Middha S., Bockol M., Yan H., and Kocher J. P. (2014) CAP-miRSeq: a comprehensive analysis pipeline for microRNA sequencing data. BMC Genomics 15, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An J., Lai J., Lehman M. L., and Nelson C. C. (2013) miRDeep*: an integrated application tool for miRNA identification from RNA sequencing data. Nucleic Acids Res. 41, 727–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths-Jones S., Grocock R. J., van Dongen S., Bateman A., and Enright A. J. (2006) miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34, D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis B. P., Burge C. B., and Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 50.Huang da W., Sherman B. T., and Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 51.Huang da W., Sherman B. T., Zheng X., Yang J., Imamichi T., Stephens R., and Lempicki R. A. (2009) Extracting biological meaning from large gene lists with DAVID. Curr. Protoc. Bioinformatics 10.1002/0471250953.bi1311s27 [DOI] [PubMed] [Google Scholar]
- 52.Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., Katayama T., Kawashima S., Okuda S., Tokimatsu T., and Yamanishi Y. (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanehisa M., Goto S., Kawashima S., Okuno Y., and Hattori M. (2004) The KEGG resource for deciphering the genome. Nucleic Acids Res. 32, D277–D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okuda S., Yamada T., Hamajima M., Itoh M., Katayama T., Bork P., Goto S., and Kanehisa M. (2008) KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 36, W423–W426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mi H., Guo N., Kejariwal A., and Thomas P. D. (2007) PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucleic Acids Res. 35, D247–D252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi-Tope G., Gillespie M., Vastrik I., D'Eustachio P., Schmidt E., de Bono B., Jassal B., Gopinath G. R., Wu G. R., Matthews L., Lewis S., Birney E., and Stein L. (2005) Reactome: a knowledgebase of biological pathways. Nucleic Acids Res. 33, D428–D432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S. E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., and Mootha V. K. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revelle L., Solan V., Londowski J., Bollman S., and Kumar R. (1984) Synthesis and biologic activity of a C-ring analogue of vitamin D3: biologic and protein binding properties of 11 α-hydroxyvitamin D3. Biochemistry 23, 1983–1987 [DOI] [PubMed] [Google Scholar]
- 59.Chan D. C. (2006) Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 22, 79–99 [DOI] [PubMed] [Google Scholar]
- 60.Chan D. C. (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 [DOI] [PubMed] [Google Scholar]
- 61.Kao S. H., Yen M. Y., Wang A. G., Yeh Y. L., and Lin A. L. (2015) Changes in mitochondrial morphology and bioenergetics in human lymphoblastoid cells with four novel OPA1 mutations. Invest. Ophthalmol. Vis. Sci. 56, 2269–2278 [DOI] [PubMed] [Google Scholar]
- 62.Chan D. C. (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125, 1241–1252 [DOI] [PubMed] [Google Scholar]
- 63.Jeong J. Y., Jeoung N. H., Park K. G., and Lee I. K. (2012) Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab. J. 36, 328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krebs E. G., and Beavo J. A. (1979) Phosphorylation-dephosphorylation of enzymes. Annu. Rev. Biochem. 48, 923–959 [DOI] [PubMed] [Google Scholar]
- 65.Lee I. K. (2014) The role of pyruvate dehydrogenase kinase in diabetes and obesity. Diabetes Metab. J. 38, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel M. S., Nemeria N. S., Furey W., and Jordan F. (2014) The pyruvate dehydrogenase complexes: structure-based function and regulation. J. Biol. Chem. 289, 16615–16623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roche T. E., and Hiromasa Y. (2007) Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia, and cancer. Cell. Mol. Life Sci. 64, 830–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ezkurdia I., Juan D., Rodriguez J. M., Frankish A., Diekhans M., Harrow J., Vazquez J., Valencia A., and Tress M. L. (2014) Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum. Mol. Genet. 23, 5866–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iqbal S., and Hood D. A. (2014) Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am. J. Physiol. Cell Physiol. 306, C1176–C1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khacho M., Tarabay M., Patten D., Khacho P., MacLaurin J. G., Guadagno J., Bergeron R., Cregan S. P., Harper M. E., Park D. S., and Slack R. S. (2014) Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat. Commun. 5, 3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sukhorukov V. M., Dikov D., Reichert A. S., and Meyer-Hermann M. (2012) Emergence of the mitochondrial reticulum from fission and fusion dynamics. PLoS Comput. Biol. 8, e1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ventura-Clapier R., Garnier A., and Veksler V. (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc. Res. 79, 208–217 [DOI] [PubMed] [Google Scholar]
- 73.Chen H., Chomyn A., and Chan D. C. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185–26192 [DOI] [PubMed] [Google Scholar]
- 74.Frier B. C., Jacobs R. L., and Wright D. C. (2011) Interactions between the consumption of a high-fat diet and fasting in the regulation of fatty acid oxidation enzyme gene expression: an evaluation of potential mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R212–R221 [DOI] [PubMed] [Google Scholar]
- 75.Majer M., Popov K. M., Harris R. A., Bogardus C., and Prochazka M. (1998) Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol. Genet. Metab. 65, 181–186 [DOI] [PubMed] [Google Scholar]
- 76.Sugden M. C., Bulmer K., Augustine D., and Holness M. J. (2001) Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-α: implications for glucose-stimulated insulin secretion. Diabetes 50, 2729–2736 [DOI] [PubMed] [Google Scholar]
- 77.Sugden M. C., Bulmer K., and Holness M. J. (2001) Fuel-sensing mechanisms integrating lipid and carbohydrate utilization. Biochem. Soc. Trans. 29, 272–278 [DOI] [PubMed] [Google Scholar]
- 78.Thomas G. W., Mains C. W., Slone D. S., Craun M. L., and Bar-Or D. (2009) Potential dysregulation of the pyruvate dehydrogenase complex by bacterial toxins and insulin. J. Trauma 67, 628–633 [DOI] [PubMed] [Google Scholar]
- 79.Huang B., Gudi R., Wu P., Harris R. A., Hamilton J., and Popov K. M. (1998) Isoenzymes of pyruvate dehydrogenase phosphatase: DNA-derived amino acid sequences, expression, and regulation. J. Biol. Chem. 273, 17680–17688 [DOI] [PubMed] [Google Scholar]
- 80.Huang B., Wu P., Popov K. M., and Harris R. A. (2003) Starvation and diabetes reduce the amount of pyruvate dehydrogenase phosphatase in rat heart and kidney. Diabetes 52, 1371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.