Abstract
Li-Fraumeni Syndrome (LFS) is an autosomal dominant genetic disorder associated with mutations in the TP53 gene and characterized by a propensity to develop a variety of malignancies resulting in a shortened lifespan. We report a case of prostate cancer in a 50 year old male with LFS. Experimental studies suggest that TP53 mutations in prostate cancer are associated with therapeutic resistance to radiation, chemotherapy, and anti-androgens, implying that LFS men may experience more aggressive cancer biology with implications for therapeutic decisions. The potential of prostate cancer to develop earlier in LFS favors institution of screening at earlier ages.
Keywords: TP53, Li-Fraumeni Syndrome, p53, Prostate cancer
Introduction
Li-Fraumeni Syndrome (LFS) is a cancer predisposition syndrome with dominant inheritance in which affected individuals display TP53 gene mutations and diverse tumor types.1 The specific type of TP53 gene mutation, combined with unique environmental and lifestyle exposures, may account for the heterogenous patterns of cancer at different ages. Familial TP53 mutations may be as high as 1:5000.1 As recognition of LFS improves and cancer screening/surveillance strategies for impacted individuals are instituted, many malignancies will be detected earlier with higher rates of cure and enhanced longevity.2 Thus, those caring for urologic cancers are likely to observe a rise in the number of LFS-Prostate Cancer (PCa) patients secondary to longer life expectancies. If PCa risk is indeed increased by LFS, a diagnosis at a younger age with more aggressive biology may be predicted. We report a case of clinically localized PCa in a 50-year-old LFS male and discuss implications for clinical assessment, ongoing surveillance, and medical management.
Case presentation
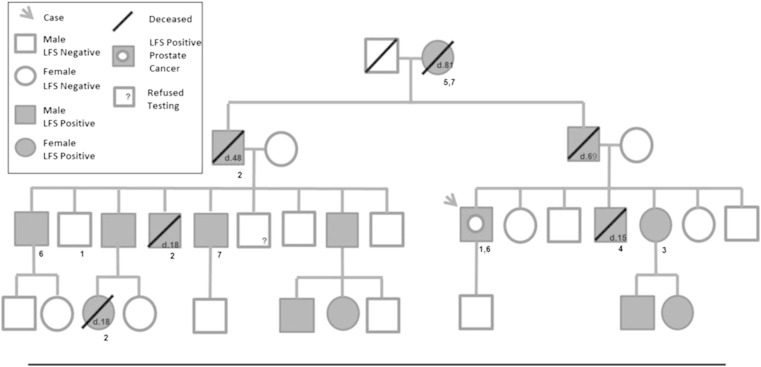
A 50-year-old male presented for primary care evaluation and underwent his first PCa screening with a normal DRE and PSA of 4.41 ng/mL. The family history was significant for PCa in a paternal uncle (72y) and cousin (60y). His family history was also significant for LFS, having been genotyped at age 49. The limited family pedigree shows the individuals, their cancer diagnoses, age, survival status, and genetic test results if known (Fig. 1). Our case was free of other cancers except for a solitary 4 cm skin lesion consistent with limited stage mycosis fungoides (T-cell lymphoma) treated with topical steroids and in remission (Fig. 2).
Figure 1.
Partial Li-Fraumeni Syndrome (LFS) case pedigree. Offspring of LFS positive individuals included in pedigree; LFS negative offspring excluded. Age of death noted within geometric shape. Diagnosed cancers listed under shapes: 1 = prostate; 2 = brain; 3 = breast; 4 = lymphosarcoma; 5 = melanoma; 6 = lymphoma; 7 = renal.
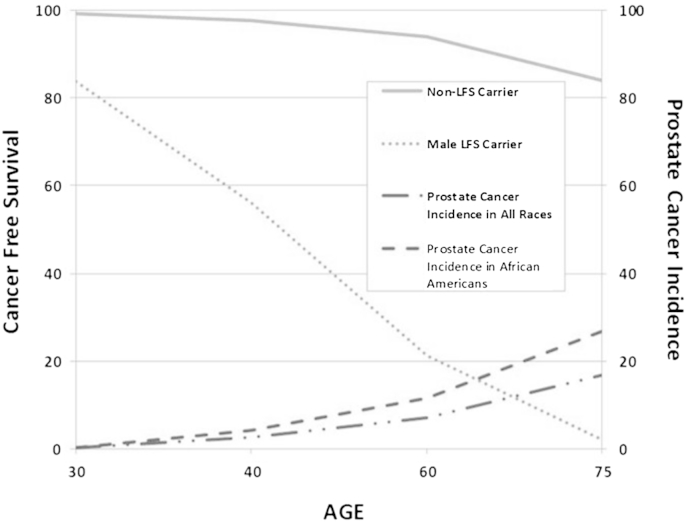
Figure 2.
Estimated cancer-free survival curves and prostate cancer incidence by age. Cancer-free survival plotted for non-LFS carriers against male LFS carriers. Prostate cancer incidence for all races and African Americans.
Transrectal biopsy revealed two of eight cores with adenocarcinoma, one on the left lateral base, Gleason grade 3 + 4 = 7 involving 40% of the specimen, and the left base with a 3 + 3 = 6 carcinoma involving 1% of the sample. After considering options, he proceeded with an uncomplicated radical robotic-assisted prostatectomy and lymph node resection. Pathologic staging showed adenocarcinoma, Gleason 3 + 3 = 6, involving 5% of the gland, multifocal, bilateral cancer apex to base, within a background of diffuse high grade prostatic intraepithelial neoplasia without nodal involvement or extracapsular extension and a staging of pT2cN0Mx.
Discussion
Caregivers involved in management of genitourinary cancers should examine family history for evidence of multiple and early-onset cancers. The prompt recognition of heredity cancer syndromes, such as LFS, may have important management and prognostic value including more effective screening for at-risk family members. Adrenocortical cancer and Wilm's tumor/nephroblastoma are two urological malignancies established as core cancers of LFS (others include: sarcoma, osteosarcoma, pre-menopausal breast cancer, brain cancer, and leukemia), and their detection should trigger a careful evaluation of the family history.1, 3
Improved awareness and identification of LFS is providing an opportunity for aggressive cancer screening and tailored surveillance in LFS families. This approach is allowing LFS patients to successfully treat cancers at curable stages, achieve greater longevity, and men are surviving to ages over 40 where PCa rates dramatically increase over 100-fold during the subsequent three decades.4 At present, data is insufficient to define prostate cancer as an LFS core cancer, particularly with sporadic prostate cancer being the most common visceral cancer among men in Western nations.4 The high prevalence of genetic defects in p53 signaling pathways based upon genomic analysis of human prostate cancer5 suggests a potential for prostate cancer to be one of the LFS core malignancies. Future monitoring of LFS families through a national/international database will address this issue.4
Decisions regarding the age at which to begin prostate cancer screening and how frequently to repeat such assessments will need to be incorporated into a comprehensive cancer LFS surveillance program, which continues to evolve. Although public health guidelines for PCa screening remain controversial, we strongly favor treating LFS men as high-risk and empirically initiating the screening program no later than age 40 until data accumulates to guide our efforts more precisely. Obviously, in an asymptomatic male whose life expectancy is less than 5 years (if an active lethal cancer or comorbidities are present), the more urgent health issues may supersede the importance of screening and detection of an early PCa.
Surgical interventions for cancer have been successfully employed with curative and palliative intent in LFS without evidence of unexpected complications. Thus, otherwise healthy men with substantial life expectancies are candidates for a radical prostatectomy with curative intent.6 We do not favor ionizing radiation (IR) therapy, either external beam or brachytherapy, for treatment for clinically localized prostate cancer in LFS, as p53 dysfunction is associated with relative radio-resistance in cancer.7 More critically, mutant p53 contributes to defective DNA repair, and LFS patients experience greater risk of developing secondary cancers due to radiation-induced oncogenic transformation.1 The risk to individuals with LFS of developing a second primary cancer is 57%, and the risk of a third is approximately 38%.1 Limiting exposure to radiation therapy and judicious utilization of diagnostic radiology is recommended.1
Chemotherapy is employed in neoadjuvant and adjuvant clinical trials for those with locally advanced prostate cancer with a high risk of relapse following single modality, such as surgical therapy. Unfortunately, many therapeutic agents can induce DNA damage, increasing the risk of future malignancy.8 Not surprisingly, p53 protein mutations also confer drug resistance in multiple cancers.9 Reported chemoresistance in dysfunctional p53 cancers include docetaxel, doxorubicin, cisplatin, anthracyclines, and mitoxantrone.9, 10 Thus far, the efficacy of taxane-based chemotherapeutics in LFS are largely unknown.10
Conclusion
LFS is an inherited cancer syndrome with urologic cancers (adrenocortical cancer and nephroblastoma) as core malignancies. The improved screening, detection, and treatment of cancers in LFS families is allowing LFS men to live longer lives and survive into the decades where prostate cancer is more prevalent. It is prudent to consider men with LFS as high risk and institute screening at ages earlier than for the general population. Surgical intervention for localized disease, as opposed to radiotherapy and systemic chemotherapy or hormonal therapy, is preferable when cure is possible. The optimal management of LFS patients is early detection of prostate cancer at a stage where curative surgical interventions are likely to be successful and the subsequent need for chemotherapy and radiotherapy will be low.
Conflict of interest
The authors have no conflicts of interest.
Acknowledgment
This work was financially supported by The Ohio State University Food Innovation Center, Columbus, Ohio. The project described was also supported by Award Number Grant UL1TR001070 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Informed consent: Informed consent for use of patient information was obtained by the authors.
References
- 1.Schneider K., Zelley K., Nichols K.E., Garber J. Li-Fraumeni Syndrome. In: Pagon R.A., Adam M.P., Ardinger H.H., editors. GeneReviews(®) University of Washington, Seattle; Seattle (WA): 1993. http://www.ncbi.nlm.nih.gov/books/NBK1311/ Accessed 19.12.14. [Google Scholar]
- 2.Antoniou A.C., Easton D.F. Risk prediction models for familial breast cancer. Future Oncol. 2006;2(2):257–274. doi: 10.2217/14796694.2.2.257. [DOI] [PubMed] [Google Scholar]
- 3.Jefferson K.P., Gillatt D.A. Hereditary urological cancer syndromes. Nat Clin Pract Urol. 2007;4(4):218–226. doi: 10.1038/ncpuro0761. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health . National Cancer Institute; 2014. Genetics of Prostate Cancer (PDQ®)http://www.cancer.gov/cancertopics/pdq/genetics/prostate/HealthProfessional/page1 Accessed 27.12.14. [Google Scholar]
- 5.Taylor B.S., Schultz N., Hieronymus H. Integrative genomic profiling of human prostate Cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bill-Axelson A., Holmberg L., Ruutu M. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.M., Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993;90(12):5742–5746. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roos W.P., Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12(9):440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Masciarelli S., Fontemaggi G., Di Agostino S. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene. 2014;33(12):1601–1608. doi: 10.1038/onc.2013.106. [DOI] [PubMed] [Google Scholar]
- 10.Knappskog S., Lønning P.E. P53 and its molecular basis to chemoresistance in breast cancer. Expert Opin Ther Targets. 2012;16(S1):S23–S30. doi: 10.1517/14728222.2011.640322. [DOI] [PubMed] [Google Scholar]