Abstract
We have recently identified a new clinical syndrome in patients receiving warfarin for anticoagulation therapy. This syndrome has been named warfarin-related nephropathy (WRN), and patients with chronic kidney disease (CKD) appear to be particularly susceptible. WRN is defined as an acute increase in international normalized ratio (INR) to >3.0, followed by evidence of acute kidney injury (AKI) within 1 week of the INR increase. AKI was defined as a sustained increase in serum creatinine of greater than or equal to 0.3 mg/dL. The AKI cannot be explained by any other factors, and the kidney biopsy demonstrates extensive glomerular hemorrhage with tubular obstruction by red blood cells (RBCs). Beyond AKI, WRN is a significant risk factor for mortality within the first 2 months of diagnosis and it accelerates the progression of CKD. We demonstrated that 5/6 nephrectomy in rats is a suitable experimental model to study WRN. Animals treated with warfarin showed an increase in serum creatinine and morphologic findings in the kidney similar to those in humans with WRN. Our recent evidence suggests that novel oral anticoagulants may induce AKI. Diagnosis of WRN may be challenging for a renal pathologist. A few cases with suspected WRN and pathologic considerations are described.
Keywords: Acute kidney injury, Anticoagulation therapy, Chronic kidney disease, Warfarin-related nephropathy
Introduction
Anticoagulant therapy is vital for patients with thromboembolic disorders, most notably atrial fibrillation or deep vein thrombosis. An estimated 4 million patients in the US and almost 7 million worldwide are receiving long-term therapy with oral anticoagulants, primarily warfarin or other coumarin derivatives, for prevention and treatment of venous and arterial thromboembolism [1]. Vitamin K antagonists, such as warfarin (Coumadin), provide reliable protection against thromboembolic events. However, this benefit comes at a cost, which is a risk of hemorrhage resulting from coagulopathy. Recently, evidence that warfarin-related coagulopathy may lead to kidney complications, including acute kidney injury (AKI), was reported [2], [3], [4]. This complication was reported in the USA [2], [3], [4] and Korea [5]. Worrisome data that even novel oral anticoagulants may also affect the kidney were recently reported [6], [7].
This review focuses on the discovery of anticoagulant-related kidney injury, describes an animal model to study it, and provides guidance for nephrologists and renal pathologists who may encounter this disease.
Clinical aspects of anticoagulation and AKI
Chronic kidney disease (CKD) is one of the major health problems in the world [8], where between 10% and 15% of the population are diagnosed with this condition [9], [10]. Anticoagulation therapy is commonly required in CKD patients for treatment or prevention of thromboembolic disorders. It has been shown that CKD patients are at high risk of developing atrial fibrillation, which requires anticoagulation therapy, and as many as 21% of nondialysis CKD patients may have atrial fibrillation, as compared with 1.5–6.2% in the general population [11]. Among the patients diagnosed with atrial fibrillation, more than a third have CKD Stage 3 and above [12]. Warfarin is the most prescribed anticoagulant in the world and more than 2 million people start warfarin therapy every year in the USA alone. Warfarin treatment requires constant monitoring of the international normalized ratio (INR) and dose adjustments should be made to keep the INR within the therapeutic range. Monitoring of INR is difficult, costly, and there is a level of noncompliance. CKD is associated with decreased anticoagulation stability in patients on warfarin therapy, which, in turn, requires more frequent and intensive clinical management [13]. Avoiding over-anticoagulation in CKD appears to be a frequent problem. Limdi et al [14] compared complications of warfarin therapy among 578 patients with different stages of CKD. They reported that patients with severe CKD were at higher risk for both over-anticoagulation and major hemorrhage, as compared with patients with mild or moderate CKD. Chan et al [15] reported that warfarin therapy is associated with higher mortality among hemodialysis patients than in non–end-stage renal disease (ESRD) CKD patients. Their study included 41,425 hemodialysis patients; 8.3% were receiving warfarin therapy. Warfarin therapy was associated with a 27% increase in mortality.
Several adverse effects of warfarin overdose on kidney function have been described, including hemorrhages, vasculitis, interstitial nephritis, and hematuria [16], [17]. Our group described AKI associated with warfarin treatment [2], [3], [4]. We named this condition warfarin-related nephropathy (WRN).
It has been suggested that warfarin can cause AKI by inducing glomerular hematuria with subsequent widespread tubular obstruction. This was first reported in a patient with severe warfarin coagulopathy and thin glomerular basement membrane (GBM) disease [18]. Later, evidence of a similar syndrome in a patient with inactive systemic lupus erythematosus (SLE), with an abnormally thick GBM, was reported [19].
The mechanism of the AKI in these patients likely involved the unusual combination of very severe warfarin coagulopathy (INR in the 6–9 range) in which the patient had either abnormally thin GBM [18] or abnormally thick GBM [19], both of which are risk factors for spontaneous gross hematuria [20]. Thus, based on these cases, there was no compelling reason to believe that AKI would be a common complication of warfarin therapy. This notion, however, was dispelled by our kidney biopsy study of nine instances of WRN in seven patients who developed unexplained AKI during only moderate over-anticoagulation with warfarin (mean INR 4.4±0.7) [4]. The kidney biopsies showed widespread and severe tubular obstruction by red blood cell (RBC) casts. Although these patients had CKD, their glomeruli were normal or showed only minor changes. Thus, the renal biopsy evidence of severe glomerular hematuria was unexpected. Also, most of the WRN patients showed little or no recovery of kidney function [4].
The next work by our group was a retrospective analysis of 103 consecutive warfarin-treated CKD patients followed in our Nephrology program from January 2005 until June 2009. Of these, 49 experienced at least one episode of INR >3.0. Of these, 18 (37%) developed an unexplained increase in serum creatinine ≥ 0.3 mg/dL within 1 week of the INR >3.0, and their CKD progression was accelerated [2].
Another evidence of the broad clinical significance of WRN is an analysis of 4,006 warfarin-treated patients [3]. Each experienced an INR >3.0 and serum creatinine was measured within 1 week of the increased INR. The analysis of this large database (4,006 patients) [3] supports the analysis of the smaller database (103 patients) [2] that showed that WRN is common in CKD patients (37% develop AKI at first onset of INR >3.0). The new insight of this study is that WRN is also common in no-CKD patients and is significantly associated with an increased risk of mortality.
Based on these publications, the key clinical features of WRN are: (1) evidence of AKI appears shortly after the INR acutely increases to>3.0; (2) kidney biopsies in these patients show acute tubular injury associated with severe and widespread occlusive RBC casts; (3) WRN accelerates the progression of CKD; (4) WRN occurs in approximately 37% (CKD) to 16% (no-CKD) of warfarin-treated patients whose INR acutely rises to>3.0; (5) patients with WRN have a significantly increased mortality rate.
We observed that WRN may occur in some patients who do not have the clinical diagnosis of CKD. Our experimental data show that control animals treated with warfarin do not develop AKI even if their prothrombin time (PT) increases more than five times [21], [22]. This raises the possibility that the people who develop WRN without apparent CKD probably had some underlying kidney injury.
Several other groups have described WRN in patients on warfarin therapy [5], [23]. Their data confirmed our observations, including a high incidence of WRN. The main conclusions from published data are:
-
•
The risk of AKI occurs at an INR threshold of >3; AKI risk is not a function of the level of INR beyond 3.
-
•
The kidney biopsy findings in those with AKI and INR >3 are consistent with catastrophic glomerular hemorrhage causing tubular injury.
-
•
An abnormally elevated INR is not sufficient by itself to cause WRN; we postulate that WRN occurs in the setting of pre-existing glomerular damage (which may not have been clinically known/diagnosed) coupled with over-anticoagulation. Normal patients who develop WRN likely have undiagnosed CKD/glomerular injury.
-
•
When specifically analyzed, WRN is associated with progression of CKD and an increased risk of subacute mortality.
-
•
The true incidence of WRN is difficult to determine from the mainly retrospective studies published thus far, but it appears to be high. The only prospective study [23] suggests an incidence of 60%, at least in the elderly population examined.
Adding to this public health problem is emerging evidence that WRN is only a subset of a broader syndrome, that probably is anticoagulant-related nephropathy (ARN) in which other, and possibly all, currently used anticoagulants may cause AKI. Indeed, AKI associated with dabigatran (direct thrombin inhibitor) use has been reported in humans [6], [24], [25] and was recently demonstrated by our group in experimental animals [7]. In addition, anticoagulants may aggravate an underlying kidney disease and induce hematuria and AKI [26].
Experimental models of WRN
First, our group demonstrated that excessive anticoagulation by superwarfarin (brodifacoum) reproduces morphologic features of WRN in 5/6 nephrectomized rats [22]. These rats had increased serum creatinine levels, glomerular hemorrhage, occlusive RBC casts, and acute tubular injury in the kidney, which closely resembled the findings in kidney biopsies from patients with WRN [4]. By contrast, treatment with brodifacoum did not affect renal function in control animals. It appears that kidney injury develops shortly after the INR increase [22]. Later, our group demonstrated that the 5/6 nephrectomy in rats is a suitable model to study WRN. Warfarin resulted in a dose-dependent increase in serum creatinine in 5/6 nephrectomy rats [21]. The increase in serum creatinine associated with warfarin treatment was greater in animals at 3 weeks and 19 weeks after the ablative surgery. The serum creatinine increase was correlated with the PT increase. Kidney morphology in 5/6 nephrectomy rats showed acute tubular injury with RBCs and RBC casts in the tubules. Treatment with vitamin K prevented serum creatinine increase and morphologic changes in the kidney associated with warfarin treatment [21].
The next step in understanding of the pathogenesis of WRN was to investigate the role of oxidative stress. Our group demonstrated that treatment with the antioxidant N-acetylcysteine (NAC) does not prevent glomerular hemorrhage but does prevent AKI in 5/6 nephrectomy rats [27]. We found that NAC in a dose-dependent manner prevents serum creatinine increase associated with excessive anticoagulation by warfarin. However, NAC could not prevent either hematuria or RBC cast formation in 5/6 nephrectomy rats, suggesting that oxidative stress plays a little, if any, role in the glomerular filtration barrier injury and glomerular hemorrhage associated with warfarin treatment. Nevertheless, NAC in a dose-dependent manner prevented serum creatinine increase in 5/6 nephrectomy rats treated with warfarin, suggesting that acute tubular injury seen in WRN is diminished. Indeed, morphologically animals treated with NAC had lesser acute tubular injury as compared with warfarin-only treated 5/6 nephrectomy rats [27]. Therefore, we believe that oxidative stress plays an important role in the deterioration of renal function and acute tubular necrosis in WRN. Our data suggests the following mechanisms of increased oxidative stress in the kidney in WRN: (1) free hemoglobin released by RBCs in the tubular lumen affects tubular epithelial cells by generating reactive oxygen species and increased lipid peroxidation [28]; (2) free hemoglobin incorporates into the tubular epithelial cells via several surface receptors, such as megalin-cubilin receptors [29]; intracellularly, free hemoglobin activates caspases and induces apoptosis [30]; (3) intracellular hemoglobin dissociates into globin and heme, while the latter is also a potent oxidant and activates proinflammatory pathways [29], [31]. It is unlikely that free iron plays a significant role in the pathogenesis of acute tubular injury, because staining for iron is negative in tubular epithelial cells and treatment with the iron chelator deferoxamine (DFO) does not affect WRN in 5/6 nephrectomy rats. Our findings indicated that glomerular hematuria is a necessary but not sufficient mechanism for the AKI in WRN. The dominant mechanism of WRN is probably tubular obstruction by RBC casts, which leads to increased oxidative stress in the kidney [27].
We recently reported worrisome evidences that novel oral anticoagulants, such as the direct thrombin inhibitor dabigatran, may also induce AKI in experimental animals [7]. We demonstrated that dabigatran increases serum creatinine in a dose-dependent manner in 5/6 nephrectomy rats, similar to the effects of warfarin in this experimental model, as we reported earlier [21]. Unexpectedly, similar elevation in serum creatinine was seen in control, nonoperated rats treated with dabigatran. The 5/6 nephrectomy rats were more sensitive to dabigatran and developed hematuria earlier and after lower doses of dabigatran as compared with control rats. Morphologically, the findings in 5/6 nephrectomy rats treated with dabigatran were similar to those found in animals with WRN [21], [22].
Our findings, that dabigatran affects kidney function, change our view on the pathogenesis of WRN. After the first publications of our works based on vitamin K antagonists [4], [21], [22], there was no compelling evidence that other anticoagulants might affect the kidney. Taking our findings with vitamin K antagonists and dabigatran together, we currently hypothesize that the pathogenesis of WRN does not include vitamin K-dependent proteins. The common result of both vitamin K antagonists and direct thrombin inhibitors is diminished thrombin activity. By acting on thrombomodulin, which is expressed on endothelial cells, thrombin activates protein C and modulates the anticoagulation cascade. Another important receptor for thrombin, which is also expressed on endothelial cells, is proteinase activated receptor 1 (PAR-1). PAR-1 is a G protein-coupled receptor and it participates in the regulation of endothelial functions, vascular permeability, leukocyte migration, and adhesion [32], [33]. In vitro studies indicate that PAR-1 activation changes endothelial monolayer integrity [34]. We propose that thrombin plays an important role in the glomerular filtration barrier function, and its decreased activity (secondary to anticoagulation) results in glomerular filtration barrier abnormalities. Indeed, our data indicate that treatment with selective PAR-1 inhibitor SCH79797 results in increased serum creatinine, hematuria, and tubular RBC casts. Interestingly, these findings were more pronounced in 5/6 nephrectomy than in control rats, indicating that ablative nephropathy by itself makes kidneys more sensitive to changes in coagulation disturbances and it is probably related to changes in PAR-1. In fact, decreased PAR-1 protein expression was found in several human kidney diseases, such as crescentic glomerulonephritis and thrombotic microangiopathy [35], [36]. Of note, despite the fact that changes in coagulation parameters were similar to those that are recommended for patients [37], [38], dabigatran affected renal function and induced hematuria in concentrations significantly higher than those used for humans [38]. These differences may be explained by different pharmacodynamics and pharmacokinetics of dabigatran in rats, which require a significantly higher dose of this drug than humans to achieve the same degree of anticoagulation [39], [40]. This raises the possibility of direct nephrotoxic effects of dabigatran [7].
Our latest data indicate that anticoagulants increase blood pressure (BP) in rats [41]. Warfarin and dabigatran both increased systolic BP in control and 5/6 nephrectomy rats in a dose-dependent manner. SCH79797 also increased systolic BP in a dose-dependent manner. Vitamin K prevented the warfarin-induced increase in BP. NAC delayed the warfarin-associated increase in BP. Interestingly, the warfarin effects on BP were similar in 5/6 nephrectomy rats at different CKD stages. A few early reports suggest that warfarin treatment may be associated with hypertension in animals [42] and humans [43]. Liu et al described a warfarin-associated increase in systolic BP in rats, but this study focused mainly on arterial calcification induced by long-term treatment with warfarin, which was presumed to cause the increase in BP [42]. Similar conclusions were reported by Dao et al [44] and Essalihi et al [45], who also performed chronic treatment with warfarin and vitamin K. Krishnan et al [43] analyzed the Stroke Prevention in Non-Rheumatic Atrial Fibrillation (SPINAF) trial, which included 525 subjects, and they found a significant elevation in the pulse pressure of warfarin-treated patients with hypertension. However, no significant changes in BP were seen by these [42], [43] or other authors [46] in patients receiving long-term warfarin treatment (2–3 years). This discrepancy between our data and previously published data may be explained by the duration of the treatment (acute vs. chronic) and by possible adjustment of antihypertensive drugs for better BP control in the patients.
Based on our data, the hypertensive effect of warfarin does not change with CKD progression. Indeed, the BP increase from baseline was similar in sham-operated rats and in 5/6 nephrectomy rats at different stages of CKD progression [41]. These data suggest that CKD patients may not be at a higher risk for hypertension associated with warfarin.
Considerations for a renal pathologist to suspect WRN in a kidney biopsy
There is a challenge for a renal pathologist to recognize WRN in kidney biopsy. Renal pathologists often do not recognize WRN because of an underlying kidney disease. Acute tubular injury and RBC casts are usually associated with those conditions. In our first description of WRN, a variety of underlying kidney disease was found (Table 1) [4].
Table 1.
Demographics, laboratory data, and morphologic findings in patients with warfarin-related nephropathy (WRN)⁎
| Patient | Age | Gender | Race | Maximal INR (IU) | SCr change from BL (mg/dL) | Urinalysis | ATN | RBC casts (%) | Immunofluorescence | GBM thickness (nm)† | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | F | AA | 8.0 | 2.5 | 3+ hematuria, 1+ proteinuria | 1+ | 2.8 | 1+ mesangial IgG, IgM, C1q, C3 | 560±120 | Renal function recovery |
| 2 | 76 | F | W | 7.0 | 2.5 | 2+ hematuria | 3+ | 20.9 | Nonspecific | 350±63 | Dialysis |
| 3 | 61 | M | W | 2.0 | 1.8 | 2+ hematuria, 3+ proteinuria | 2+ | 4.4 | Nonspecific | 357±101 | Dialysis, expired |
| 4 | 80 | M | W | 5.2 | 2.6 | 2+ hematuria, 2+ proteinuria | 3+ | 16.8 | 1+ mesangial IgA | 429±85 | Dialysis |
| 5 | 38 | F | W | 3.9 | 1.3 | 1+ hematuria, 1+ proteinuria | 1+ | 2.3 | 1+ mesangial IgA | 277±73 | Renal function recovery |
| 6 | 63 | M | W | 3.7 | 1.5 | 2+ hematuria, RBC casts | 1+ | 16.3 | Nonspecific | 430±99 | Partial renal function recovery |
| 7 | 82 | F | W | 2.8 | 3.4 | 1+ hematuria | 2+ to 3+ | 17.8 | 1+ mesangial IgA | 289±76 | Renal function recovery |
| 8 | 73 | M | W | 3.0 | 3.9 | 2+ hematuria, 3+ proteinuria | 3+ | 10.9 | Nonspecific | 310±89 | Dialysis |
| 9 | 55 | M | W | 3.8 | 8.3 | 1+ hematuria, 1+ proteinuria | 3+ | 11.4 | 1+ mesangial IgG, C1q, C3 | 343±148 | Renal function recovery |
†The mean GBM thickness established in our laboratory for males was 373±56 nm and for females was 351±40 nm.
AA, African American; ATN, acute tubular necrosis; BL, baseline; C, complement; GBM, glomerular basement membrane; Ig, immunoglobulin; INR, international normalized ratio; IU, international units; RBC, red blood cell; SCr, serum creatinine; W, white.
Morphological findings were scored semiquantitatively using the following criteria: 0, absent; 1+, mild; 2+, moderate; 3+, prominent. If changes were minimal but not absent, the score of +/− was applied.
No guidelines have been established yet to diagnose WRN. Here are two recent examples of WRN diagnosis from our practice.
Case 1
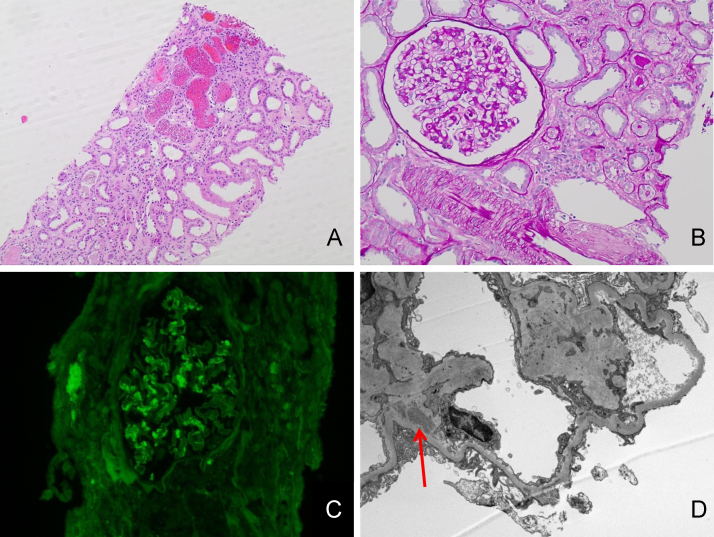
A 61-year-old Caucasian female with recently diagnosed diabetes mellitus. Her baseline serum creatinine was normal. She presented to hospital following episodes of diarrhea with serum creatinine of 3.2 mg/dL. She developed deep vein thrombosis and she was started on warfarin therapy. Her INR was as high as 5. Her serum creatinine increased up to 6.6 mg/dL within 2 weeks after initiation of warfarin therapy. Serologies showed positive antinuclear antibody (ANA; 1:640), but her complement levels were normal. Kidney biopsy findings by light microscopy included numerous RBC casts and acute tubular necrosis (Fig. 1A). The glomeruli were unremarkable (Fig. 1B). Immunofluorescence showed mild smudgy staining for immunoglobulin G (IgG) (Fig. 1C). Electron microscopy showed scattered small electron dense immune-type complex deposits (Fig. 1D). There was a disproportion between the number of RBC casts, the degree of acute tubular necrosis, and relatively small immune complex deposits in the absence of proliferative glomerular lesions or cellular crescents.
Case 2
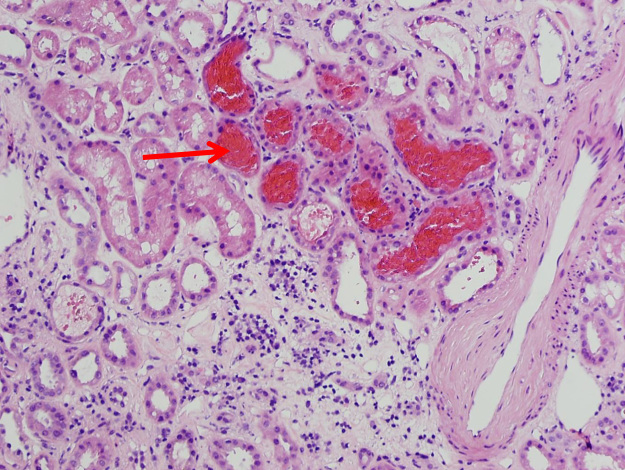
A 41-year-old Caucasian female with history of aortic bifurcation thrombosis. She underwent a graft placement and started on warfarin therapy. Her INR was as high as 27. Her baseline serum creatinine was 1.0 mg/dL, but this increased to 6.7 mg/dL shortly after the high INR. Urinalysis showed hematuria with RBC casts. Her serologies [ANA; antineutrophil cytoplasmic antibodies (ANCA)] were negative and her complement levels were normal. In a kidney biopsy, immunofluorescence did not show positive staining. Electron microscopy showed normal GBM thickness. However, by light microscopy there were numerous tubular RBC casts and acute tubular necrosis (Fig. 2).
Figure 1.
Light microscopy, immunofluorescence, and electron microscopy in a patient with suspicious warfarin-related nephropathy (WRN) (Case 1). (A) Light microscopy shows numerous red blood cell (RBC) casts in the tubules and acute tubular necrosis. Hematoxylin and Eosin, magnification 40×. (B) Glomeruli are unremarkable by light microscopy. Periodic acid–Schiff stain, magnification 200×. (C) Direct immunofluorescence with an antibody to IgG shows mild mesangial staining. Magnification 200×. (D) Electron microscopy shows scattered mesangial electron-dense immune-type deposits (arrow). Magnification 10,000×.
Figur 2.
Light microscopy in a patient with suspicious warfarin-related nephropathy (WRN) (Case 2). Light microscopy shows numerous red blood cell (RBC) casts in the tubules and acute tubular necrosis. Hematoxylin and Eosin, magnification 200×.
It is not clear whether other anticoagulants, including new oral drugs, can induce AKI. A case of dabigatran-induced AKI has been reported [6]. Our own observations include a kidney biopsy from a patient on heparin therapy, where we have seen numerous RBC casts and acute tubular necrosis as well. We believe that anticoagulant-related kidney injury should be suspected in a patient on anticoagulation therapy, if there is a disproportion between the number of RBC tubular casts, acute tubular necrosis, and the degree of an underlying kidney lesion (such as glomerular immune complex depositions, GBM thickness abnormalities, etc.) in kidney biopsy. Detailed evaluation of coagulation data and medications is recommended for all patients with RBC casts and AKI.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgments
The author expresses his gratitude to Dr Hebert and Dr Rovin, Division of Nephrology, Ohio State University Wexner Medical Center for their help and consultations in patients with WRN.
References
- 1.Nutescu EA, Wittkowsky AK, Burnett A, Merli GJ, Ansell JE, Garcia DA. Delivery of optimized inpatient anticoagulation therapy: consensus statement from the anticoagulation forum. Ann Pharmacother. 2013;47:714–724. doi: 10.1345/aph.1R634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, Wu H, Bhatt U, Nadasdy T, Hebert LA. Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115:c142–146. doi: 10.1159/000312877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–189. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54:1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 5.An JN, Ahn SY, Yoon CH, Youn TJ, Han MK, Kim S, Chin HJ, Na KY, Chae DW. The occurrence of warfarin-related nephropathy and effects on renal and patient outcomes in korean patients. PLoS One. 2013;8:e57661. doi: 10.1371/journal.pone.0057661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadiyala D, Brewster UC, Moeckel GW: Dabigatran induced acute kidney injury. In: The American Society of Nephrology Annual Meeting, November 1–4, 2012, San Diego, CA, FR-PO1122, 2012
- 7.Ryan M, Ware K, Qamri Z, Satoskar A, Wu H, Nadasdy G, Rovin B, Hebert L, Nadasdy T, Brodsky SV. Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant. 2013 Sep 5 doi: 10.1093/ndt/gft380. Available at: 10.1093/ndt/gft380. (Epub) 2013 Sep 5. [DOI] [PubMed] [Google Scholar]
- 8.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, Locatelli F, MacLeod A, Vanholder R, Walker R, Wang H. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–1314. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang QL, Koenig W, Raum E, Stegmaier C, Brenner H, Rothenbacher D. Epidemiology of chronic kidney disease: results from a population of older adults in Germany. Prev Med. 2009;48:122–127. doi: 10.1016/j.ypmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, Lerma EV. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:173–181. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kooiman J, van de Peppel WR, van der Meer FJ, Huisman MV. Incidence of chronic kidney disease in patients with atrial fibrillation and its relevance for prescribing new oral antithrombotic drugs. J Thromb Haemost. 2011;9:1652–1653. doi: 10.1111/j.1538-7836.2011.04347.x. [DOI] [PubMed] [Google Scholar]
- 13.Kleinow ME, Garwood CL, Clemente JL, Whittaker P. Effect of chronic kidney disease on warfarin management in a pharmacist-managed anticoagulation clinic. J Manag Care Pharm. 2011;17:523–530. doi: 10.18553/jmcp.2011.17.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limdi NA, Beasley TM, Baird MF, Goldstein JA, McGwin G, Arnett DK, Acton RT, Allon M. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol. 2009;20:912–921. doi: 10.1681/ASN.2008070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapoor KG, Bekaii-Saab T. Warfarin-induced allergic interstitial nephritis and leucocytoclastic vasculitis. Intern Med J. 2008;38:281–283. doi: 10.1111/j.1445-5994.2008.01646.x. [DOI] [PubMed] [Google Scholar]
- 17.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414–1419. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 18.Abt AB, Carroll LE, Mohler JH. Thin basement membrane disease and acute renal failure secondary to gross hematuria and tubular necrosis. Am J Kidney Dis. 2000;35:533–536. doi: 10.1016/s0272-6386(00)70209-5. [DOI] [PubMed] [Google Scholar]
- 19.Kabir A, Nadasdy T, Nadasdy G, Hebert LA. An unusual cause of gross hematuria and transient ARF in an SLE patient with warfarin coagulopathy. Am J Kidney Dis. 2004;43:757–760. doi: 10.1053/j.ajkd.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Spetie DN, Nadasdy T, Nadasdy G, Agarwal G, Mauer M, Agarwal AK, Khabiri H, Nagaraja HN, Nahman NS, Jr, Hartman JA, Hebert LA. Proposed pathogenesis of idiopathic loin pain-hematuria syndrome. Am J Kidney Dis. 2006;47:419–427. doi: 10.1053/j.ajkd.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Ozcan A, Ware K, Calomeni E, Nadasdy T, Forbes R, Satoskar AA, Nadasdy G, Rovin BH, Hebert LA, Brodsky SV. 5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. Am J Nephrol. 2012;35:356–364. doi: 10.1159/000337918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware K, Brodsky P, Satoskar AA, Nadasdy T, Nadasdy G, Wu H, Rovin BH, Bhatt U, Von Visger J, Hebert LA, Brodsky SV. Warfarin-related nephropathy modeled by nephron reduction and excessive anticoagulation. J Am Soc Nephrol. 2011;22:1856–1862. doi: 10.1681/ASN.2010101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim AK, Campbell DA. Haematuria and acute kidney injury in elderly patients admitted to hospital with supratherapeutic warfarin anticoagulation. Int Urol Nephrol. 2013;45:561–570. doi: 10.1007/s11255-012-0364-0. [DOI] [PubMed] [Google Scholar]
- 24.Berger R, Salhanick SD, Chase M, Ganetsky M. Hemorrhagic complications in emergency department patients who are receiving dabigatran compared with warfarin. Ann Emerg Med. 2013;61:475–479. doi: 10.1016/j.annemergmed.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Nishio S, Tsuboi N, Kurashige M, Tanaka M, Ueda H, Yokoo T, Miyazaki Y, Utsunomiya Y, Hosoya T. A case of acute kidney injury during warfarin therapy. Nihon Jinzo Gakkai Shi. 2013;55:966–971. [PubMed] [Google Scholar]
- 26.August C, Atzeni A, Koster L, Heidenreich S, Lang D. Acute renal failure in IgA nephropathy: aggravation by gross hematuria due to anticoagulant treatment. J Nephrol. 2002;15:709–712. [PubMed] [Google Scholar]
- 27.Ware K, Qamri Z, Ozcan A, Satoskar AA, Nadasdy G, Rovin BH, Hebert LA, Nadasdy T, Brodsky SV. N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am J Physiol Renal Physiol. 2013;304:F1421–F1427. doi: 10.1152/ajprenal.00689.2012. [DOI] [PubMed] [Google Scholar]
- 28.Patel RP, Svistunenko DA, Darley-Usmar VM, Symons MC, Wilson MT. Redox cycling of human methaemoglobin by H2O2 yields persistent ferryl iron and protein based radicals. Free Radic Res. 1996;25:117–123. doi: 10.3109/10715769609149916. [DOI] [PubMed] [Google Scholar]
- 29.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–420. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 30.Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69:1385–1392. doi: 10.1038/sj.ki.5000315. [DOI] [PubMed] [Google Scholar]
- 31.Tsiftsoglou AS, Tsamadou AI, Papadopoulou LC. Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol Ther. 2006;111:327–345. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86:298–307. [PubMed] [Google Scholar]
- 34.Carlile-Klusacek M, Rizzo V. Endothelial cytoskeletal reorganization in response to PAR1 stimulation is mediated by membrane rafts but not caveolae. Am J Physiol Heart Circ Physiol. 2007;293:H366–H375. doi: 10.1152/ajpheart.01044.2006. [DOI] [PubMed] [Google Scholar]
- 35.Rondeau E, Vigneau C, Berrou J. Role of thrombin receptors in the kidney: lessons from PAR1 knock-out mice. Nephrol Dial Transplant. 2001;16:1529–1531. doi: 10.1093/ndt/16.8.1529. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Zacharias U, Peraldi MN, He CJ, Lu C, Sraer JD, Brass LF, Rondeau E. Constitutive expression and modulation of the functional thrombin receptor in the human kidney. Am J Pathol. 1995;146:101–110. [PMC free article] [PubMed] [Google Scholar]
- 37.Favaloro EJ, Lippi G. Laboratory testing and/or monitoring of the new oral anticoagulants/antithrombotics: for and against? Clin Chem Lab Med. 2011;49:755–757. doi: 10.1515/CCLM.2011.126. [DOI] [PubMed] [Google Scholar]
- 38.Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–295. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
- 39.Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. Effects of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate, on thrombus formation and bleeding time in rats. Thromb Haemost. 2007;98:333–338. [PubMed] [Google Scholar]
- 40.Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98:155–162. [PubMed] [Google Scholar]
- 41.Ware KM, Vance JC, Muni N, Hebert LA, Satoskar AA, Nadasdy G, Ivanov I, Nadasdy T, Rovin BH, Brodsky SV. Oral warfarin and the thrombin inhibitor dabigatran increase blood pressure in rats: hidden danger of anticoagulants? Am J Hypertens. 2014 doi: 10.1093/ajh/hpu129. , 10.1093/ajh/hpu129, 2014, in press. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, Wan J, Yang Q, Qi B, Peng W, Chen X. Effects of atorvastatin on warfarin-induced aortic medial calcification and systolic blood pressure in rats. J Huazhong Univ Sci Technolog Med Sci. 2008;28:535–538. doi: 10.1007/s11596-008-0510-1. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan S, Chawla N, Ezekowitz MD, Peixoto AJ. Warfarin therapy and systolic hypertension in men with atrial fibrillation. Am J Hypertens. 2005;18:1592–1599. doi: 10.1016/j.amjhyper.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Dao HH, Essalihi R, Graillon JF, Lariviere R, De Champlain J, Moreau P. Pharmacological prevention and regression of arterial remodeling in a rat model of isolated systolic hypertension. J Hypertens. 2002;20:1597–1606. doi: 10.1097/00004872-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 45.Essalihi R, Dao HH, Yamaguchi N, Moreau P. A new model of isolated systolic hypertension induced by chronic warfarin and vitamin K1 treatment. Am J Hypertens. 2003;16:103–110. doi: 10.1016/s0895-7061(02)03204-1. [DOI] [PubMed] [Google Scholar]
- 46.Lim MA, Shafique S, See SY, Khan FN, Parikh CR, Peixoto AJ. Effects of warfarin on blood pressure in men with diabetes and hypertension—a longitudinal study. J Clin Hypertens (Greenwich) 2007;9:256–258. doi: 10.1111/j.1524-6175.2007.06383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]