Abstract
A 68-year-old man was introduced to our hospital for the treatment of lung and mediastinum lymph node metastases that originated from an urachal carcinoma 4 years after a partial cystectomy. First-line chemotherapy with an S-1 and cisplatin combination was ineffective. The patient received FOLFIRI plus bevacizumab chemotherapy as salvage chemotherapy. Stability was achieved after eight cycles of FOLFIRI plus bevacizumab therapy. We conducted a biopsy of the metastatic tumor, and the pathology of the biopsy tissue was partially necrotic. To our knowledge, this case represents the first report of a metastatic urachal carcinoma treated with FOLFIRI plus bevacizumab.
Keywords: Urachal carcinoma, Chemotherapy, FOLFIRI, Bevacizumab
Introduction
To date, no randomized trials and fewer than 300 cases of urachal carcinomas have been reported in the literature. As a result, limited information exists regarding the effective management of these cancers, especially knowledge concerning the most effective chemotherapeutic agents against atypical bladder cancers with histological and biological features similar to those of bowel cancers. Most urachal carcinomas are mucin-producing enteric type adenocarcinomas that occasionally may exhibit signet ring cells morphology.1 The prognosis of patients with recurrent urachal carcinoma is extremely poor. We report a case of metastatic urachal carcinoma in which the immunohistochemical profile's similarities to that of colon cancer led to treatment with FOLFIRI plus bevacizumab, which is a specific first-line chemotherapy of colon cancer.
Case report
A 68-year-old man was introduced to our hospital for the treatment of lung and mediastinum lymph node metastases originating from an urachal carcinoma. He had undergone a partial cystectomy for this urachal carcinoma 4 years ago. After no evidence of recurrence for 2 years, he ceased follow-up examinations. The patient then presented dyspnea, and computed tomography (CT) revealed multiple lung tumors and an anterior mediastinum mass (Fig. 1), which was diagnosed as metastases of urachal carcinoma by thoracoscopic biopsy. Colonoscopy denied primary colon cancer and the serum levels of carcinogen embryonic antigen and carbohydrate antigen 19-9 were within normal ranges. This pathology revealed a mucinous adenocarcinoma with a pathology similar to that of the primary urachal carcinoma (Fig. 2(a and b)). On immunohistochemical staining, the tumor was positive for cytokeratin-20, cytoleratin-7, and caudal–type homeobox transcription factor 2. However, the tumor was negative for thyroid transcription factor-1 and napsin A. From the results of immunohistochemical staining, the patient was diagnosed with a recurrence of urachal carcinoma.
Figure 1.
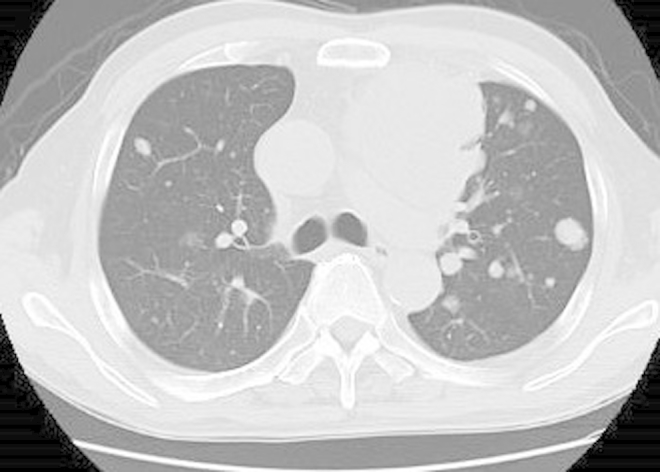
Chest computed tomography conducted before chemotherapy shows multiple lung tumors and the anterior mediastinum mass.
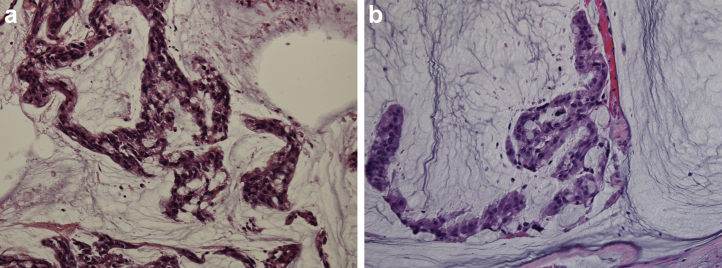
Figure 2.
(a) Pathology of primary urachal carcinoma stained with hematoxylin and eosin (magnification ×400). (b) Pathology of a thoracoscopic biopsy of the lung tumor stained with hematoxylin and eosin (magnification ×400).
The patient was treated with S-1 and cisplatin combination (S-1/CDDP) chemotherapy (S-1 was administered orally at a dose of 120 mg/day for 14 days, and CDDP was infused at a dose of 120 mg on day 8 of each 28-day cycle) as a first-line chemotherapy. After three cycles of S-1/CDDP, CT revealed an increase of lung metastases, and we assessed progressive disease (PD). We changed the treatment regimen to FOLFIRI + bevacizumab as a salvage chemotherapy (bevacizumab 5 mg/kg, irinotecan 180 mg/m2, l-leucovorin 200 mg/m2, fluorouracil 400 mg/m2, and bolus 2400 mg/m2/46 h of each 14 days cycle) under careful informed consent and the approval of the institutional review board of Osaka City University. Radiographic evaluation, performed every two cycles, revealed stable disease (SD) in the lung and anterior mediastinum metastases. After eight cycles, the lung and anterior mediastinum metastases remained stable, and we performed a biopsy of the anterior mediastinum metastases to evaluate viable tumor cells. The pathological findings showed a mix of viable cells and necrotic tissue with a large amount of mucin, and we thus assessed the chemotherapy as partially effective. However, we had to give the patient a washout period because of general fatigue (grade 2, CTCAE v4.0) and severe diarrhea (grade 3, CTCAE v4.0) despite using the maximum doses of loperamide hydrochloride and hangeshashinto (TJ-14, a Kampo medicine), which reduces the level of prostaglandin E2 and affects cyclooxygenase activity, thereby alleviating chemotherapy-induced oral mucositis.2 After 6 months, CT revealed increased lung metastasis and we recommended further chemotherapy, but the patient declined this suggestion because of the severe diarrhea caused by irinotecan and decided to undergo palliative care. After 8 months, he died of further disease progression. The second-line chemotherapy regimen of FOLFIRI plus bevacizumab led to 12 months of progression-free survival and 20 months of overall survival after the failure of first-line chemotherapy.
Discussion
To date, no recommended chemotherapy regimen has been established for metastasis or unresectable urachal carcinoma. We choose S-1/CDDP for first-line chemotherapy in this case because S-1 is an oral 5-FU derivative consisting of tegafur, gimeracil, and oteracil. The clinical effect of 5-FU is known to be enhanced by combination with CDDP, and effective response rates for S-1/CDDP combination chemotherapy have been reported for esophageal, gastric, and lung cancer; two cases have shown good responses to S-1/CDDP chemotherapy for urachal carcinoma.3 Unfortunately, S-1/CDDP chemotherapy led to no positive response in the present case.
Recently, several cases of urachal carcinoma chemotherapy based on irinotecan or oxaliplatin have been reported; these treatments were employed because urachal adenocarcinomas are often histologically similar to adenocarcinomas from the gastrointestinal tract. Irinotecan is a topoisomerase 1 inhibitor that disrupts cell division by interfering with DNA replication and has demonstrated preclinical activity in adenocarcinomas from a variety of tumor types, including gastric, colorectal, pancreatic, lung, and breast carcinomas. Currently, FOLFIRI (irinotecan in combination with 5-FU/leucovorin) with or without bevacizumab is indicated as a first-line chemotherapy for metastatic colon cancer,4 and FOLFOX (oxaliplatin, leucovorin, and fluorouracil) with or without bevacizumab is another first-line chemotherapy for metastatic colon cancer. The efficacy of FOLFOX against urachal carcinoma has recently been reported in several case reports.5 Because we used platinum-based chemotherapy as the first-line treatment in this case, we choose FOLFIRI rather than FOLFOX for second-line chemotherapy. Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the biological activity of the human vascular endothelial growth factor (VEGF). The NCCN Guidelines for colon cancer recommend that bevacizumab should be added to FOLFIRI or FOLFOX for advanced or metastatic colon cancer.4 We therefore chose to add bevacizumab to FOLFIRI in this case.
Unfortunately, we were unable to perform a pathological autopsy, but the biopsy tissue from the anterior mediastinum metastases after eight cycles of FOLFIRI plus bevacizumab chemotherapy revealed a mix of viable cells and necrotic tissue, with a large amount of mucin. Therefore we conclude that FOLFIRI plus bevacizumab led to 12 months of progression-free survival and 20 months of overall survival after the failure of first-line chemotherapy. This case represents the first report of metastatic urachal carcinoma treated with FOLFIRI plus bevacizumab, indicating its potential as a second-line chemotherapy for this disease.
Conflict of interest
None declared.
References
- 1.Gopalan A., Sharp D.S., Fine S.W. Urachal carcinoma: A clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol. 2009;33:659. doi: 10.1097/PAS.0b013e31819aa4ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori K., Kondo T., Kamiyama Y. Preventive effect of Kampo medicine (Hangeshashin-to) against irinotecan-induced diarrhea in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51:403. doi: 10.1007/s00280-003-0585-0. [DOI] [PubMed] [Google Scholar]
- 3.Kojima Y., Yamada Y., Kamisawa H. Complete response of a recurrent advanced urachal carcinoma treated by S-1/cisplatin combination chemotherapy. Int J Urol. 2006;13:1123. doi: 10.1111/j.1442-2042.2006.01487.x. [DOI] [PubMed] [Google Scholar]
- 4.Colon Cancer . 2014. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) 2. [Google Scholar]
- 5.Tran B., McKendrick J. Metastatic urachal cancer responding to FOLFOX chemotherapy. Can J Urol. 2010;17:5120. [PubMed] [Google Scholar]