Abstract
Medullary carcinoma is a rare malignant tumor of the kidney. It affects individuals of African descent and all cases reported show evidence of sickle cell trait. We reviewed an unusual carcinoma arising in a white man, the ninth in the literature. The tumor demonstrated features associated with renal medullary carcinoma, or unclassified renal cell carcinoma, medullary phenotype as recently described; the presence of sickle cell trait confirmed the diagnosis of medullary carcinoma. This case is helpful in the differential diagnosis with non-sickle cell associated “renal cell carcinoma, unclassified with medullary phenotype,” and study of this spectrum of tumors is ongoing.
Keywords: Kidney, Medullary carcinoma, Unclassified renal cell carcinoma, Sickle cell trait
Introduction
Renal medullary carcinoma (RMC) is a rare aggressive subtype of renal tumor, with 182 cases reported in the English literature,1, 2, 3, 4, 5 originating from the medulla of the kidney and associated with sickle cell trait and disease.1 The great majority of patients reported have been African-Americans. Herein we describe the clinicopathologic features of an RMC arising in a white man with sickle cell trait. RMC has never been reported in Italy, highlighting the degree of clinical suspicion necessary to identify such a case.
Case presentation
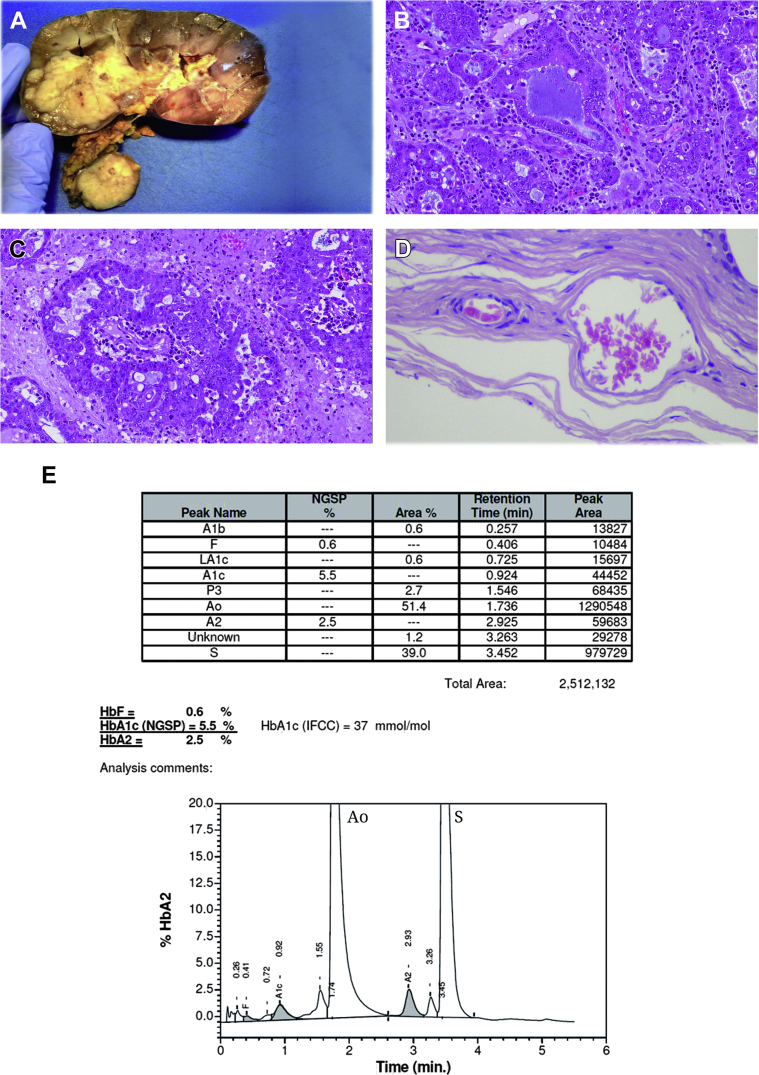
A 23-year old white male presented with left-sided loin pain, without hematuria. Ultrasound examination showed a renal mass. Abdominal computed tomography (CT) scan confirmed the sonographic findings demonstrating a 42 mm mass in the upper pole of the left kidney, with enlargement of regional lymph-nodes. The histology of CT-guided needle biopsy revealed necrotic tissue and only a focus of viable neoplastic proliferation of atypical epithelial cells, primarily compatible to renal cell carcinoma, not otherwise specified. Subsequently patient underwent to a staging full body CT, which revealed multiple bilateral lung metastases. Radical nephrectomy with regional lymphadenectomy was performed. At gross examination, the specimen revealed a yellowish-white mass in the upper renal pole, 5.5 cm in diameter, with invasion of both perirenal and renal sinus fat (Fig. 1A).
Figure 1.
Gross and microscopic characteristics of the tumor and sickle cell status (A–E). A, Note that the tumor is located in the cortico-medullary region, with lymph node hilar metastasis. B and C, Glandular differentiation and desmoplastic stroma with inflammatory cells in the medullary carcinoma. D, Drepanocytes identified between and at the periphery of the carcinoma. E, Electrophoretic analysis documented high level of mutated Hemoglobin (S).
Histologically, the tumor showed proliferation of epithelioid cells, arranged in tubular and cribriform structures, in desmoplastic and myxoid stroma (Fig. 1B). There were multiple foci of necrosis (∼40% of the tumor), and a rich acute inflammatory infiltrate (Fig. 1C). There was also a massive metastasis in one hilar lymph node. Drepanocytes (sickle forms) were histologically noted, and taken with the tumor morphologic characteristics, tests were ordered to screen for hemoglobinopathies (Fig. 1D). A peripheral blood hemoglobin electrophoresis, performed in the Clinical Analysis Laboratory, uncovered a sickle cell trait, confirming the histological suspicion (Fig. 1E).
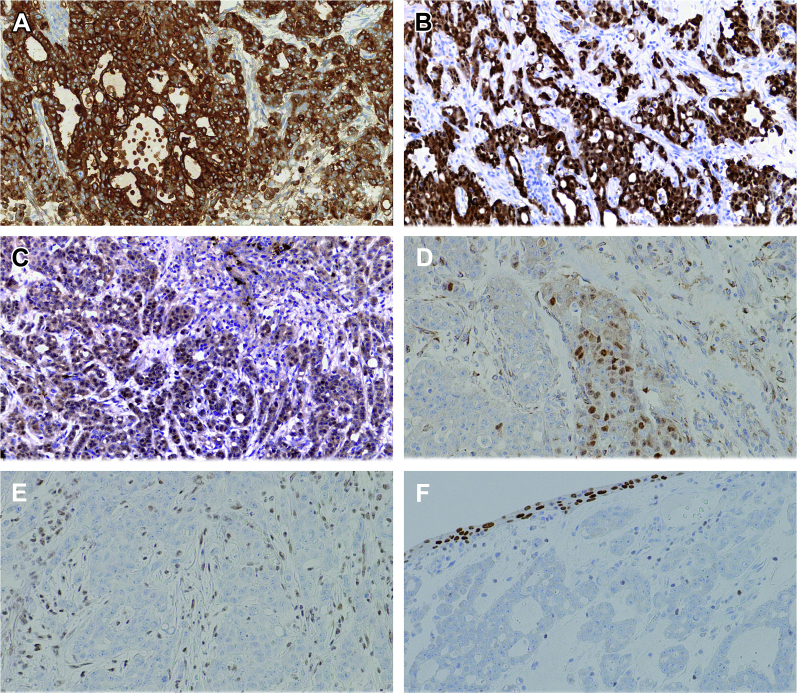
Given the rarity of this tumor in Caucasians, extensive immunohistochemical studies were performed, showing reactivity for cytokeratins, polyclonal CEA, PAX8, PAX2, AMACR, S100A1, and OCT3/4. An immunostain for the chromatin-modifying protein SMARCB1 (also known as INI-1) was negative (Fig. 2). In the light of these findings taken together, a diagnosis of RMC was made.
Figure 2.
Immunohistochemical status in medullary carcinoma (A–F). Tumor cells are diffusely positive for CK AE1/AE3 (A), S100A1 (B), PAX2 (C), and focally positive for OCT3/4 (D). No expression for INI1 (E) and GATA3 (F) is evident.
Disease progressed, under treatment with Sunitinib and Sorafenib, and the patient died at 10 months of follow-up with multiple pulmonary metastases.
Discussion
Since the original studies by Davis et al, 182 cases of renal RMC have been reported, and the great majority in African-American patients, with only 5% described in Caucasians.1, 2, 3, 4, 5 The exceptional nature of the case presented concerns the tumor arising in a white male without apparent genealogic link to African or American populations, as well as the unusual laterality of disease, in the left kidney. To the best of our knowledge, this is the first Italian case of RMC reported in the English literature.
RMC usually presents with hematuria, pain, weight loss, and fever; our patient had left-sided loin pain, without hematuria, confirming that RMC bleeding is more typical for right sided tumors.1 Though CT scan is the imaging modality of choice for RMC, both ultrasound and abdominal CT scan performed identified the mass. Importantly, when a core biopsy was performed, only necrotic material with a focus of neoplastic tissue was obtained, limiting histological diagnosis to renal cell carcinoma without the possibility of further classification.
The mean RMC size is 7 cm (1.8 to 13).1, 2 The most consistent histological pattern described in the literature includes anastomosing tubules and cords forming microcystic structures with reticular appearance; less frequently with cribriform, solid, micropapillary, and rhabdoid morphologies, associated with a desmoplastic stromal reaction. A brisk inflammatory component is often seen, and intraluminal mucin is frequently documented (∼70% of cases). The overall most prevalent tumor that can simulate RMC is high grade urothelial carcinoma of the pelvicalyceal mucosa with glandular differentiation. Fortunately, urothelial carcinoma is often associated to dysplastic change, or carcinoma in situ (CIS) in the residual adjacent mucosa. The expression of SMARCB1 is quite helpful to support diagnosis of urothelial carcinoma. Similarly, metastatic deposits of adenocarcinoma in the renal parenchyma from other organs may also be quite challenging in the differential diagnosis. Reviewing the literature, a principal area of concern has been the overlapping clinicopathologic features between RMC and collecting duct carcinoma (CDC), given that both demonstrate male predominance, aggressive clinical course with high stage at presentation, prevalent right-sided disease, and intratumoral desmoplastic and inflammatory reactions.2 RMC has been considered distinct from CDC in that it is documented in a younger population than CDC (mean age 19 years versus 50 years in CDC) with a male to female ratio of 2:1; furthermore, all patients have sickle cell trait or disease (0% in CDC) and more than 90% are African-Americans (10% in CDC).1
Recently, absence of SMARCB1/INI-1 protein expression has been demonstrated in RMC by immunohistochemistry and loss of heterozygosity of the SMARCB1 gene by microsatellite analysis.4 SMARCB1 is a tumor suppressor gene located on chromosome 22, involved in cell cycle control and regulation of the cytoskeleton. Immunohistochemistry of the current case showed absence of nuclear staining of SMARCB1 (Fig. 2). Recently, some authors have documented that OCT3/4, a sensitive and specific marker for germ-cell tumors, was expressed in 10/14 RMC analyzed, as in our case, underlining the potential diagnostic pitfall with using it, particularly when a clear history of sickle cell trait is not available.
This particular conundrum, the CDC versus RMC differential, has been the subject of a recent editorial, written by two of the authors (MBA, SCS) in consultation with an international panel of kidney experts.5 With increasing use of allegedly “specific” markers such as SMARCB1 and OCT3/4, cases have been identified that show convincing features of medullary carcinoma in individuals where sickle cell trait has been rigorously excluded. The term “renal cell carcinoma, unclassified, with medullary phenotype” (RCCU-MP) has been proposed to provide terminology for such cases, with the recommendation that the criteria used to make that designation and clinical data used in exclusion of sickle cell trait (and therefore diagnosis of RMC) be recorded in the pathologist's comment.
Conclusion
We emphasize to consider a broad differential diagnosis with careful clinical and laboratory correlation to secure the correct diagnosis even in the rarest of circumstances. We hence recommend availing of the latest published literature in this rapidly evolving area of kidney pathology, hoping that emerging technologies and understanding will make a better diagnosis, classification, and treatment. Additional study is needed to understand the relationship between CDC, RMC and RCCU-MP and to characterize, and hopefully identify targets within, the molecular circuitry leading to the pathogenesis of these diseases.
Conflicts of interst
The authors declare that there are no conflicts of interest.
References
- 1.Davis C.J., Jr., Mostofi F.K., Sesterhenn I.A. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol. 1995;19:1–11. doi: 10.1097/00000478-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Srigley J.R., Moch H. Carcinoma of the collecting ducts of Bellini. In: Eble J.N., Sauter G., Epstein J.I., editors. World Health Organization Classification of Tumors Pathology & Genetics: Tumors of the Urinary System and Male Genital Organs. IARC; Lyons: 2004. pp. 33–34. [Google Scholar]
- 3.Rao P., Tannir N.M., Tamboli P. Expression of OCT3/4 in renal medullary carcinoma represents a potential diagnostic pitfall. Am J Surg Pathol. 2012;36:583–588. doi: 10.1097/PAS.0b013e3182417d78. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q., Galli S., Srinivasan R. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol. 2013;37:368–374. doi: 10.1097/PAS.0b013e3182770406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin M.B., Smith S.C., Agaimy A. Collecting duct carcinoma versus renal medullary carcinoma: an appeal for nosologic and biological clarity. Am J Surg Pathol. 2014;38:871–874. doi: 10.1097/PAS.0000000000000222. [DOI] [PubMed] [Google Scholar]