Abstract
Little is known about mtDNA replication during oocyte maturation and its regulation by extracellular factors. The present study determined the effects of supplementation of maturation medium with sus scrofa follicular fluid (pFF, 0, 10, 20, and 30%) on mtDNA copy number and oocyte maturation in Experiment 1; the effects on EGF (10 ng/mL), neuregulin 1 (NRG1, 20 ng/mL) and NRG1+IGF1(insulin-like growth factor 1, 100 ng/mL + NRG1 20 ng/mL), on mtDNA copy number, oocyte maturation, and embryo development after parthenogenic activation in Experiment 2, and effects on embryo development after in vitro fertilization in Experiment 3. Overall, mtDNA copy number increased from GV to MII stage oocytes after in vitro maturation (GV: 167,634.6 ± 20,740.4 vs MII: 275,131.9 ± 9,758.4 in Experiment 1, P<0.05; GV: 185,004.7 ± 20,089.3 vs MII: 239,392.8 ± 10,345.3 in Experiment 2, P<0.05). Supplementation of IVM medium with pFF inhibited mtDNA replication (266,789.9 ± 11,790.4 vs 318,510.1 ± 20,377.4, P<0.05) and oocyte meiotic maturation (67.3 ± 0.7% vs 73.2 ± 1.2, for the pFF supplemented and zero pFF control, respectively, P<0.01). Compared to the control, addition of growth factors enhanced oocyte maturation. Furthermore, supplementation of NRG1 stimulated mitochondrial replication, resulted in more mtDNA copies in MII oocytes than GV oocytes, and increased percentage of blastocysts in both parthenogenetic and in vitro fertilized embryos. This study demonstrated that mitochondrial biogenesis in oocytes is stimulated during in vitro maturation. Oocyte mtDNA copy number was associated with its developmental competence. Supplementation of maturation medium with NRG1 increased mtDNA copy number, and thus provides a means to improve oocyte quality and developmental competence in pigs.
Keywords: Oocyte, Mitochondrial DNA, Follicular fluid, EGF, Neuregulin 1
1. Introduction
In the pig, the mitochondrial genome (mtDNA) is 16.6 kb in size [1] and encodes 13 subunits of the electron transfer chain that are essential for cellular energy production [2], with the number of copies being directly proportional to the amount of ATP synthesized [2,3]. The mammalian oocyte typically contains about 100,000 mitochondria that can occupy up to 30% of the cytoplasmic space [4]. During oocyte growth within follicles and the early stages of maturation, oocytes are coupled to cumulus cells which provide metabolic support through the provision of ATP and pyruvate [5,6]. After ovulation, mammalian oocytes become uncoupled from cumulus cells and they rely on their own mitochondria to generate ATP [5,7]. From ovulation until the morula stage, the embryo relies on mitochondrial oxidative phosphorylation to supply most of the ATP demands for development [7,8].
However, during preimplantation development, mtDNA replication is limited, which on a per cell basis results in a progressive reduction in mtDNA content in cleaving blastomeres. Individual blastomeres in later-staged embryos, therefore, contain fewer mtDNA genomes than in earlier stages. The lack of mtDNA replication during early embryo development suggests that mtDNA copy number needs to be amplified to sufficient levels prior to fertilization. Studies have shown that there is an increase in ATP during maturation in mouse [9], cattle [10], and pig [11], and this ATP increase is correlated with the embryo development in cattle [10]. A decrease in ATP content during maturation has been suggested to lead to developmental arrest at cleavage stages in human [12]. These data suggest that the status and activity of mitochondria in the mammalian oocyte is a determining factor of oocyte quality and viability, leading to the speculation that mtDNA copy number could be used as a marker of viability. A critical threshold of approximately 100,000 copies in the MII oocyte has been proposed for the mouse [13], human [14] and pig [15].
In a specific cell type, mtDNA copy number can change in response to environmental signals including temperature, energy deprivation, and availability of nutrients and growth factors [16]. In Schwann cells, neuregulin and insulin-like-growth factor 1 (IGF 1) synergize to drive mitochondrial biogenesis, resulting in an increase of mtDNA copy number [17]. Furthermore, It has been suggested that low mtDNA content and oocyte viability may result from deficient oocyte maturation, since immature oocytes have considerably fewer numbers of mtDNA as compared to mature oocytes [18,19].
Follicular fluid within the ovarian follicle provides a unique micro-environment necessary for oocyte growth and maturation. Follicular fluid is composed of transudates from the serum through the blood-follicle barrier, but it also contains locally produced molecules such as estrogens and insulin-like growth factor 1 (IGF1) [20,21]. The reports on the effects of pFF supplementation in IVM medium are controversial. Some reported that pFF enhances meiotic maturation and blastocyst formation [22], decreases polyspermic fertilization [23], and has an anti-oxidative effect that may result in improved oocyte developmental competence [24]. On the other hand, some reports found no effects [25] or negative effects [26,27] on oocyte maturation and embryo development. However, it is not known if pFF supplementation during maturation has any effects on mtDNA copy number in oocytes.
Epidermal growth factor (EGF) has been used in IVM systems for decades and it has proved to have beneficial effects on oocyte maturation and cumulus expansion [25,28]. However, recent studies using mouse genetic models indicate that EGF-like growth factors, rather than EGF itself, accumulate in the follicular fluid at the time of ovulation. The EGF-like growth factor mRNA is regulated by luteinizing hormone, and its genes and proteins are detected in the follicle [29,30]. The EGF-like growth factors include several members such as neuregulin (NRG). They play essential roles in oocyte meiotic maturation in primates [31] and rodents [32]. But, their roles in regulation of mtDNA copy number and embryonic development in pigs are not known. We determined mtDNA copy number changes during oocyte maturation from GV to MII stage and examined the effects of supplementation of maturation medium with various concentrations of pFF (0, 10, 20, and 30%), EGF (10 ng/mL), NRG1 (20 ng/mL), and NRG1 (20 ng/mL) + IGF1 (100 ng/mL) on mtDNA copy number, oocyte meiotic maturation, and subsequent embryo developmental competence.
2. Materials and methods
2.1. Experimental design
The overall objective of this study was to determine the regulation of mtDNA copy number in the oocyte by porcine follicular fluid and extracellular growth factors (EGF, neuregulin 1, and IGF 1) and the importance of its regulation in embryo development. A series of 3 experiments were conducted.
2.1.1. Experiment 1: Effects of porcine follicular fluid (pFF) supplementation on mtDNA copy number and oocyte maturation
To study the effects of various doses of pFF supplementation in in vitro maturation (IVM) medium on mtDNA copy number and oocyte meiotic maturation, 4 concentrations of pFF at 0, 10, 20, and 30% were added in IVM medium. The IVM medium was TCM 199 (Gibco BRL, Grand Island, NY) supplemented with 0.1% polyvinylalcohol (w/v), 3.05 mM D-glucose, 0.91 mM sodium pyruvate, 10 μg/mL gentamicin, 0.57 mM cysteine, 0.5 μg/mL luteinizing hormone (from sheep pituitary), 0.5 μg/mL follicle-stimulating hormone (from porcine pituitary), and 10 ng/mL EGF [33]. The cumulus-oocyte complexes (COCs) were collected and cultured as described below. Sixteen GV stage oocytes before IVM and 16 MII oocytes with an extruded polar body after IVM from each pFF group were collected for mtDNA copy number assay. The number of MII oocytes was also recorded for determining meiotic maturation.
2.1.2. Experiment 2: Effects of epidermal growth factor (EGF), neuregulin 1(NRG1), and NRG1+IGF1 supplementation on mtDNA copy number, oocyte maturation, and parthenogenetic embryo development
Experiment 2 determined the effects of EGF, NRG1, and NRG1+IGF1 treatment during in vitro maturation on mtDNA copy number and embryo development. The basic maturation medium was the same as for Experiment 1. Four treatment groups were included: 10 ng/mL EGF, 20 ng/mL NRG1 (neuregulin-1, R&D Systems, Minneapolis, MN), NRG1 (20 ng/mL) + IGF1 (insulin-like growth factor 1, 100 ng/mL), and no growth factor supplementation as the control. For mtDNA analysis, 16 GV oocytes before IVM and 16 MII oocytes from each treatment group after IVM were collected. To study the effects of growth factors on embryo development, the MII oocytes were activated electrically and cultured in PZM3 as described below. All embryos were evaluated for cleavage on Day 2 (day of activation = 0). Blastocyst formation out of total embryos cultured and the number of nuclei in blastocyst stage embryos were determined on Day 7.
2.1.3. Experiment 3: Effects of EGF and NRG1supplementation in IVM medium on oocyte maturation and development of in vitro fertilized embryos
The objective of this experiment was to determine the effects of EGF and NRG1 on embryo development by in vitro fertilization. The IVM medium and experimental design was the same as experiment 2. Since NRG1+IGF1 had no effects on mtDNA copy number or blastocyst formation, this treatment group was not included. MII oocytes from the control, EGF and NRG1 groups were in vitro fertilized and cultured in PZM3 as described below. All embryos were evaluated for cleavage on Day 2, blastocyst formation and the number of nuclei in blastocyst stage embryos on Day 7 (IVF day = 0).
2.2. Chemicals and follicular fluid preparation
All chemicals were from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated.
For the collection of porcine follicular fluid (pFF), ovaries were obtained from prepubertal gilts slaughtered at a local abattoir. Follicular fluid was collected from medium-sized [33] (3–6 mm in diameter) healthy follicles with clear follicular fluid and high vascularity, with an 18-gauge needle, which was attached to a disposable syringe. Aspirates were pooled in 15-mL conical tubes and centrifuged at 1000×g for 10 min to remove the oocytes and cellular debris. The supernatant was aspirated, filtered through a 0.22 um filter and stored at −20 °C until use.
2.3. Collection of porcine oocytes and in vitro maturation
Ovaries were collected from prepubertal gilts at a local abattoir, transported to the laboratory at 37 °C, and washed with saline solution containing 10 μg/mL gentamicin. Cumulus oocyte complexes were aspirated from antral follicles (3–6 mm in diameter) with an 18-gauge needle attached to a 10-mL syringe. The cumulus-oocyte complexes (COCs) in the follicular fluid were allowed to settle by gravity. The COCs were rinsed three times in Hepes-buffered Tyrode medium containing 0.01% PVA [33]. Only the COCs with multiple layers of intact cumulus cells and uniform ooplasm were selected for IVM. After washing three times in IVM medium, a group of 50–60 COCs was placed into each well of four-well plates (Nunc, Roskilde, Denmark) containing 500 μL of IVM medium and cultured for 22 h at 38.5 °C, 5% CO2, in humidified air. COCs were transferred into basic maturation medium without FSH and LH and cultured for another 22 h. Matured oocytes were then vortexed in 0.1% hyaluronidase in Hepes-buffered Tyrode medium containing 0.01% PVA for 4 min to remove the cumulus cells. Matured oocytes having an extruded first polar body with uniform cytoplasm were used for experiments.
2.4. Activation and culture of oocytes
In Experiments 2, MII oocytes were activated electrically as is used for somatic cell nuclear transfer [34]. Activation was accomplished with two direct current pulses of 1.2 kv/cm for 30 microseconds with 1-sec interval between the 2 pulses, provided by a BTX Electro-cell Manipulator 200 (BTX, San Diego, CA) in fusion medium (0.3 M mannitol, 1.0 mM CaCl2, 0.1 mM MgCl2, and 0.5 mM Hepes, pH 7.4). After electrical activation, embryos were washed and incubated in four-well culture plates (Nunc) containing 500 μL of PZM3 [35] at 38.5 °C and 5% CO2 in humidified air.
2.5. In vitro fertilization
The in vitro fertilization was performed as previously described [34]. Briefly, oocytes with an extruded polar body at 44 h of IVM were washed three times in mTBM medium. Approximately 30–35 oocytes were transferred into 50-μL droplets of IVF medium covered with mineral oil that had been equilibrated for 4 h at 38.5 °C in 5% CO2 in air. A frozen semen pellet was thawed at 38.5 °C in 10 mL of sperm washing medium. After washing twice by centrifugation (1,900 × g for 4 min), cryopreserved ejaculated spermatozoa were suspended in fertilization medium to a concentration of 1 × 106 cells/mL. Fifty microliters of the sperm solution was added to the fertilization droplets containing the oocytes, giving a final sperm concentration of 0.5×106 cells/mL. Oocytes were co-incubated with sperm for 5 h. After fertilization, oocytes were washed three times and cultured in 500 μL PZM3 in 4-well plate (Nunc) at 38.5 °C in 5% CO2 in humidified air.
2.6. Number of nuclei in blastocyst stage embryos
On Day 7 of culture, embryos were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature, and mounted on slides in mounting medium containing 4,6-diamidino-2-phenyl- indole. Slides were analyzed using an epifluorescent microscope (Nikon, Natick, MA) equipped with a digital camera. Images were captured and processed using Nikon NIS-Elements software.
2.7. Oocyte preparation for real-time PCR
The zona pellucida was removed by treating the oocyte in acidic PBS solution (pH 1.8 to 1.9) for 2 seconds and washing in Hepes-buffered Tyrode medium. An individual oocyte was transferred to a 0.5 mL tube containing 1 μL DEPC-treated PBS with 0.1% PVA and snap frozen. The contents were repeated pipetted up and down to lyse the oocytes and release the DNA.
2.8. External DNA standards for real time PCR
The external standard of 369 bp was generated as previously described [15] using primers designed to amplify the mitochondrial cytochrome B gene (forward primer: 5′-GGA ATC TCA TCA GAC ATA GAC-3′; reverse primer: 5′-GAG GTC TGC TAC TAG TAT TC-3′). Reactions were performed in 15 μL using 7.5 μL GeneAmp® Fast PCR Master Mix (ABI, Foster City, CA), 0.5 mM each primer, and 1 μL DNA template. Plasmid standard concentrations were determined on a NanoDrop 1000 Spectrophotometer (NanoDrop products, Wilmington, DE). It was assumed that 1 ng of the plasmid standard (3 kb Vector + 0.369 kb PCR product ) contains 2.72× 108 molecules. These samples were serially diluted 10-fold in order to construct a standard curve for PCR quantification.
2.9. Quantitative real-time PCR (Q-PCR)
To quantify mtDNA copy number in oocytes, the primers were redesigned inside of the 369 bp cytochrome B gene using Integrated DNA Technology (Coralville, IA) software, with forward primer as 5′-ACC TAC TAG GAG ACC CAG ACA ACT-3′ and reverse primer as 5′-TGA ACG TAG GAT AGC GTA GGC GAA-3′. The single-cell lysates were used as the template and Q-PCR reactions were carried out using iQ™ SYBR® Green supermix (Bio-Rad Laboratories, Hercules, CA) on the My iQ® single color real-time PCR detection systems (Bio-Rad Laboratories) according to the following conditions: 1 cycle at 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s. Melt-curve analyses reported on the specificity of the PCR products were performed at the same time to check for the absence of mispriming and the quality of amplifications. A linear regression analysis of all standard curves for sample with copy number between 102 and 107 showed a high correlation coefficient (r2 = 99.9%). A water control was included within each assay. Absolute mtDNA copy number in a single oocyte was calculated using a standard curve derived from Q-PCR amplifications with Microsoft Excel.
From the same day aspirated COC pool, 16 to 17 GV and 16 to 17 MII oocyte samples from each of 4 treatment groups in experiment 1 and 2 were assayed with the known-amount of standards and water control on the same PCR plates to control temporal and cohort variations.
2.10. Statistical analysis
All dependent variables were analyzed for normality using the Wilk-Shapiro test [36]. Data for the dependent variables, mtDNA copy number in the oocyte, percent oocyte meiotic maturation, percent cleavage, percent blastocyst formation, and number of nuclei within the blastocysts were analyzed by the MIXED procedure of SAS ® software (v9.2) [36], with treatment and aspiration day in class as the main effects. Percent oocyte meiotic maturation, percent cleavage, and percent blastocyst formation were Arcsine transformed, while the number of nuclei in blastocyst stage embryos and mtDNA data were logarithmically transformed to approach a normal distribution. Drop in the dish was the experimental unit and used as the error term. Mean differences were determined by using the Fisher Least Significant Difference. The correlations between mtDNA copy number and percent blastocyst formation were analyzed by using CORR procedure of SAS software [36]. A value of P ≤ 0.05 was considered to be statistically significant. In the results, the least-squares means and the standard errors of means are presented.
3. Results
3.1. mtDNA copy number change during in vitro maturation
In order to determine whether there was a mtDNA copy number change during the 44 h in vitro maturation, 69 GV-stage and 314 metaphase II (MII)-stage oocytes from 5 replicates were collected from the same oocyte cohort and assayed in Experiment 1; 105 GV- and 394 MII-stage oocytes from 7 replicates assayed in Experiment 2. The hypothesis tested was that mtDNA copy number in GV stage oocytes (μGV) was equal to the number in MII oocytes (μMII) (H0: μGV= μMII, α=0.05). A pooled mean contrast test was then performed by using MIXED procedure of SAS software within each experiment, letting the day by treatment interaction serve as the error term. The average mtDNA copy number was 167,634.6 ± 20,740.4, and 275,131.9 ± 9,758.4 for GV and MII oocytes, respectively, in Experiment 1; 185,004.7 ± 20,089.3 and 239,392.8 ± 10,345.3 for GV and MII oocytes in Experiment 2. In both experiments, the resulting probability values were less than 0.05, indicating higher mtDNA copy numbers in MII oocytes than the GV oocytes.
3.2. Experiment 1: Effects of porcine follicular fluid (pFF) supplementation on mtDNA copy number and oocyte maturation
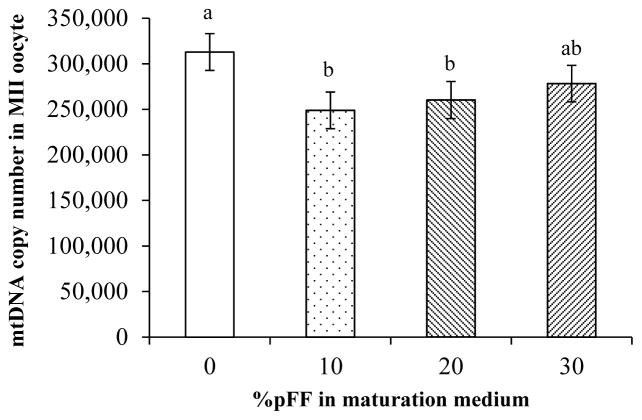
An effect of pFF treatment on mtDNA copy number was detected (P<0.01). Further paired comparison analysis indicated that mtDNA copy number in both 10% and 20% pFF groups was lower than the 0% pFF control (P<0.01). But there was no difference between 30% pFF and the control or among the three pFF supplemented groups (Fig. 1). To further test if pFF-supplemented group (10% to 30%) was different from the no pFF supplementation control in mtDNA copy number and in oocyte maturation rate, the data from 10, 20, and 30% pFF groups were pooled and compared to the 0% pFF control. The average mtDNA copy number in the pFF-supplemented group was lower than in the zero control (266,789.9 ± 11,790.4 vs 318,510.1 ± 20,377.4, P<0.05). There was no difference in oocyte meiotic maturation rate among the 4 groups, with the average of 73.2 ± 6.4, 71.9 ± 6.4, 64.1 ± 6.4, and 65.8 ± 6.4% for the 0, 10, 20, and 30% pFF groups, respectively. However, when all pFF-supplemented groups were pooled and compared to the 0% pFF control, maturation rate in pFF-supplemented group was lower than the 0% pFF control (73.2 ± 1.2 vs 67.3 ± 0.7%, P<0.01).
Figure 1.
A: Mitochondrial DNA copy number in the 0, 10, 20, and 30% pFF groups. Compared to the 0% pFF control, addition of 10 and 20% pFF in maturation medium reduced mtDNA copy number in MII oocytes. (a,b: columns with different letters differ, P<0.05).
3.3. Experiment 2: Effects of epidermal growth factor (EGF), neuregulin 1(NRG1), and NRG1+IGF1 supplementation on mtDNA copy number, oocyte maturation, and parthenogenetic embryo development
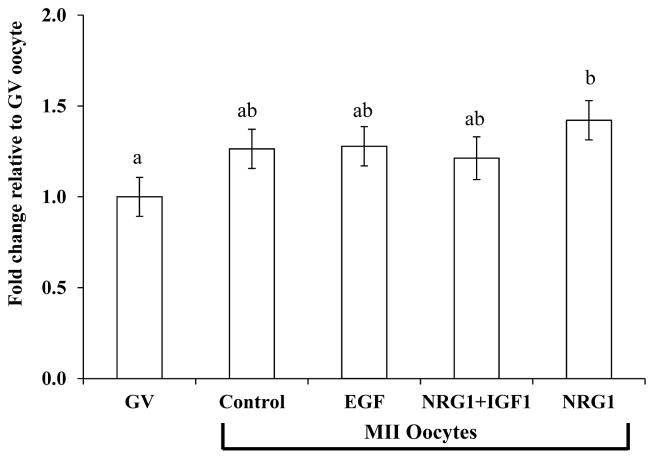
There was no difference in mtDNA copy number in MII oocyte among the EGF, NRG1, NRG1+IGF1, and control groups. The mtDNA copy number in GV oocytes from the same oocyte pool provides a base line before in vitro maturation. A total of 105 GV oocytes were assayed side by side with the MII oocytes and used to test the hypothesis that there was a change in mtDNA copy number in MII oocytes matured in EGF-, NRG1- or NRG1+IGF1-supplemented medium compared to GV oocytes before maturation, i.e. to test the 4 IVM systems with different hormone combinations. Mitochondrial DNA copy number in the NRG1- supplemented group was higher, compared to the GV oocytes (281,293 ± 22,893.5 vs 192,288.7 ± 21,675.4, P<0.05, Fig. 2).
Figure 2.
LSmean ± SEM of mitochondrial DNA copy number changes during oocyte maturation in vitro compared to GV-stage oocytes. Values were obtained from seven replicates and are reported as fold changes relative to that of GV-stage oocytes that were collected from the same cohort and ran side by side with MII oocytes. mtDNA copy number in NRG1-treated MII oocytes was higher compared to GV-stage oocytes (a,b: columns with different letters differ, P<0.05).
A total of 3,558 oocytes were processed to determine the treatment effects on oocyte meiotic maturation. Oocyte meiotic maturation in the EGF, NRG1, and NRG1+IGF1 groups was higher than the control (P<0.01), though there was no difference among the EGF, NRG1, and NRG1+IGF1 groups (Table 1). To study the effects of growth factors on embryo development, 1,070 MII oocytes from 9 replicates were activated and cultured in PMZ3. There was a higher percent cleavage from EGF and NRG1+IGF1 groups than the control or NRG1 (P<0.05). Blastocyst formation was determined on day 7 of culture. Only embryos with 16 or more nuclei were considered to be true blastocysts and used for blastocyst formation and cell number analysis. Percentage of blastocyst formation out of total activated oocytes in the NRG1 treatment group was higher than the control (P<0.05, Table 1). There was no difference in percentage of blastocyst formation among EGF, NRG1 and NRG1+IGF1 groups. There was also no difference in the cell number within the blastocyst-stage embryos among the 4 groups (Table 1).
Table 1.
Oocyte maturation, embryo cleavage, blastocyst formation, and cell number in the parthenogenic embryos of the Control, EGF, NRG1, and NRG1+IGF1 treatment groups.
| Oocyte maturation
|
Embryo development
|
||||||
|---|---|---|---|---|---|---|---|
| Treatment | n | MII oocytes | Maturation, % | n | Cleavage (%) | Blastocyst (%) | Cell # |
| Control | 973 | 621 | 63.4±1.4a | 295 | 215(70.1±4.2)a | 44(15.1±2.1)c | 37.5±2.3 |
| EGF | 800 | 601 | 74.7±1.5b | 219 | 179(83.1±4.9)ab | 42(22.2±2.8)cd | 32.0±2.7 |
| NRG1 | 945 | 679 | 73.4±1.4b | 261 | 225(71.1±4.2)a | 61(23.8±2.4)d | 31.1±2.3 |
| NRG1+IGF1 | 840 | 650 | 76.4±1.5b | 250 | 225(90.8±4.9)b | 37(19.4±2.8)cd | 31.3±2.7 |
Means with different letters differ (a,b: P<0.01; c,d: P<0.05).
3.4. Experiment 3: Effects of epidermal growth factor (EGF) and neuregulin 1(NRG1) on oocyte maturation and development of in vitro fertilized embryos
A total of 3,294 oocytes from 12 replicates were used to determine the treatment effects on oocyte meiotic maturation. Again, EGF and NRG1 treatment increased percent maturation compared to the control (P<0.01), though there was no difference between the EGF and NRG1 groups (Table 2).
Table 2.
Oocyte maturation, embryo cleavage, blastocyst formation, and cell number in the in vitro fertilized embryos of the Control, EGF, and NRG1 treatment groups.
| Oocyte maturation
|
Embryo development
|
||||||
|---|---|---|---|---|---|---|---|
| Treatment | n | MII oocytes | Maturation, % | n | Cleaved (%) | Blastocyst (%) | Cell # |
| Control | 1044 | 662 | 62.8±2.1a | 655 | 355(54.5±2.7)a | 121(19.3±1.7)a | 34.2±1.5 |
| EGF | 1097 | 829 | 75.8±2.1b | 827 | 509(64.8±2.7)b | 191(25.7±1.7)b | 36.8±1.2 |
| NRG1 | 1153 | 828 | 71.5±2.1b | 825 | 543(69.3±2.7)b | 203(26.5±1.7)b | 34.6±1.1 |
Means with different letters differ (P<0.05).
To test the effects of EGF and NRG1treatment during in vitro maturation on subsequent development in in vitro fertilized embryos, a total of 2,307 MII oocytes from 12 replicates were in vitro fertilized and cultured in this experiment. Cleavage at day 2 and blastocyst formation, and cell number in embryos at day 7 are summarized in Table 2. Percentage of cleavage at day 2 was higher in the EGF and NRG1 groups than the control (P<0.05). Again, embryos with 16 or more nuclei were considered to be true blastocysts and were included in the analysis. Compared to the control, EGF and NRG1 treatment increased the percentage of blastocysts (P<0.05, Table 2), and there was no difference between EGF and NRG1 groups. There was no difference in cell number of blastocyst-stage embryos among the three treatment groups.
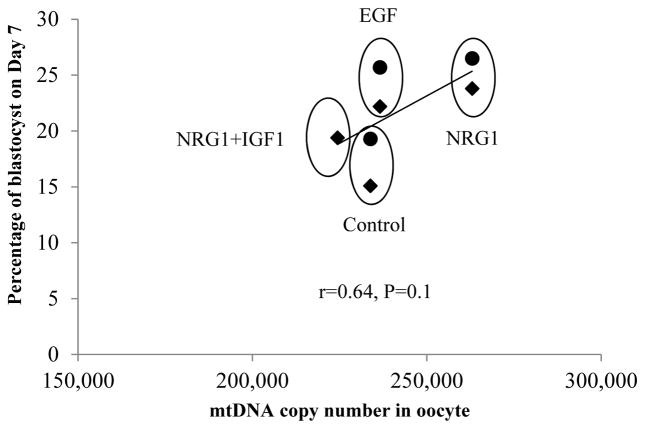
To link mtDNA copy number with percentage of blastocyst formation in Experiments 2 and 3, the average mtDNA copy number of the Control, EGF, NRG1 and NRG1+IGF1 groups were plotted against the means of percent blastocyst formation in both parthenogenetic and in vitro fertilized embryos (Fig. 3). There was a general positive linear correlation between mtDNA copy number and percentage blastocyst formation (r=0.64, P=0.1). NRG1 group observed a higher mtDNA copy number and higher percentage of blastocyst formation in both parthenogenic and IVF embryos compared to the control group (P<0.05).
Figure 3.
Relationship plotting between mtDNA copy number and percentage of blastocyst based on the means of the Control, EGF, NRG1 and NRG1+IGF1 groups. Overall positive linear regression is presented. NRG1 group observed a higher mtDNA copy number and higher percentage of blastocyst formation compared to the control group (P<0.05). Diamonds (◆) represent parthenogenetic embryos, and dots (●) represent in vitro fertilized embryos.
4. Discussion
The importance of mitochondria and its DNA genome number in oocytes is well established. The high number of mtDNA copies in oocytes and the fact that mtDNA is not replicated after fertilization and before the blastocyst stage have led to the assumption that the mtDNA copy number in MII oocytes might be used as a marker of development competence [37]. To determine the dynamic change of mtDNA copy number during oocyte maturation from GV to metaphase II (MII) stage and the possibility of regulating mtDNA copy number, we examined the mtDNA copy number in GV oocytes, and the effects of porcine follicular fluid, EGF, NRG1, and NRG1+IGF1 on mtDNA copy number and subsequent oocyte maturation and embryo development capability after parthenogenetic activation and in vitro fertilization. It was found that mtDNA copy number increased during in vitro maturation and this number could be regulated by porcine follicular fluid (pFF) and NRG1 addition in IVM medium. Supplementation of pFF inhibited mtDNA replication and oocyte meiotic maturation while NRG1 stimulated its replication, enhanced oocyte maturation, and blastocyst formation. The effects of different treatments on mtDNA copy number are mirrored by the effects on oocyte meiotic maturation and subsequent embryo development.
To the best of our knowledge, there is no report on mtDNA copy number in the porcine GV stage oocyte. It was found that the average mtDNA copy number in GV oocytes was 167,634.6 ± 20,740.4 in Experiment 1 and was 185,004.7 ± 20,089.3 in Experiment 2. The mean mtDNA copy number in MII oocyte ranged from 239,392 to 425,886 in the present study, which agrees with the data reported by EL Shourbagy et al [15] and Spikings et al. [19]. Oocyte maturation is the process during which oocyte acquires the developmental competence. It involves both nuclear and cytoplasmic maturation [38,39]. Nuclear maturation involves germinal vesicle breakdown, chromosomal condensation and segregation, and polar body extrusion. Cytoplasmic maturation is less well characterized but involves organelle reorganization, increases in the content of Ca++ stores, and the storage of mRNAs and proteins [39]. Mitochondria play an important role in both of these processes since they provide the main supply of ATP during oocyte maturation [10]. During the final oocyte maturation, it was observed that there is an increase in ATP content in oocyte in cattle [10], pig [11], and mouse [9]. Our observation of a significant increase in mtDNA copies after in vitro maturation confirmed these findings, and also support the suggestion that mitochondrial ATP production is stimulated during oocyte maturation. Furthermore, this ATP increase is correlated with the success of embryo development in cattle [10]. Higher oocyte meiotic maturation and higher embryonic development to blastocysts were also observed in the current study in the oocytes with higher mtDNA copy numbers in the NRG1-supplemented group, which supports the findings in cattle.
The role of pFF supplementation in maturation medium has been studied for years. In most studies, pFF is added to the IVM medium at concentrations between 10% and 25% [11,40,41]. It acts on different aspects of oocyte maturation and fertilization and it has been reported to be beneficial to the in vitro embryo production systems [41–43]. However, in the present study, negative effects of pFF on both mtDNA copy number and oocyte maturation were observed. The follicular fluid is a complex mixture of iron, steroid and peptide hormones, growth factors and lipids from serum and locally produced such as IGF1 [20,21]. The discrepancy between positive and negative or no effects from pFF as described by different researchers may be caused from different properties of follicular fluid due to follicular size, physiological stage of follicles, and if the donors were gilts or sows. On the other hand, the effect of pFF on mtDNA copy number may provide a new approach to pre-select superior pFF that could be beneficial for in vitro embryo production. In addition, the concentrations of pFF in maturation medium are other critical factors that should be taken into account. Brevini et al (2003) [26] reported that blastocyst development remained low in 10% pFF group, while 25% pFF treatment significantly enhanced blastocyst development. Both Brevini’s [11,26] and data of the current study indicate that, to avoid the inhibitory effects of low pFF concentration, a high concentration should be considered during in vitro maturation. The inhibitory effects of pFF on meiotic maturation and embryonic development have been reported by others [26,27]. It is shown that the inhibitory effects are caused mainly by inhibin. In addition, nitric oxide in the follicular fluid inhibits meiotic maturation [44] and other inhibitory factors may be involved as well (as reviewed by Baka and Malamitsi-Puchner [45]).
Recently, research by using mouse genetic models discovered that the preovulatory LH surge induces the transient and sequential expression of the EGF-like peptides, such as amphiregulin (AREG), epiregulin (EREG), Beta-cellulin (BTC) [30] and neuregulin (NRG) [29], in the follicles. These peptides are initially produced by the granulosa cells and have autocrine effects on granulosa cells and paracrine actions on cumulus cells since their receptors (ERBB1) are expressed in both granulosa cells and cumulus cells [29]. There are many members in EGF-family, including EGF itself, heparin-binding EGF-like growth factor, transforming growth factor-α, AREG, BTC, and NRG. All have highly similar structural and functional characteristics. Several lines of evidence have shown that they play important roles in final oocyte maturation. In primates, the EGF-like peptides induced oocyte meiotic resumption [31]. In rodents, mutant mice null for Areg and homozygous for Egfrwa2 exhibited significantly reduced phosphorylation of ERBB1 in cumulus cells, impaired COC expansion and oocyte meiotic arrest at the GV stage [32]. Downs and Chen [46] showed that in the presence of neutralizing antibodies to ERBB1, the meiotic resumption of oocytes was suppressed. Although the expression of the EGF-like factors was transiently increased after hCG stimulation in vivo, it was also demonstrated that sustained activity of ERBB1 and ERK1/2 was required for the induction of cumulus expansion and oocyte meiotic resumption [47]. In addition, NRG1 is a granulosa cell survival factor by its inhibitory effects on apoptosis [29]. To add to the essential role in oocyte maturation, in the current study, we provided further evidence to show that NRG1 addition in IVM medium stimulated mtDNA replication during IVM, and enhanced embryo development to the blastocyst stage.
Replication of mtDNA is dependent on nuclear-encoded transcription and replication factors being translocated to the mitochondria [48]. It utilizes an RNA primer generated through transcription by mitochondrial transcription factor A (TFAM) [49] and the mitochondrial-specific polymerase gamma [50], consisting of catalytic (POLG) and accessory (POLG2) subunits [51]. Previously, it was shown that TFAM accumulates in the cytoplasm during oocyte maturation in the pig (30 times higher in MII oocytes than GV oocytes) [52] and this is shown here to correlate with mtDNA copy number. These two factors regulate mtDNA copy number, which is also correlated to the OXPHOS requirements for specific cells [53]. But how NRG1 regulated TFAM and POLG, and then mtDNA copy number is not known. NRG1 has been show to phosphorylate and induce GA-binding protein mediated mitochondrial gene expression in muscle cells [54].
Though NRG1 and IGF1synergitically stimulated mitochondrial biogenesis in Schwann cells as reported by Echave et al. [17], which was why the NRG1+IGF1 treatment group was included in this study, we did not find any effect on mtDNA copy number, even though they increased oocyte meiotic maturation and cleavage in this study. Neuregulin, on its own, promotes cell-cycle progression but not cell growth, whereas IGF1, on its own, promotes cell growth but not cell-cycle progression. However, when the NRG1 and IGF1 are added together, they synergize to drive cell-cycle progression [55,56], which may explain the higher cleavage in the NRG1+IGF1 group. Nevertheless, it was found that NRG1 treatment increased mtDNA copy number in the MII oocyte compared to the GV oocyte. Furthermore, a positive linear association between mtDNA copy number in the MII oocyte and percentage of blastocyst formation was observed. In the NRG1 group, mtDNA copy number in oocytes was higher, and their blastocyst formation was also higher compared to the control.
Brilliant cresyl blue (BCB) is a dye that can be broken down by glucose-6-phosphate dehydrogenase (G6PD) [57]. The G6PD activity is high in growing oocytes and very low in oocytes that have completed their growth [57]. Thus, BCB staining could distinguish these two groups of oocytes, with BCB+ oocytes being developmentally competent [15]. The findings of current study confirmed the observation that competent BCB+ oocytes contain more copies of mtDNA, and are more likely to fertilize than incompetent BCB− oocytes in the pig. And supplementation of BCB− oocytes with purified mitochondria from BCB+ oocytes improved post-fertilization developmental competence [15]. In bovine, oocytes with higher mtDNA copy numbers also have higher blastocyst development [2,58]. A threshold of 100,000 mtDNA copies was proposed for developmental competence [13]. More recently, using a heterozygous TFAM knockout approach, Wai et al. (2010) [59] demonstrated that mouse oocytes with as few as 4,000 mtDNA copies could be fertilized and develop to the blastocyst stage. However, a higher mtDNA copy number in mature oocytes was still a critical threshold for subsequent post-implantation development. Furthermore, the considerable increase in mtDNA copy number as oocytes mature from GV to MII as found in the present study indicates that an increase in mitochondria is required to provide sufficient energy to support pre-implantation embryo development.
5. Conclusion
The present study shows that there was significant mtDNA replication during oocyte maturation from GV to MII oocytes. Supplementation of IVM medium with porcine follicular fluid suppressed mtDNA copy number and oocyte meiotic maturation. EGF-like growth factor, NRG1, enhanced mtDNA replication, oocyte maturation, and blastocyst formation. This study demonstrated that oocyte mitochondrial biogenesis is stimulated during in vitro maturation. Supplementation of maturation medium with NRG1 provides a means to increase mtDNA copy number, and then improve oocyte viability and developmental competence.
Acknowledgments
The authors would like to thank Dr. Mark Ellersieck for help with the data analysis. This research was funded by NIH U42 RR018877 and Food for the 21st Century Program.
References
- 1.Ursing BM, Arnason U. The complete mitochondrial DNA sequence of the pig (Sus scrofa) J Mol Evol. 1998;47:302–6. doi: 10.1007/pl00006388. [DOI] [PubMed] [Google Scholar]
- 2.May-Panloup P, Chretien M, Malthiery Y, Reynier P. Mitochondrial DNA in the Oocyte and the Developing Embryo. In: Justin CSJ, editor. Current Topics in Developmental Biology. Academic Press; 2007. pp. 51–83. [DOI] [PubMed] [Google Scholar]
- 3.Dumollard R, Duchen M, Carroll J. The Role of Mitochondrial Function in the Oocyte and Embryo. In: Justin CSJ, editor. Current Topics in Developmental Biology. Academic Press; 2007. pp. 21–49. [DOI] [PubMed] [Google Scholar]
- 4.Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–80. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 5.Downs SM. The influence of glucose, cumulus cells, and metabolic coupling on ATP levels and meiotic control in the isolated mouse oocyte. Dev Biol. 1995;167:502–12. doi: 10.1006/dbio.1995.1044. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod. 2007;77:2–8. doi: 10.1095/biolreprod.106.059899. [DOI] [PubMed] [Google Scholar]
- 7.Dumollard R, Duchen M, Sardet C. Calcium signals and mitochondria at fertilisation. Semin Cell Dev Biol. 2006;17:314–23. doi: 10.1016/j.semcdb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Leese HJ. Metabolic control during preimplantation mammalian development. Hum Reprod Update. 1995;1:63–72. doi: 10.1093/humupd/1.1.63. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224:672–80. doi: 10.1002/jcp.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–9. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- 11.Brevini TA, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005;72:1218–23. doi: 10.1095/biolreprod.104.038141. [DOI] [PubMed] [Google Scholar]
- 12.Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–24. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 13.Pikó L, Taylor KD. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Developmental Biology. 1987;123:364–74. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- 14.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–91. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 15.El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233–45. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- 16.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–38. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 17.Echave P, Machado-da-Silva G, Arkell RS, Duchen MR, Jacobson J, Mitter R, Lloyd AC. Extracellular growth factors and mitogens cooperate to drive mitochondrial biogenesis. J Cell Sci. 2009;122:4516–25. doi: 10.1242/jcs.049734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piko L, Taylor KD. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987;123:364–74. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- 19.Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod. 2007;76:327–35. doi: 10.1095/biolreprod.106.054536. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RG. Follicular fluid. J Reprod Fertil. 1974;37:189–219. doi: 10.1530/jrf.0.0370189. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CJ, Hammond JM. Gonadotropins and estradiol stimulate immunoreactive insulin-like growth factor-I production by porcine granulosa cells in vitro. Endocrinology. 1987;120:198–207. doi: 10.1210/endo-120-1-198. [DOI] [PubMed] [Google Scholar]
- 22.Grupen CG, Armstrong DT. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: temporal effects of follicular fluid during IVM. Reprod Fertil Dev. 2010;22:1100–9. doi: 10.1071/RD09307. [DOI] [PubMed] [Google Scholar]
- 23.Bijttebier J, Van Soom A, Meyer E, Mateusen B, Maes D. Preovulatory follicular fluid during in vitro maturation decreases polyspermic fertilization of cumulus-intact porcine oocytes in vitro maturation of porcine oocytes. Theriogenology. 2008;70:715–24. doi: 10.1016/j.theriogenology.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 24.Tatemoto H, Muto N, Sunagawa I, Shinjo A, Nakada T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biol Reprod. 2004;71:1150–7. doi: 10.1095/biolreprod.104.029264. [DOI] [PubMed] [Google Scholar]
- 25.Abeydeera L, Wang W, Cantley T, Rieke A, Murphy C, Prather R, Day B. Development and viability of pig oocytes matured in a protein-free medium containing epidermal growth factor. Theriogenology. 2000;54:787–97. doi: 10.1016/S0093-691X(00)00390-3. [DOI] [PubMed] [Google Scholar]
- 26.Brevini TAL, Francisci C, Vassena R, Bagg MA, Grupen CG, Armstrong DT, Gandol F. Follicular fluid concentration during pig IVM affects oocyte parthenogenetic development and mitochondria distribution. Theriogenology. 2003;59:440. [Google Scholar]
- 27.Tsafriri A, Channing CP. An inhibitory influence of granulosa cells and follicular fluid upon porcine oocyte meiosis in vitro. Endocrinology. 1975;96:922–7. doi: 10.1210/endo-96-4-922. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med. 2009;27:52–61. doi: 10.1055/s-0028-1108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noma N, Kawashima I, Fan HY, Fujita Y, Kawai T, Tomoda Y, Mihara T, Richards JS, Shimada M. LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol. 2011;25:104–16. doi: 10.1210/me.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 31.Nyholt de Prada JK, Lee YS, Latham KE, Chaffin CL, VandeVoort CA. Role for cumulus cell-produced EGF-like ligands during primate oocyte maturation in vitro. Am J Physiol Endocrinol Metab. 2009;296:E1049–58. doi: 10.1152/ajpendo.90930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–24. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katayama M, Rieke A, Cantley T, Murphy C, Dowell L, Sutovsky P, Day BN. Improved fertilization and embryo development resulting in birth of live piglets after intracytoplasmic sperm injection and in vitro culture in a cysteine-supplemented medium. Theriogenology. 2007;67:835–47. doi: 10.1016/j.theriogenology.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Ross JW, Hao Y, Spate LD, Walters EM, Samuel MS, Rieke A, Murphy CN, Prather RS. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol Reprod. 2009;81:525–30. doi: 10.1095/biolreprod.109.077016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod. 2002;66:112–9. doi: 10.1095/biolreprod66.1.112. [DOI] [PubMed] [Google Scholar]
- 36.Statistical Analysis Systems. SAS/STAT User's Guid (V9.2) Cary, NC: Statistical Analysis Systems Institute, Inc; 2008. 9.2. [Google Scholar]
- 37.Chiaratti MR, Meirelles FV. Mitochondrial DNA copy number, a marker of viability for oocytes. Biol Reprod. 2010;83:1–2. doi: 10.1095/biolreprod.110.084269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun QY, Nagai T. Molecular mechanisms underlying pig oocyte maturation and fertilization. J Reprod Dev. 2003;49:347–59. doi: 10.1262/jrd.49.347. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PA. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology. 2009;71:836–48. doi: 10.1016/j.theriogenology.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida M, Ishizaki Y, Kawagishi H, Bamba K, Kojima Y. Effects of pig follicular fluid on maturation of pig oocytes in vitro and on their subsequent fertilizing and developmental capacity in vitro. J Reprod Fertil. 1992;95:481–8. doi: 10.1530/jrf.0.0950481. [DOI] [PubMed] [Google Scholar]
- 41.Vatzias G, Hagen DR. Effects of porcine follicular fluid and oviduct-conditioned media on maturation and fertilization of porcine oocytes in vitro. Biol Reprod. 1999;60:42–8. doi: 10.1095/biolreprod60.1.42. [DOI] [PubMed] [Google Scholar]
- 42.Daen FP, Sato E, Naito K, Toyoda Y. The effect of pig follicular fluid fractions on cumulus expansion and male pronucleus formation in porcine oocytes matured and fertilized in vitro. J Reprod Fertil. 1994;101:667–73. doi: 10.1530/jrf.0.1010667. [DOI] [PubMed] [Google Scholar]
- 43.Funahashi H, Day BN. Effects of follicular fluid at fertilization in vitro on sperm penetration in pig oocytes. J Reprod Fertil. 1993;99:97–103. doi: 10.1530/jrf.0.0990097. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura Y, Yamagata Y, Sugino N, Takayama H, Kato H. Nitric Oxide Inhibits Oocyte Meiotic Maturation. Biology of Reproduction. 2002;67:1588–92. doi: 10.1095/biolreprod.102.005264. [DOI] [PubMed] [Google Scholar]
- 45.Baka S, Malamitsi-Puchner A. Novel follicular fluid factors influencing oocyte developmental potential in IVF: a review. Reprod Biomed Online. 2006;12:500–6. doi: 10.1016/s1472-6483(10)62005-6. [DOI] [PubMed] [Google Scholar]
- 46.Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75:105–14. doi: 10.1002/mrd.20781. [DOI] [PubMed] [Google Scholar]
- 47.Reizel Y, Elbaz J, Dekel N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol. 2010;24:402–11. doi: 10.1210/me.2009-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clayton DA. Transcription and replication of mitochondrial DNA. Hum Reprod. 2000;15(Suppl 2):11–7. doi: 10.1093/humrep/15.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- 49.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–6. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 50.Hubscher U, Kuenzle CC, Spadari S. Functional roles of DNA polymerases beta and gamma. Proc Natl Acad Sci U S A. 1979;76:2316–20. doi: 10.1073/pnas.76.5.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray H, Wong TW. Purification and identification of subunit structure of the human mitochondrial DNA polymerase. J Biol Chem. 1992;267:5835–41. [PubMed] [Google Scholar]
- 52.Antelman J, Manandhar G, Yi YJ, Li R, Whitworth KM, Sutovsky M, Agca C, Prather RS, Sutovsky P. Expression of mitochondrial transcription factor A (TFAM) during porcine gametogenesis and preimplantation embryo development. J Cell Physiol. 2008;217:529–43. doi: 10.1002/jcp.21528. [DOI] [PubMed] [Google Scholar]
- 53.Moyes CD, Battersby BJ, Leary SC. Regulation of muscle mitochondrial design. J Exp Biol. 1998;201:299–307. [PubMed] [Google Scholar]
- 54.Fromm L, Burden SJ. Neuregulin-1-stimulated phosphorylation of GABP in skeletal muscle cells. Biochemistry. 2001;40:5306–12. doi: 10.1021/bi002649m. [DOI] [PubMed] [Google Scholar]
- 55.Conlon IJ, Dunn GA, Mudge AW, Raff MC. Extracellular control of cell size. Nat Cell Biol. 2001;3:918–21. doi: 10.1038/ncb1001-918. [DOI] [PubMed] [Google Scholar]
- 56.Echave P, Conlon IJ, Lloyd AC. Cell size regulation in mammalian cells. Cell Cycle. 2007;6:218–24. doi: 10.4161/cc.6.2.3744. [DOI] [PubMed] [Google Scholar]
- 57.Tian WN, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem. 1998;273:10609–17. doi: 10.1074/jbc.273.17.10609. [DOI] [PubMed] [Google Scholar]
- 58.Hua S, Zhang Y, Li XC, Ma LB, Cao JW, Dai JP, Li R. Effects of granulosa cell mitochondria transfer on the early development of bovine embryos in vitro. Cloning Stem Cells. 2007;9:237–46. doi: 10.1089/clo.2006.0020. [DOI] [PubMed] [Google Scholar]
- 59.Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]