Abstract
Though cortical abnormalities have been demonstrated in moderate and severe traumatic brain injured (TBI) patients, there have been no studies examining cortical changes following blast related mild TBI (mTBI). The purpose of this study was to determine the effects and functional relevance of blast mTBI on cortical thickness in a small cohort of carefully screened blast injured US Service Members (SM). Twelve SM with mTBI acquired through blast injury were compared to 11 demographically matched control SM without TBI. Both mTBI and control participants were active duty and had completed a combat deployment. Subjects underwent MRI examination and the T1 weighted anatomic images were processed using the FreeSurfer suite of tools. Cortical thickness maps were compared between groups and examined for relationships with time since injury (TSI). Utilizing a large database of functional imaging results (BrainMap), significant regions of interest (ROI) were used to determine the behavioral profiles most consistently associated with the specific ROI. In addition, clinical variables were examined as part of post-hoc analysis of functional relevance. Group comparisons controlling for age demonstrated several significant clusters of cortical thinning for the blast injured SM. After multiple comparisons correction (False Discovery Rate (FDR)), two left hemisphere clusters remained significant (left superior temporal (STG) and frontal (SFG) gyri). No clusters were significantly correlated with TSI after FDR correction. Behavioral analysis for the STG and SFG clusters demonstrated three significant behavioral/cognitive sub-domains, each associated with audition and language. Blast injured SMs demonstrated distinct areas of cortical thinning in the STG and SFG. These areas have been previously shown to be associated with audition and language. Post-hoc analyses of clinical records demonstrated significant abnormal audiology reports for the blast injured SM suggesting that the thinning in these ROIs might be related to injury to the external auditory system rather than direct injury to the brain from the blast. It is clear that additional replication is needed in much larger cohorts. Importantly, the combination of imaging tools and methods in this study successfully demonstrated the potential to define unique ROIs and functional correlates that can be used to design future studies.
Keywords: TBI, Blast Injury, Mild TBI, Cortical thickness, Cognition, Behavior, FreeSurfer, MANGO
Introduction
Traumatic brain injury (TBI) as the result of exposure to a blast is a common source of acquired brain injury in the United States military (Owens et al. 2008; Warden 2006; Wilk et al. 2010). Currently, it is widely accepted that blast exposure poses a risk to the central nervous system (CNS) of Service Members (SM), though there remains much to be learned about the severity and functional impact. For this reason, blast injury remains a primary focus of many research endeavors across the spectrum of clinical, mechanical, and neuropathology research (Brenner et al. 2012; Goeller et al. 2012; Lange et al. 2012; Panzer et al. 2012).
Conceptually, blast exposure is thought to produce injury in a number of ways but typically involves a complex combination of blast overpressure exposure, blunt impact, and/or acceleration/deceleration of the head (Panzer et al. 2012). Fluid filled organs and soft tissue are particularly susceptible to injury when initially exposed to the violent localized changes in atmospheric pressure. This phenomenon results in rapid compression and decompression forces that mechanically deform the soft tissue. In addition, shrapnel or debris propelled by the blast and/or the acceleration of the head into an object can result in additional risks for injury. Combined, these forces can result in TBI with important clinical and functional consequences ranging from subtle to profound.
Blast exposure has been linked to a variety of clinical and cognitive outcomes in SM, including headaches (Eskridge et al. 2013), nausea, tinnitus (Fausti et al. 2009), chronic pain (Ramasamy et al. 2011), gait and balance difficulties (Akin and Murnane 2011; Sylvia et al. 2001), and sleep disturbances (Ruff et al. 2009). Cognitive/behavioral symptoms are commonly reported in blast-exposed SMs and include forgetfulness, inattention, and generally slowed cognitive processing (Cooper et al. 2011, 2012). The relative contribution of blast exposure, acoustic trauma and combat stress to mTBI symptoms in SM is not known, and objective physiologic measures are needed to sort out this complex relationship.
Medical imaging (especially MRI) demonstrates abnormalities in TBI populations that correspond with severity of injury (Tate and Bigler 2000) and particular MRI sequences may prove to be sensitive biomarkers of mTBI (for Reviews see (Lin et al. 2012; McDonald et al. 2012; Shenton et al. 2012; Slobounov et al. 2012)). In addition, differences in cortical thickness measured by MRI correlate with functionally relevant behavioral/cognitive dysfunction in moderate and severe TBI cohorts (McCauley et al. 2010; Merkley et al. 2008; Palacios et al. 2013; Wilde et al. 2012). These findings emphasize the potential of cortical thickness measures to elucidate functionally relevant brain-behavior relationships that could lead to improved treatment in these patients. In an attempt to extend previous findings from moderate and severe TBI cohorts, we focused on the relationship between mTBI from blast injury and measures of cortical thickness, as this measure has yet to be examined in this population. Further, we sought to examine the clinical relevance of any cortical abnormalities observed using new functional imaging database tools and post-hoc analysis of clinical data and self-reported post-concussive symptoms.
Methods
Participants
Data from a pilot study of blast injury was used in this study. Our sample included both well-defined blast injured mild TBI (N =12) and healthy, non-injured recently deployed (N =11) controls matched for both age and gender. TBI and healthy non-injured deployed controls were males between the ages of 18 and 40. The protocol and consent process were approved and monitored by the local institutional review boards. All participants underwent the consent process and provided written approval for participation.
All potential participants were identified based on their responses to questionnaires routinely administered by the Defense and Veterans Brain Injury Centers (DVBIC) personnel to returning SM at a large military medical center (Brooke Army Medical Center). Based on their responses, potential participants identified as having been injured by blast during deployment in Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) were provided information regarding the study as part of the consent process.
Blast mild TBI participants
mTBI participants were 12 male active duty SMs recruited from the Traumatic Brain Injury Clinic at San Antonio Military Medical Center. Potential participants were thoroughly screened and diagnosed with mTBI by semi-structured clinical interview and record review using the Veterans Affairs (VA)/Department of Defense (DoD) criteria (see Table 1). To minimize the likelihood of including patients without objective evidence of a brain injury (i.e., including subjective reporting of momentary confusion at the time of blast exposure) in this small sample, only subjects who had a documented loss of consciousness less than 30 min were included in this sample. Individuals with moderate, severe or penetrating brain injuries were excluded. In addition, mTBI participants were required to have been injured within the past 18 months during an OIF/OEF deployment, have a Shipley Intelligence Scale score within the normal range (T ≥40), and no history of head injury prior to deployment.
Table 1.
Basic demographic information for the two groups
| Variable | Blast injured group | Non-injured controls |
Statistical differences |
|---|---|---|---|
| Age | 26.53 (6.16) | 27.73 (2.41) | P=0.55 |
| Sex | 100 % Male | 100 % Male | N/A |
| Education | 13.13 (1.55) | 13.27 (1.56) | P=0.82 |
| Time Since Injury | 103 (51) Days | N/A | N/A |
| Shipley Total | 54.29 (5.81) | 57.09 (5.19) | P=0.22 |
| NSI Score | 18.79 (13.77) | N/A | N/A |
| CES-D | 15.63 (18.60) | 11.73 (8.74) | p=0.55 |
| PCL-M | 35.29 (13.72) | 31.64 (10.28) | p=0.47 |
NSI Neurobehavioral Symptom Inventory; CES-D Center for Epidemiologic Studies-Depression Scale; PCL-M PTSD Check List-Military version
Control participants
Control participants were 11 healthy non-injured male active duty SM recently returned from deployment (within 18 months) and screened by DVBIC as described above. Control subjects were recruited through the Post-Deployment Health Re-Assessment process. Additional criteria for control participants included Shipley Intelligence Scale score in the normal range (i.e., T ≥40).
Exclusion criteria for both groups included MRI contraindications (i.e., shrapnel), prior psychiatric diagnoses, and certain pain medications including long acting preparations (i.e., fentanyl patch).
Behavioral and imaging data
Each participant completed a brief battery of psychometric and self-reported behavioral measures and underwent magnetic resonance imaging (MRI) examination. Behavioral assessment included measures of intelligence (Shipley Intelligence Scale), psychiatric co-morbidity (Center for Epidemiological Study-Depression (CES-D) and PTSD Checklist-Military version (PCL-M)) and symptom reporting (Neurobehavioral Symptom Inventory (NSI)).
MRI assessment included high-resolution structural imaging acquired using a 3T Siemens TIM Trio. Six MPRAGE T1 weighted images were acquired for each participant utilizing the following parameters: TR (repetition time)=2200 msec, TE (echo time)=2.8 msec, slice thickness=0.8, in plane resolution 0.8×0.8, FOV=256×256×192, and a Flip angle=13°.
MRI post processing
After each scan was visually inspected for artifacts that might affect processing (i.e., motion, enlarged ventricles, anatomy coverage, inhomogeneity) and examined for consistent scan acquisition (same scan parameters), the six images were motion corrected, linear registered, and averaged to produce a high-resolution anatomical image for analysis. This step produces images with excellent gray/white boundaries and has been shown to improve segmentation results. Each new averaged image was then processed using the FreeSurfer (MGH Martinos Center, Boston, MA) suite of processing tools described in more detail elsewhere (Fischl and Dale 2000). For our data, we used the recon-all pipeline resulting in fully segmented images and cortical thickness maps. Using FreeSurfer 5.1, data were first processed using the recon-all command. Upon completion, pial and white matter surface maps were visually inspected for each participant and corrected manually when required to ensure the validity of the measurements (it should be noted that no significant consistent corrections were required in this data set). After inspection and manual correction, the cortical thickness maps were then smoothed using the -qcache command resulting in standardized maps for processing.
Statistical procedures
Statistical procedures included a combination of traditional and image based statistical methods.
MRI group comparisons
Group comparisons of cortical thickness maps were accomplished using both the –qdec and –glmfit tools available in FreeSurfer. Main effects for cortical thickness were initially examined with group membership as a fixed factor and age as a nuisance factor. A full width half max (FWHM) of 15 mm was utilized resulting in “heat maps” indicating cortical areas that were significantly different. Finally, correction for multiple comparisons was accomplished using false discovery rate (FDR) feature built into the qdec processing tool.
In addition, the correlations with time since injury (TSI) was examined using TSI as continuous variable and age as a nuisance factor for the blast injured group only. Again, a FWHM of 15 mm was utilized as a smoothing kernel and “heat maps” were derived showing cortical clusters where the TSI and cortical thickness were significantly correlated.
Functional relevance procedures
To determine the functional relevance of cortical thickness differences, the Behavioral Analysis tool in the public domain Multi-image Analysis GUI (MANGO; Research Imaging Institute, UTHSCSA, San Antonio, TX) software was used (see (Lancaster et al. 2012) for a description of methods). This software was developed to provide an automated way to determine behavioral domains typically associated with specific brain regions by utilizing data from the BrainMap database (http://www.brainmap.org/). This database contains metadata from approximately 2100 peer reviewed papers and 10,000 experiments. Each experiment is assigned to one or more behavioral classifications across five domains (action, cognition, emotion, interoception, and perception) along with the x-y-z coordinates for the functional imaging activations. Using 3-D images, the spatial probability distribution of an activation can be calculated and statistical validity is provided as z-scores testing the null hypothesis that the distribution of an activated foci observed within an ROI is not different from a spatially uniform random distribution across the brain (Lancaster et al. 2012).
Utilizing the Talairach coordinates and cluster size from the FreeSurfer cortical thickness comparisons above, ROIs indicating significant differences between the groups were identified and delineated within MANGO. The Behavioral Analysis tool then automatically lists each sub-domain associated with that ROI and calculates the associated z-score for each behavior. A positive z-score ≥3.0 was considered significant after a Bonferroni correction for multiple comparisons (p =0.05 for 51 behavior sub-domains).
Results
Demographics
Descriptive statistics are provided for the mTBI and control groups in Table 1. There was no significant difference between the two groups for age or education. In addition, measures of intelligence, depression, and PTSD symptomatology were also similar between the two groups. Generally speaking, the blast mTBI group endorsed more symptoms for depression and PTSD, although the scores were not statistically different. The mean CES-D score for the mTBI group was just below the clinical cut-off of 16, which when exceeded is indicative of clinically significant or mild depressive symptomatology.
Group comparisons
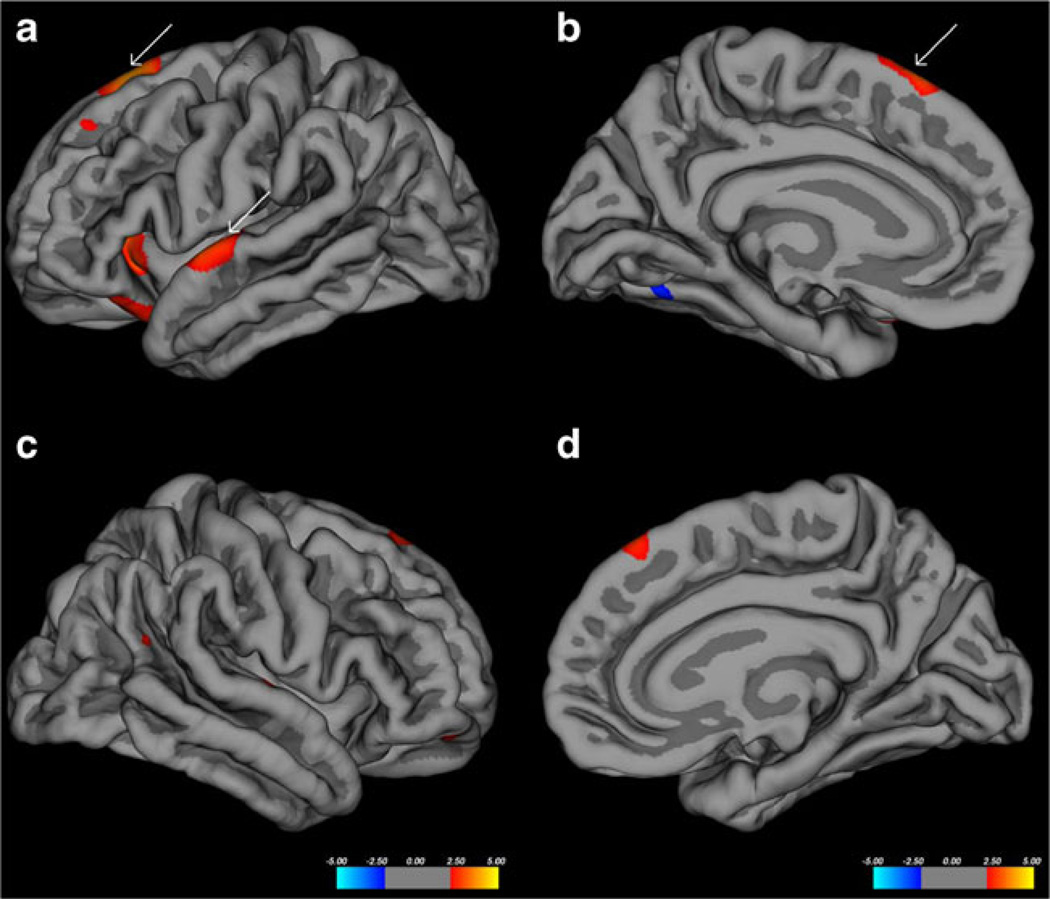
Before multiple comparisons correction, several significant clusters of cortical thinning were noted between the groups with the blast injured group demonstrating significant thinning when controlling for age (see Fig. 1). Notably, there were four significant clusters greater than 200 mm2 noted in the left hemisphere including the superior temporal/transverse gyri, superior frontal gyrus, and two areas in the lateral orbitofrontal gyri. The remaining four significant areas were all less than 50 mm2 (fusiform, superiorfrontal, caudal middle frontal, and precuneus). There were two significant clusters greater than 100 mm2 in the right hemisphere including the transverse temporal and superior frontal gyri. Six additional areas less than 100 mm2 were noted in the precentral (two separate areas), lateral orbitofrontal gyrus (two separate areas), inferior parietal, and precuneus.
Fig. 1.
Pial surface maps showing areas (clusters) of significant thinning (red) between the two groups before multiple comparison correction. a and b show right lateral and medial surfaces respecitively. c and d show right lateral and medial surfaces respectively. White arrows show two clusters that remain significant after FDR correction
After FDR correction, only two areas remained significant in the left hemisphere. The superior temporal and superior frontal gyri remained significant (see Fig. 1 white arrows) though the size of the clusters was greatly reduced (497.90 to 103.14 mm2 and 350.52 to 55.00 mm2 respectively). No areas in the right hemisphere remained significant.
Time since injury
There were multiple areas of significant positive and negative correlations between time since injury and cortical thickness in the blast injured participants. There were three areas in the left hemisphere that were larger than 100 mm2 including the insula, pars triangularis, and fusiform. Seven additional areas less then 75 mm2 were also noted. For the right hemisphere, there were no areas of significant correlation between time since injury and cortical thickness. After FDR correction, no significant relationships in the left hemisphere remained.
Functional relevance
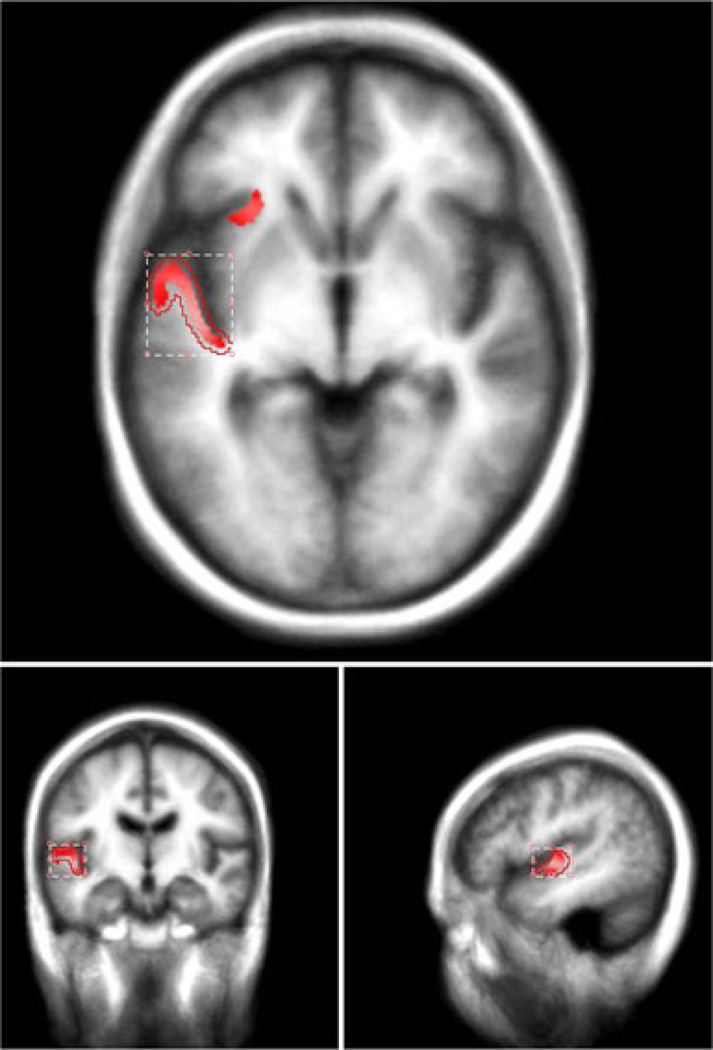
For the purposes of this study, we only examined the two statistically significant left hemisphere clusters identified in the group comparisons analyses described above (see Fig. 2). For the left superior temporal and transverse gyri, three (3) significant associations were noted with audition (z =5.09), language/speech (z =4.50), and speech execution (z =3.47). For the left superior frontal gyrus, three (3) significant associations were also noted, audition (z =5.46), language/speech (z =4.20), and language/phonology (z =3.18). Behavioral domains associated with each significant ROI are listed along with their z-scores in Tables 2 (superior temporal gyrus) and 3 (superior frontal gyrus).
Fig. 2.
Label map from FreeSurfer cortical thickness analyses projected onto a template brain in MANGO for behavioral analyses
Table 2.
Positive associations of behavioral test and domain data from MANGO for the left superior temporal/transverse gyri
| Category | Domain | Z-score |
|---|---|---|
| Audition | Perception | 5.093 |
| Language (Speech) | Cognition | 4.500 |
| Execution (Speech) | Action | 3.470 |
| Language (Phonology) | Cognition | 2.752 |
| Music | Cognition | 2.048 |
| Language (Other) | Cognition | 1.490 |
| Language (Semantics) | Cognition | 1.380 |
| Happiness (Other) | Emotion | 1.071 |
| Sleep | Interoception | 0.931 |
| Soma | Cognition | 0.895 |
| Language (Syntax) | Cognition | 0.807 |
| Hunger | Interoception | 0.733 |
| Sadness | Emotion | 0.651 |
| Language (Orthography) | Cognition | 0.610 |
| Air-hunger | Interoception | 0.431 |
| Reasoning | Cognition | 0.081 |
Significant tests and domains are in bold italicized text
Table 3.
Positive associations of behavioral test and domain data from MANGO for the left superior frontal gyrus
| Category | Domain | Z-score |
|---|---|---|
| Audition | Perception | 5.464 |
| Language (Speech) | Cognition | 4.196 |
| Language (Phonology) | Cognition | 3.176 |
| Execution (Speech) | Action | 2.621 |
| Happiness (Other) | Emotion | 1.996 |
| Motor (Learning) | Action | 1.726 |
| Language (Semantics) | Cognition | 1.527 |
| Language (Syntax) | Cognition | 1.318 |
| Music | Cognition | 1.293 |
| Language (Other) | Cognition | 1.209 |
| Social | Cognition | 1.187 |
| Soma | Cognition | 1.184 |
| Sleep | Interoception | 0.917 |
| Sadness | Emotion | 0.540 |
| Happiness (Humor) | Emotion | 0.384 |
| Hunger | Interoception | 0.159 |
| Memory (Explicit) | Cognition | 0.158 |
| Space | Cognition | 0.120 |
| Language (Orthography) | Cognition | 0.040 |
Significant tests and domains are in bold italicized text
Post-hoc clinical chart review
Based on the anatomical location of significant ROI’s and the functional findings described above, a retrospective clinical chart review was conducted to determine the presences and degree of audiology abnormalities in this sample. Medical record review revealed that each blast-injured participant was screened and/or tested by the Audiology Department in the hospital as part of their clinical workup. Results from the clinical chart review indicated that all but two of the blast-exposed participants were diagnosed with audiology problems including tinnitus, ruptured tympanic membrane, conductive and/or sensorineural hearing loss, and/or reduction in hearing (see Table 4). It should be noted that no hearing abnormalities were charted previous to the blast exposure for the mTBI participants and that these hearing abnormalities were temporally tied to the blast event. Clinical chart review for the control participants did not reveal any diagnostic codes for hearing abnormalities or referrals for screening/testing by the Audiology Department. Additional subjective reporting of hearing abnormalities was obtained from responses to the Neurobehavioral Symptom Inventory (NSI) for each group and indicated that the blast-exposed participants reported moderate hearing difficulty (Likert scale mode response = 2; symptoms; range 0 to 4) compared to no hearing difficulty in non-injured control participants (Likert scale mode response = 0; no symptoms; range 0–1).
Table 4.
Results from the clinical chart review showing the subjective/objective reporting of audiology clinical symptoms
| Patient ID | Subjective tinnitus | Ruptured TM | Conductive neural loss | Sensorineural hearing loss | Hearing loss | NSI reporting item #8 |
|---|---|---|---|---|---|---|
| 002 | − | + | − | − | − | 1 |
| 004 | − | ++ | − | − | + | 2 |
| 005 | − | − | − | − | − | 0 |
| 006 | − | − | − | − | + | 2 |
| 007 | ++ | − | − | − | ++ | 0 |
| 008 | ++ | − | − | − | 1 | |
| 009 | − | − | − | − | ++ | 0 |
| 010 | ++ | ++ | ++ | ++ | − | 3 |
| 011 | − | − | − | ++ | − | 2 |
| 012 | − | − | − | − | − | 1 |
| 013 | ++ | − | − | − | − | 2 |
| 014 | ++ | − | − | − | − | 1 |
| 015 | ++ | − | − | − | ++ | 4 |
The NSI column show the individual’s response to item #8 asks the participant to indicate the degree of disturbance in the last two weeks with regards to hearing difficulty. A score of 0 indicates no problem, 1 indicates mild, 2 indicates moderate (occasionally disrupts activities), 3 indicates severe (frequently disrupts activities), and 4 indicates very severe (almost always present and unable to perform daily activities)
Discussion
The primary finding of this study is the identification of regional cortical thinning in blast injured SMs compared to age-and gender-matched deployed service member controls. This study is one of the first to demonstrate statistically significant cortical thinning in blast injured mild TBI patients. Notably, several bilateral areas of thinning are observed, most pronounced in the superior temporal, superior frontal, and lateral orbitofrontal gyri with the left superior temporal and frontal gyri clusters remaining statistically significant after correction for multiple comparisons.
Using the BrainMap database and behavioral analysis tools in MANGO, the functional relevance of the significant clusters from the left superior/frontal gyri were examined. Results from this analysis demonstrated that these two areas are highly involved in speech perception and/or language. This finding was expected as the functional relevance of the transverse gyrus, also known as Heschl’s gyrus, has long been documented in the literature as primary auditory cortex. Though the two clusters were examined independently using the behavioral analysis tool, it is clear from the overlapping functional findings and recent functional connectivity research using resting state functional MRI (fMRI) that the superior temporal and frontal gyri are part of an integrated network involved in speech perception and understanding (Yue et al. 2013).
The spatial distribution of thinning and the functional relevance of these areas in this small sample is noteworthy because TBI is often characterized as having a diverse injury distribution with no two injuries occurring in the same location. Moreover, spatially unique injuries are hypothesized as one of the reasons why between groups differences (especially between mTBI and controls) are difficult to observe (Bigler and Maxwell 2012; Shenton et al. 2012). Though only the left hemisphere findings remained significant after FDR correction, it is noteworthy that there is important overlap between regions affected bilaterally (especially Heschl’s gyri). As noted above, this observation prompted us to re-examine patients’ clinical records for possible explanations. Evaluation of the clinical records led us to discover consistent audiology complaints and medical findings including subjective tinnitus, ruptured tympanic membranes, and/or conductive/sensorineural hearing loss in the majority of our research participants (see Table 4). In fact, 6/13 (~46 %) mTBI patients had a medical record history of subjective tinnitus, 3/13 (~23 %) had a ruptured tympanic membrane, 1/13 (~8 %) had conductive hearing loss, 2/13 (~15 %) had sensorineural hearing loss, and 5/13 (~38 %) had other types of hearing loss (i.e., shifts in the range of hearing). In total, all but two of the patients (85 % of our sample) had clinically verified audiology complaints. These findings are notable, as research has shown that up to 60 % of SMs exposed to blast manifest audiology abnormalities, especially tinnitus (Fausti et al. 2009; Lew et al. 2007).
With these facts in mind, the cortical thinning in the STG and SFG observed in this study is likely the result of hearing loss or injury rather than the direct effects of the blast event and may take time to develop in these patients. Animal and human research demonstrating that audiology abnormalities/ symptoms result in cortical reorganization support this hypothesis. For example, because tinnitus is such a significant clinical phenomenon in the general population (~14 % of US population; Saunders 2007), it has been the focus of much research and these studies demonstrate a variety of pathological (Lanting et al. 2009; Yang et al. 2012) and volumetric (Schecklmann et al. 2013; Schneider et al. 2009) changes that mirror the current findings. For example, Schecklmann et al., (2013) show a significant reduction of grey matter volume in Heschl’s gyrus that is negatively associated with tinnitus distress. Though our finding is made in a small sample, the significant ROI findings in the primary auditory cortex, the rigorous quality control procedures (i.e., use of average T1 image and visual inspection), and corroborating findings from the current literature increase our confidence that the thinning observed in this group is real and represents gross morphological changes related to audiologic impairment.
Though the combination of these results is compelling, there are several weaknesses that temper enthusiasm for the findings. First and foremost, the sample size is a clear limitation imposed by the pilot nature of the study. However, there were efforts to carefully characterize and document that individuals in the TBI group sustained a blast injury by reviewing medical records for evidence of LOC. We also attempted to recruit a control group that shared as many demographic (age, gender, active duty, recently deployed) variables as possible. Regardless, it is clear that replication in a much larger sample of patients and controls is required. Currently, there are ongoing efforts to reproduce these findings in larger samples of patients with blast injury. Second, the cross-sectional nature of the study limits our ability to definitively state what is the cause of the abnormal thinning or the temporal timing of cortical thinning in relation to the injury. On average, our cohort is approximately 3 months post injury and there is a concern that this time frame is short and may not be sufficient to produce this type of change. However, there is a growing literature that suggests cortical thickness changes occur at shorter time frames (6 weeks to 3 months) in the context of intensive learning activities (Engvig et al. 2010; Haier et al. 2009; Martensson et al. 2012). Regardless, cross-sectional examination is, in fact, a major limitation of many studies of mTBI and future studies would benefit from a prospective approach that could elucidate the evolution and progression of injury after mTBI and answer questions about when various changes might occur in relation to injury and/or the development of injury related symptoms. Third, our study was not designed to address audiology findings per se. In fact, it was the spatial location of cortical thinning that led to a retrospective chart review that identified the degree of audiology-related symptoms in this group. Given the nature of the injury mechanism investigated, it makes intuitive sense to examine related audiology factors and future studies of blast injury should include measures and/or examination of audiology examination findings and related symptoms.
Regardless of these limitations, this study provides a foundation on which to base future research efforts in the study of blast mTBI. It clearly illustrates that cortical thinning is observable/quantifiable and is functionally relevant in this group of patients.
Acknowledgments
The views, opinions and/or findings contained in this manuscript are those of the authors and should not be construed as an official Department of Defense or Veterans Affairs position, policy or decision unless so designated by other documentation. Research on this article was done under Contract #W91YTZ-12-C-0132. In the conduct of this research, the investigators adhered to the policies regarding the protection of human subjects as prescribe by Code of Federal Regulations (CFR) Title 45, Volume 1, Part 46; Title 32, Chapter1, Part 219; and Title 21, Chapter 1, Part 50 (Protection of Human Subjects).
Contributor Information
D. F. Tate, Email: dftatephd@mac.com, Defense and Veterans Brain Injury Center, Contractor for the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., San Antonio Military Medical Center (SAMMC), San Antonio, TX, USA.
G. E. York, LTC, MC, San Antonio Military Medical Center, San Antonio, TX, USA
M. W. Reid, Defense and Veterans Brain Injury Center, Contractor for the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., San Antonio Military Medical Center (SAMMC), San Antonio, TX, USA
D. B. Cooper, Defense and Veterans Brain Injury Center, Contractor for the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., San Antonio Military Medical Center (SAMMC), San Antonio, TX, USA
L. Jones, Department of Neurology, Mayo Clinic, Rochester, MN, USA
D. A. Robin, Research Imaging Institute, University of Texas Health Science Center-San Antonio, San Antonio, TX, USA
J. E. Kennedy, Defense and Veterans Brain Injury Center, Contractor for the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., San Antonio Military Medical Center (SAMMC), San Antonio, TX, USA
J. Lewis, LtCol, MC, Air Force Medical Support Agency, Falls Church, VA, USA
References
- Akin FW, Murnane OD. Head injury and blast exposure: vestibular consequences. Otolaryngologic Clinics of North America. 2011;44(2):323–334. doi: 10.1016/j.otc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging and Behavior. 2012;6(2):108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Bahraini N, Hernandez TD. Perspectives on creating clinically relevant blast models for mild traumatic brain injury and post traumatic stress disorder symptoms. Frontiers in Neurology. 2012;3:31. doi: 10.3389/fneur.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DB, Chau PM, Armistead-Jehle P, Vanderploeg RD, Bowles AO. Relationship between mechanism of injury and neurocognitive functioning in OEF/OIF service members with mild traumatic brain injuries. Military Medicine. 2012;177(10):1157–1160. doi: 10.7205/milmed-d-12-00098. [DOI] [PubMed] [Google Scholar]
- Cooper DB, Kennedy JE, Cullen MA, Critchfield E, Amador RR, Bowles AO. Association between combat stress and post-concussive symptom reporting in OEF/OIF service members with mild traumatic brain injuries. Brain Injury. 2011;25(1):1–7. doi: 10.3109/02699052.2010.531692. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, et al. Effects of memory training on cortical thickness in the elderly. NeuroImage. 2010;52(4):1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Eskridge SL, Macera CA, Galarneau MR, Holbrook TL, Woodruff SI, Macgregor AJ, et al. Influence of combat blast-related mild traumatic brain injury acute symptoms on mental health and service discharge outcomes. Journal of Neurotrauma. 2013 doi: 10.1089/neu.2012.2537. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. Journal of Rehabilitation Research and Development. 2009;46(6):797–810. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeller J, Wardlaw A, Treichler D, O’Bruba J, Weiss G. Investigation of cavitation as a possible damage mechanism in blast-induced traumatic brain injury. Journal of neurotrauma. 2012;29(10):1970–1981. doi: 10.1089/neu.2011.2224. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Research Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Laird AR, Eickhoff SB, Martinez MJ, Fox PM, Fox PT. Automated regional behavioral analysis for human brain images. Frontiers in Neuroinformatics. 2012;6:23. doi: 10.3389/fninf.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange RT, Pancholi S, Brickell TA, Sakura S, Bhagwat A, Merritt V, et al. Neuropsychological outcome from blast versus non-blast: mild traumatic brain injury in U.S. military service members. Journal of the International Neuropsychological Society. 2012;18(3):595–605. doi: 10.1017/S1355617712000239. [DOI] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hearing Research. 2009;255(1–2):1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Lew HL, Jerger JF, Guillory SB, Henry JA. Auditory dysfunction in traumatic brain injury. Journal of Rehabilitation Research and Development. 2007;44(7):921–928. doi: 10.1682/jrrd.2007.09.0140. [DOI] [PubMed] [Google Scholar]
- Lin AP, Liao H, Merugumala S, Prabhu S, Meehan W, Ross B. Metabolic imaging of mild traumatic brain injury. Brain Injury and Behavior. 2012;6(2):208–223. doi: 10.1007/s11682-012-9181-4. [DOI] [PubMed] [Google Scholar]
- Martensson J, Eriksson J, Bodammer NC, Lindgren M, Johansson M, Nyberg L, et al. Growth of language-related brain areas after foreign language learning. NeuroImage. 2012;63(1):240–244. doi: 10.1016/j.neuroimage.2012.06.043. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Merkley TL, Schnelle KP, Bigler ED, Hunter JV, et al. Patterns of cortical thinning in relation to event-based prospective memory performance three months after moderate to severe traumatic brain injury in children. Developmental Neuropsychology. 2010;35(3):318–332. doi: 10.1080/87565641003696866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Saykin A, McAllister T. Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging and Behavior. 2012;6(2):193–207. doi: 10.1007/s11682-012-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkley TL, Bigler ED, Wilde EA, McCauley SR, Hunter JV, Levin HS. Diffuse changes in cortical thickness in pediatric moderate-to-severe traumatic brain injury. Journal of Neurotrauma. 2008;25(11):1343–1345. doi: 10.1089/neu.2008.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BD, Kragh JF, Jr., Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. The Journal of Trauma. 2008;64(2):295–299. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- Palacios EM, Sala-Llonch R, Junque C, Fernandez-Espejo D, Roig T, Tormos JM, et al. Long-term declarative memory deficits in diffuse TBI: correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex. 2013;49(3):646–657. doi: 10.1016/j.cortex.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Panzer MB, Myers BS, Capehart BP, Bass CR. Development of a finite element model for blast brain injury and the effects of CSF cavitation. Annals of Biomedical Engineering. 2012;40(7):1530–1544. doi: 10.1007/s10439-012-0519-2. [DOI] [PubMed] [Google Scholar]
- Ramasamy A, Hill AM, Phillip R, Gibb I, Bull AM, Clasper JC. The modern “deck-slap” injury-calcaneal blast fractures from vehicle explosions. The Journal of Trauma. 2011;71(6):1694–1698. doi: 10.1097/TA.0b013e318227a999. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Ruff SS, Wang XF. Improving sleep: initial headache treatment in OIF/OEF veterans with blast-induced mild traumatic brain injury. Journal of Rehabilitation Research and Development. 2009;46(9):1071–1084. doi: 10.1682/jrrd.2009.05.0062. [DOI] [PubMed] [Google Scholar]
- Saunders JC. The role of central nervous system in plasticity in tinnitus. Journal of Communication Disorders. 2007;40(4):313–334. doi: 10.1016/j.jcomdis.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Lehner A, Poeppl TB, Kreuzer PM, Rupprecht R, Rackl J, et al. Auditory cortex is implicated in tinnitus distress: a voxel-based morphometry study. Brain Structure & Function. 2013 doi: 10.1007/s00429-013-0520-z. [DOI] [PubMed] [Google Scholar]
- Schneider P, Andermann M, Wengenroth M, Goebel R, Flor H, Rupp A, et al. Reduced volume of Heschl’s gyrus in tinnitus. NeuroImage. 2009;45(3):927–939. doi: 10.1016/j.neuroimage.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior. 2012;6(2):137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Gay M, Johnson B, Zhang K. Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging and Behavior. 2012;6(2):224–243. doi: 10.1007/s11682-012-9167-2. [DOI] [PubMed] [Google Scholar]
- Sylvia FR, Drake AI, Wester DC. Transient vestibular balance dysfunction after primary blast injury. Military Medicine. 2001;166(10):918–920. [PubMed] [Google Scholar]
- Tate DF, Bigler ED. Fornix and hippocampal atrophy in traumatic brain injury. Learning and Memory. 2000;7(6):442–446. doi: 10.1101/lm.33000. [DOI] [PubMed] [Google Scholar]
- Warden D. Military TBI during the Iraq and Afghanistan wars. The Journal of Head Trauma Rehabilitation. 2006;21(5):398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Merkley TL, Bigler ED, Max JE, Schmidt AT, Ayoub KW, et al. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. International Journal of Developmental Neuroscience. 2012;30(3):267–276. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk JE, Thomas JL, McGurk DM, Riviere LA, Castro CA, Hoge CW. Mild traumatic brain injury (concussion) during combat: lack of association of blast mechanism with persistent postconcussive symptoms. The Journal of Head Trauma Rehabilitation. 2010;25(1):9–14. doi: 10.1097/HTR.0b013e3181bd090f. [DOI] [PubMed] [Google Scholar]
- Yang S, Su W, Bao S. Long-term, but not transient, threshold shifts alter the morphology and increase the excitability of cortical pyramidal neurons. Journal of Neurophysiology. 2012;108(6):1567–1574. doi: 10.1152/jn.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Zhang L, Xu G, Shu H, Li P. Task-modulated activation and functional connectivity of the temporal and frontal areas during speech comprehension. Neuroscience. 2013;237:87–95. doi: 10.1016/j.neuroscience.2012.12.067. [DOI] [PubMed] [Google Scholar]