Abstract
Background
Since 2006, routine HPV vaccination has been recommended for females aged 11–12 in the US. However not much is known about the extent of and factors associated with HPV vaccination after the ages of 11–12.
Methods
Provider-verified data on 8710 females aged 13–17 were analyzed from the 2013 NIS-Teen survey. 2013 Data was utilized since it was the first year one can fully evaluate the age at vaccination through age 17 for females who could receive the HPV vaccine at age 11.
Results
Among HPV vaccinated females who were 17 in 2013, 47% (95% CI=43–50%) received their first dose after age 12, and 24% (95% CI=21–26%) received their first dose after age 14. The HPV vaccine was more likely to be initiated later than the meningococcal and Tdap vaccines (p<0.05), and later HPV vaccine initiation was more common among those having a more highly educated mother and those not receiving a check-up/well visit between the ages of 11 and 12 in adjusted analyses (p-values<0.05). Females initiating the HPV vaccine late were more likely to not receive three doses (RR=1.90, 95% CI=1.76–2.04).
Conclusions
HPV vaccination is commonly initiated after the age of 12 in the US, which could limit the vaccine׳s population-level effectiveness.
Keywords: HPV vaccines, Late initiation, Vaccination, NIS-Teen
Highlights
-
•
We examine the extent of and factors for later HPV vaccine initiation in US teens.
-
•
47% of HPV vaccinated females received the vaccine after the ages of 11–12.
-
•
HPV vaccination was initiated later than other recommended vaccines.
-
•
Late initiation was more common among those with a higher social economic status.
-
•
Later initiators were less likely to receive all three doses of the HPV vaccine.
1. Introduction
Human papillomavirus (HPV) is a common sexually transmitted infection that can lead to cervical, oropharyngeal, and other anogenital cancers as well as genital warts [1]. Prophylactic HPV vaccines have demonstrated nearly 100% efficacy against infection with certain high-risk HPV types among individuals not previously exposed to HPV [2], [3]. However, the HPV vaccines are most effective when administered prior to HPV exposure, as these vaccines do not have a therapeutic effect on active HPV infections [4].
In 2006, the US Advisory Committee on Immunization Practices (ACIP) recommended routine HPV vaccination for females aged 11–12 [5], [6]. “Catch-up” vaccination was also recommended for females ages 13–26 who have not been vaccinated previously [5]. As of 2013, 57% of females aged 13–17 in the US had received at least one dose of an HPV vaccine, and 38% received all three doses of the HPV vaccine as recommended [7].
Multiple demographic, cognitive, and contextual factors have been found to be associated with HPV vaccine uptake [7], [8], [9], [10], [11], but much less is known about factors related to the age at vaccine initiation. The age at HPV vaccine initiation is important given that HPV infection and its related sequelae can often occur at young ages with a North American study suggesting approximately one third of unvaccinated females were HPV infected in their teen years [12] and another suggesting that cervical dysplasia and genital warts can often occur in females aged 14–17 years [13].
Data are now available to examine the age at vaccination initiation through age 17 among females who were between 9 and 11 years old when the HPV vaccine was first recommended and available in 2006–2007. Our aims in the current study were to describe the age-specific prevalence of HPV vaccine initiation and identify factors that may differentiate females initiating the HPV vaccine during the recommended ages of 11–12 from females initiating the vaccine after age 12.
2. Methods
2.1. Study population and design
This study utilized the publically available de-identified National Immunization Survey-Teen (NIS-teen) surveys from 2010 to 2013 conducted by the US Center for Diseases Control׳s (CDC) National Center for Immunization and Respiratory Diseases [7]. NIS-Teen sampled parents or guardians of eligible teens, aged 13–17 at the time of the survey, from all 50 states and the District of Columbia, using a stratified random-digit-dialed dual-frame sample of landline and starting in 2011, cellular telephones [7]. When approval was granted by the parent or guardian, questionnaires regarding vaccination history including the HPV, meningococcal, and Tetanus, Diphtheria, and Pertussis (Tdap) vaccines were also sent to the teen׳s providers. Provider-reported immunization histories were synthesized with other available demographic and behavioral information and samples were weighted to estimate the general US population, as previously described [14], [15].
Our main analyses examined the age of HPV vaccine initiation among the 8710 females from the US who were in NIS-teen 2013 and had provider-reported immunization histories. Some of these analyses were restricted to the 5269 females who received at least one dose of the HPV vaccine. In addition, we focused some analyses on the 1509 females who were 17 years of age in NIS-teen 2013. The Gardasil HPV4 vaccine was approved by the US Federal Drug Administration (FDA) and recommended by the Center of Disease Control (CDC)׳s Advisory committee on Immunization Practices (ACIP) in June of 2006 and was available on the market in the subsequent months [5], [6]. The household interviews for the 2013 NIS-teen survey began on January 10, 2013 and ended on February 13, 2014 [15]. Thus, females who were 17 years of age in the NIS-teen 2013 survey were born between January 1995 and February 1997. These individuals were between the ages of 9 and 11 years old when the vaccine was recommended and available, and are thus the oldest cohort that could have been vaccinated at age 11 in accordance with recommendations for routine HPV vaccination.
In a sub-analysis that examined whether the age at HPV vaccine initiation may be changing over time, we also utilized NIS-teen survey data from 2010 to 2012 to evaluate the ages at HPV vaccine initiation among the 5465 females who were older than the routine recommended ages when the HPV vaccine was approved (i.e. those born between 1992 and 1995 who were 17 in NIS-teen 2010–2012). Additionally, we examined the age of vaccine initiation by age 15 in the 5079 females who were at least 15 in NIS-teen 2013 (i.e. those born in 1995–1998) in NIS-teen 2013. HPV vaccination was not routinely recommended for US males until 2011 [16], so they were therefore not included in any analyses.
2.2. Measures
This study׳s primary outcome of interest was age when receiving the first dose of the HPV vaccine (either Gardasil (HPV4) or Cervarix (HPV2)). We also examined the ages when receiving the Tdap and meningococcal vaccines, the two other vaccines that are routinely recommended for adolescents aged 11–12, as secondary outcomes. The routine recommendations for the meningococcal and Tdap vaccines were made in the spring of 2005 [17], [18], one year prior to the HPV vaccine recommendation. Only vaccine doses received prior to the date of the parental interview were included. While the adolescents’ exact ages in days at the time of their vaccinations and their age in years at the time of the survey were included in the NIS-teen database, the participants’ exact birthdays and date of each survey were not included. Because individuals’ exact ages when the HPV vaccine was FDA approved were unknown, age ranges for each cohort of participants when the HPV vaccine was approved are included in this manuscript.
Demographic and behavioral measures collected through the parental interview in the NIS-teen were included in this analysis [15]. Healthcare access was categorized as either: private insurance, federal Vaccines for Children (VFC), or other coverage, as previously described [8]. The VFC program covers all females under 19 who are eligible for Medicaid, uninsured, were an American Indian/Alaska Native or attended a federally qualified health center [19]. Individuals who had the State Children׳s Health Insurance Program (CHIP) coverage, but not Medicaid were categorized as having “other” coverage.
2.3. Statistical analyses
Probability sampling weights were utilized in all analyses through STATA 13 svy commands (StataCorp, College Station, TX) to obtain nationally representative estimates. The weights account for the multi-state stratified sampling design and differential response rates [15]. The HPV vaccination status and age of initiation was calculated for the 8710 females aged 13–17 in NIS-teen 2013. Potential factors associated with later HPV vaccine initiation (after age 12) among the 5269 HPV vaccinated females aged 13–17 in NIS-teen 2013 were evaluated through weighted Poisson regression with robust error variances [20]. This method was utilized instead of weighted logistic regression given the high prevalence of the outcome (vaccination after age 12).
Three different forms of multivariate adjustment were performed. We performed individual models for each covariate of interest first adjusting for only for age, then adjusting for age and further demographic factors, and finally for age, demographic factors and other exposures significant in prior age-adjusted modeling (location, check-up visit). Delayed Meningococcal/Tdap vaccine initiation was not included in this model since it was not considered an exposure; rather it was considered a separate outcome that may be correlated with late HPV vaccine initiation. Adjusted relative risks (RRs) and 95% confidence intervals (95% CIs) were reported for each of the three models. We also examined whether females received all three doses of the HPV vaccine, restricting to individuals who were followed at least 24 weeks following their first HPV vaccine dose [15]. Chi-square tests and linear p-trends were estimated using continuous values for ordinal or continuous variables, when available. All statistical tests were two-sided and considered significant at an α=0.05 level.
3. Results
3.1. Age of vaccination for females born in 1995–1996
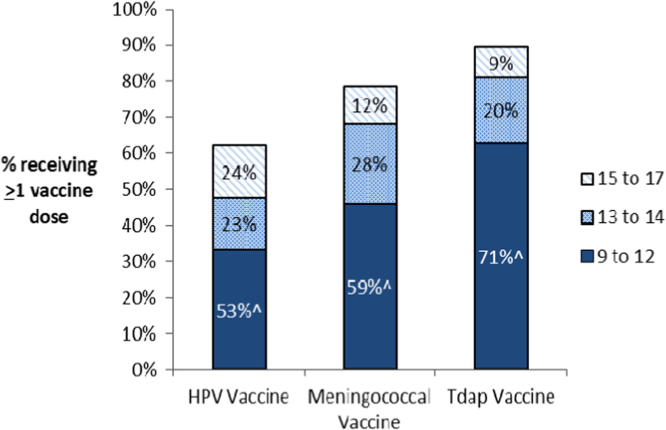
Among females in the United States who were 17 in 2013, 62% (95% CI=58–67%) received at least one dose of the HPV vaccine. Although these females born in 1995–1996 were between 9 and 11 years of age when the HPV vaccine was recommended and became available, close to half of those vaccinated received their first HPV vaccine dose after the recommended ages of 11–12 (47%, 95% CI=43–50%), and nearly a quarter (24%, 95% CI=21–26%) received their first dose between the ages of 15–17 (Fig. 1).
Fig. 1.
Vaccination status and age of vaccine initiation among 1509 females aged 17 in NIS-teen 2013*. Overall, 62% of females recived at least one HPV vaccine dose, 79% received at least one meningococcal vaccine dose, and 90% recived at least one Tdap vaccine dose. ^Percent receiving the vaccine between the ages of 9 and 12 among vaccinated females. *The ACIP recommends routine vaccination with the HPV, Meningococcal, and Tdap vaccines between the ages of 11 and 12.
The HPV vaccine was more likely to be initiated at older ages than the meningococcal and the Tdap vaccines among females aged 17 in 2013 (Fig. 1). Among those vaccinated, the HPV vaccine was more commonly initiated after age 12 (47% vs. 41% (meningococcal), p=0.02; 47% vs. 30% (Tdap), p<0.001; Fig. 1), and after age 14 (24% vs. 12% (meningococcal), p<0.001; 24% vs. 9% (Tdap), p<0.001; Fig. 1), than the other vaccines. Most HPV vaccinated females were vaccinated for both Tdap and Meningococcal disease (90%), while HPV vaccination was less common among individuals who received either a Meningococcal vaccine (67%) or the Tdap vaccine (61%).
3.2. Age of HPV vaccination over time
Most females who were 17 years of age in previous versions of NIS-teen (2008–2012) were born between 1992 and 1995 and were 12 or older when the HPV vaccine was recommended and became available. Thus the vast majority of these individuals received the vaccine after age 12 (Supplementary Fig. 1).
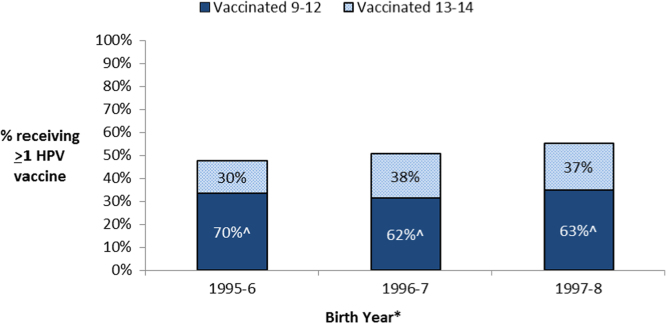
When restricting again to the 8710 females in NIS-teen 2013, we observed that a higher percentage of females born from 1995 to 2000 initiated HPV vaccination between the ages of 11–12 and between the ages of 13–14 in more recent years (Supplementary Fig. 2). HPV vaccine uptake by age 13 increased from 33% for those born in 1995–1996 to 44% for those born in 1999–2000 (p-trend<0.001), and the HPV vaccine uptake between the ages of 13–14 non-significantly increased from 14% for those born in 1995–1996 to 20% in the youngest eligible group born in 1997–1998 (p-trend=0.09; Supplementary Fig. 2). However, given the increases in both age groups, the percent of HPV vaccinated females receiving the vaccine prior to age 13 did not increase in analyses restricted to relevant populations born from 1995 to 1998 (Fig. 2). Indeed, when restricting to 2634 HPV vaccinated females who were 15 or older at the time of the survey, the percent receiving the vaccine at the ages of 11–12 modestly declined from 70% in those born in 1995–1996 to 62% and 63% for those born in 1996–1997 and 1997–1998 (p-values<0.01, Fig. 2).
Fig. 2.
Vaccination status and age of vaccine initiation by age 15 among 5079 females aged 15–17 in NIS-teen 2013. #This analysis was restricted to 5079 females who were 15 or older at the time of the survey. This analysis excluded vaccinations after age 14 given that many females born in more recent years (1996–1998) were not yet 16–17 at the time of this survey. ^Percent receiving the HPV vaccine between the ages of 9 to 12 among the 2634 females born between 1995 and 1998 and HPV vaccinated prior to age 15. *As the NIS-2013 survey was conducted between January 2013 and February 2014, years of birth ranged two years in each grouping (i.e. those aged 17 at the time of the survey were born between January 1995 and February 1997 but is classified as 1995-6 in the figure above).
3.3. Factors related to later HPV vaccine initiation
HPV vaccinated females were considerably more likely to initiate the HPV vaccine after age 12 if they also initiated the meningococcal or Tdap vaccine late (71% vs. 25%, age-adjusted RR=2.76, 95% CI=2.39–3.19; Table 1). In fact, the individuals who initiated both the meningococcal and Tdap vaccines after age 12 were the most likely to initiate HPV vaccine after age 12 (86%, adjusted RR=3.24, 95% CI=2.81–3.73).
Table 1.
Risk factors for later HPV Vaccine initiation among 5269 females in NIS-teen 2013 receiving at least one dose of the HPV vaccine.
| Demographic | n | % Receiving vaccine after the age of 12a | Age-adjusted RR (95%CI)a | Model 1a, b demo.-adj. RR (95% CI) | Model 2a, c further-adj. RR (95% CI) |
|---|---|---|---|---|---|
| Education level of mother | |||||
| HS grad or less | 1619 | 29.5% | REF | REF | REF |
| Some college | 1382 | 41.5% | 1.35 (1.12–1.63) | 1.33 (1.10–1.62) | 1.37 (1.13–1.66) |
| College grad | 2268 | 40.0% | 1.33 (1.13–1.56) | 1.25 (1.03–1.52) | 1.27 (1.05–1.54) |
| p-Trend | 0.003 | 0.02 | 0.01 | ||
| Race/ethnicity | |||||
| Caucasian | 3201 | 38.5% | REF | REF | REF |
| Hispanic | 891 | 32.8% | 0.88 (0.72–1.08) | 1.00 (0.82–1.22) | 1.04 (0.86–1.27) |
| Non-hispanic black | 520 | 32.8% | 0.84 (0.68–1.04) | 0.89 (0.72–1.10) | 0.91 (0.73–1.12) |
| Non-hispanic other | 657 | 35.1% | 0.91 (0.70–1.16) | 0.94 (0.73–1.20) | 0.83 (0.64–1.08) |
| Family income | |||||
| Below poverty | 1049 | 31.1% | REF | REF | REF |
| Above poverty, ≤$75 K/year | 1835 | 35.2% | 1.06 (0.87–1.30) | 0.98 (0.79–1.21) | 0.99 (0.80–1.23) |
| Above poverty, >$75 K/year | 2261 | 41.4% | 1.25 (1.03–1.52) | 1.08 (0.85–1.38) | 1.06 (0.82–1.35) |
| Unknown | 124 | 24.7% | 0.73 (0.44–1.19) | 0.69 (0.43–1.11) | 0.65 (0.40–1.04) |
| p-Trend | 0.02 | 0.35 | 0.49 | ||
| Well-child/check-up between ages of 11 and 12 | |||||
| Yes | 4258 | 38.3% | REF | REF | |
| No | 245 | 55.6% | 1.46 (1.16–1.84) | 1.58 (1.28–1.96) | |
| Don׳t know | 130 | 49.8% | 1.34 (0.99–1.83) | 1.41 (1.04–1.92) | |
| Location | |||||
| Northeast | 1161 | 42.1% | REF | REF | |
| Midwest | 1116 | 39.1% | 0.96 (0.83–1.10) | 0.95 (0.82–1.10) | |
| South | 1612 | 32.1% | 0.79 (0.67–0.92) | 0.78 (0.67–0.92) | |
| West | 1203 | 34.4% | 0.84 (0.68–1.05) | 0.85 (0.69–1.05) | |
| Health insurance | |||||
| Private | 2733 | 39.2% | REF | ||
| VFC eligible | 1316 | 33.4% | 0.87 (0.74–1.03) | ||
| Other | 899 | 34.6% | 0.90 (0.74–1.11) | ||
| Facility of provider | |||||
| Public | 736 | 38.0% | REF | ||
| Hospital | 536 | 31.0% | 0.79 (0.57–1.11) | ||
| Private | 2365 | 37.7% | 0.98 (0.79–1.22) | ||
| All STD/school/teen clinic | 132 | 25.1% | 0.69 (0.42–1.13) | ||
| Mixed/unknown | 1500 | 34.3% | 0.89 (0.70–1.13) | ||
| Delayed Meningococcal or Tdap vaccine initiation | |||||
| No – vaccinated both 11–12 | 3506 | 24.7% | REF | ||
| Yes – one or both | 888 | 71.2% | 2.76 (2.39–3.19) | ||
| Language | |||||
| English | 4843 | 37.1% | REF | ||
| Spanish | 381 | 28.4% | 0.78 (0.57–1.05) | ||
| Other | 45 | 47.4% | 1.25 (0.82–1.91) | ||
| Physician/HC recommendation for vaccination | |||||
| Yes | 4200 | 37.4% | REF | ||
| No | 780 | 31.0% | 0.87 (0.70–1.09) | ||
| Don׳t know | 260 | 37.1% | 1.01 (0.75–1.36) | ||
Survey weighted.
Adjusted for age, education status of mother, family income, and race/ethnicity.
Adjusted for age, education status of mother, family income, and race/ethnicity, location, and well-child/check-up between ages of 11 and 12.
Among the demographic factors examined, later HPV vaccine initiation (after age 12) was shown to be more common among females with more educated mothers (41% vs. 30%, p<0.05) and a higher family income (41% vs. 31%, p<0.05, Table 1) in age-adjusted analyses. In addition, not receiving a check-up or well visit at ages 11–12 was associated with an increased likelihood of later HPV vaccine initiation (RR=1.46, 95% CI=1.16–1.84), however the vast majority of HPV vaccinated females received a check-up or well-visit between the ages of 11 and 12 (95%). The percent of females initiating the HPV vaccine late was similar across race/ethnicity, health insurance status, type of provider, and whether a physician/healthcare provider ever recommended the vaccine (p-values>0.05, Table 1). However, HPV vaccination was less likely to be initiated late among HPV vaccinated females in the southern US compared to those in the northeast (32% vs. 42%, p<0.05, Table 1).
Even in controlled multivariate analysis, later HPV vaccine initiation was more likely among females with more educated mothers and those who did not have well child or check-up visits between the ages of 11 and 12 (p-trend=0.01 and adjusted RR=1.58, 95% CI=1.28–1.96, respectively; Table 1). In addition, HPV vaccinated individuals living in the southern US remained less likely to initiate the HPV vaccine late than HPV vaccinated females from the northeast (adjusted RR=0.78, 95% CI=0.67–0.92). Higher family income and race/ethnicity were not associated with later HPV vaccine initiation after adjustment.
3.4. HPV vaccine dose completion by age of vaccine initiation
Females who initiated the HPV vaccine after age 12 were also almost twice as likely to not complete all three doses of the vaccine compared to those who received the vaccine between the ages of 11 and 12 (age-adjusted RR=1.90, 95% CI=1.76–2.04, Table 2). Among HPV vaccinated females who received all three HPV vaccine doses, 26% received their first dose after the age of 12. This prevalence of later initiation was considerably lower than the prevalence in women receiving only two (44%) or one doses (46%) (P-values<0.001, Table 2).
Table 2.
Number of HPV doses by age of HPV vaccine initiation among 5269 HPV vaccinated females within NIS-teen 2013a.
| Demographic | N (%) | % Receiving vaccine after the age of 12b | Age adjusted RR (95%CI)b, c |
|---|---|---|---|
| Number of HPV doses | |||
| 3 | 3466 (71.2%) | 26.1% | REF |
| 2 | 786 (16.2%) | 43.8% | 1.91 (1.75–2.09)b |
| 1 | 276 (12.7%) | 45.6% | 1.88 (1.71–2.06)b |
| p-Trend | <0.001 | ||
Restricted to individuals who were followed at least 24 months following their first HPV vaccine dose.
Survey weighted.
3 vs. 2/1 doses: RR=1.90 (95% CI=1.76–2.04).
4. Discussion
This study provides evidence that HPV vaccination in the US is commonly initiated after the recommended ages of 11–12 for routine vaccination and the percentage of HPV-vaccinated females initiating after age 12 has not yet declined over time. Compared to other recommended vaccines, HPV vaccination initiation is more commonly delayed until older ages and this may limit the population-level effectiveness of the HPV vaccine in the US.
Individuals receiving the HPV vaccine at older ages may obtain less protection from the vaccine depending on the age of their sexual debut. A previous study suggested that over a quarter of females acquired at least one HPV type within the first year of their sexual debut [21] and a third of US females aged 14–19 had a least one HPV type in the pre-HPV vaccine era [12]. Additionally, other studies report that approximately half of US teens initiate sexual behavior prior to or at the age of 17 [22], [23]. Thus given that almost half of vaccinated females initiated the HPV vaccine between the ages of 13 and 17, many of them may not have obtained full protection from the vaccine.
Not only did we observe that later HPV vaccine initiation was common, but our analysis suggests that the percentage of HPV vaccinated females initiating the vaccine at older ages has not declined in more recent years. Another recent analysis of 2008–2012 NIS-Teen data reported that the mean age at HPV vaccination is declining in more recent years [24]. However counter to this analysis, Rahman and colleagues [24] included cohorts born in 1994 or earlier who were already 13 or older when the HPV vaccine was approved. Therefore, this previously reported decline in the mean age at HPV vaccination between 2010 and 2013 is likely due to the fact that a larger proportion of included females were eligible to be vaccinated at ages 11–12 each subsequent year. Our analysis differs in that we limited our analysis to those females who were eligible to be HPV vaccinated at age 11.
Our study suggests that the previously observed increase in HPV vaccine uptake in more recent cohorts [7], [25] is driven by both an increased uptake prior to age 13 and an increased uptake during the ages of 13 and above. In other words, HPV vaccine uptake appears to be increasing in cohorts born more recently, but a sizeable percentage of these cohorts continue to be receiving the vaccine at older ages (after age 12). Continued follow-up of more recent cohorts born after 1997 are necessary to determine whether later HPV vaccine initiation continues to be common.
Factors associated with later HPV vaccination initiation in this study appear to be fairly similar to those related to reduced HPV vaccine uptake [7], [8], [9], [10], [11]. For example, we found that having a higher maternal education and lack of healthcare utilization were associated with later initiation, and both of these factors have been previously related to reduced HPV vaccination uptake [7], [8], [9], [10], [11]. Additionally, we found that vaccine initiation occurs at older ages among HPV vaccinated females compared to Tdap and meningococcal vaccinated females. Previous studies have suggested lower uptake of the HPV vaccine than the Tdap and meningococcal vaccines [7]. This coupled with our observation that later meningococcal/Tdap initiation is strongly associated with later HPV vaccine initiation suggests that there are some females delaying initiation of multiple vaccines while others may just be delaying initiation of the HPV vaccine.
This study also found that the age of HPV vaccine initiation is comparable across race/ethnicity, which mirrors the finding that HPV vaccine uptake is fairly similar by race/ethnicity [7], although uptake may be modestly higher among Hispanic females [7], [9]. There were some differences between factors for HPV vaccine uptake and age of vaccine initiation. Previous analyses of the NIS-Teen data have found females living in the US south to be the least likely to initiate the HPV vaccine [26]. Our results suggest that the limited number of southern females who do initiate the vaccine are more likely to receive it prior to age 13.
Our study also found that females initiating the HPV vaccine at older ages were about two times less likely to receive all three recommended doses than those vaccinated at age 11–12. Non-completion of the HPV vaccine series is common in the US, as over a quarter of HPV vaccinated females do not receive all three doses [7]. A recent post-hoc analysis of a randomized control trial found that the HPV vaccine efficacy is similar between one, two, and three doses [27], and that antibody titers are similar in those receiving two or three doses [28]. However, further evaluation in a formal randomized clinical trial is necessary. Females who initiated the vaccine later are at a higher risk of having a prevalent HPV infection by the time of vaccination, and thus our study suggests that this factor could confound the relationship between the number of doses and HPV vaccine effectiveness in observational studies. Future observational studies examining the HPV vaccine effectiveness of fewer doses should adjust for age at the time of vaccination and other potential confounders such as number of sexual partners prior to vaccination if available.
This is one first studies to examine the extent of and factors associated with HPV vaccination occurring in the US after the recommended ages of 11–12 for routine vaccination. It utilizes a large sample that is generalizable to the US teen population. However, there are a few limitations. The household and cellphone response rate to these surveys were limited [7], which can lead to non-response/non-coverage bias. Sample weights were utilized to minimize this bias. In addition, we did not have access to individuals’ exact birthdays so we were restricted to utilizing ranges for the ages when the HPV vaccine was approved in June of 2006.
This study suggests that not only does the United States have incomplete HPV vaccine uptake, but uptake is often occurring at older ages that can limit the vaccine׳s population-level effectiveness. Close to half of HPV vaccinated females are still initiating the HPV vaccine after the routine-recommended ages of 11–12, particularly those with a higher social economic status and those not utilizing healthcare services. Further study is necessary to examine the population-level impact of later HPV vaccine initiation on HPV-related outcomes, particularly in these populations.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute (NCI) at the National Institutes of Health. The lead and second authors (D.C. Beachler and F.A. Gonzales) received funding through NCI Cancer Prevention Fellowship Program. The NIS-teen survey data is publicly available.
Acknowledgments
We thank the participants of the NIS-teen survey
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2015.12.001.
Appendix A. Supplementary material
Supplementary material
Refrences
- 1.Bosch F.X., Broker T.R., Forman D., Moscicki A.B., Gillison M.L., Doorbar J. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl. 7):SH1–S31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano A.R., Palefsky J.M., Goldstone S., Moreira E.D., Jr., Penny M.E., Aranda C. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hildesheim A., Wacholder S., Catteau G., Struyf F., Dubin G., Herrero R. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32:5087–5097. doi: 10.1016/j.vaccine.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hildesheim A., Herrero R., Wacholder S., Rodriguez A.C., Solomon D., Bratti M.C. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. J. Am. Med. Assoc. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz L.E., Dunne E.F., Saraiya M., Lawson H.W., Chesson H., Unger E.R. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration. June 8, 2006 Approval Letter-Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant, 2015, 2006.
- 7.Elam-Evans L.D., Yankey D., Jeyarajah J., Singleton J.A., Curtis R.C., MacNeil J. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years – United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014;63:625–633. [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins R.B., Lin M., Silliman R.A., Clark J.A., Hanchate A. Why are U.S. girls getting meningococcal but not human papilloma virus vaccines? Comparison of factors associated with human papilloma virus and meningococcal vaccination among adolescent girls 2008 to 2012. Womens Health Issues. 2015;25:97–104. doi: 10.1016/j.whi.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Bednarczyk R.A., Curran E.A., Orenstein W.A., Omer S.B. Health disparities in human papillomavirus vaccine coverage: trends analysis from the National Immunization Survey-Teen, 2008–2011. Clin. Infect. Dis. 2014;58:238–241. doi: 10.1093/cid/cit707. [DOI] [PubMed] [Google Scholar]
- 10.Kessels S.J., Marshall H.S., Watson M., Braunack-Mayer A.J., Reuzel R., Tooher R.L. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30:3546–3556. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 11.Feiring B., Laake I., Molden T., Cappelen I., Haberg S.E., Magnus P. Do parental education and income matter? A nationwide register-based study on HPV vaccine uptake in the school-based immunisation programme in Norway. BMJ Open. 2015;5:e006422. doi: 10.1136/bmjopen-2014-006422. ,2014-006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariri S., Unger E.R., Sternberg M., Dunne E.F., Swan D., Patel S. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J. Infect. Dis. 2011;204:566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 13.Smith L.M., Strumpf E.C., Kaufman J.S., Lofters A., Schwandt M., Levesque L.E. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics. 2015 doi: 10.1542/peds.2014-2961. [DOI] [PubMed] [Google Scholar]
- 14.Jain N., Singleton J.A., Montgomery M., Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey-Teen 2006. Publ. Health Rep. 2009;124:642–651. doi: 10.1177/003335490912400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Immunization Survey-Teen: a user’s guide for the 2013 Public-Use Data File, 2015, 2014.
- 16.Recommendations on the Use of Quadrivalent Human Papillomavirus Vaccine in Males — Advisory Committee on Immunization Practices (ACIP), 2011, 2012. [PubMed]
- 17.Bilukha O.O., Rosenstein N. National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2005;54:1–21. [PubMed] [Google Scholar]
- 18.Broder K.R., Cortese M.M., Iskander J.K., Kretsinger K., Slade B.A., Brown K.H. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2006;55:1–34. [PubMed] [Google Scholar]
- 19.Lindley M.C., Smith P.J., Rodewald L.E. Vaccination coverage among U.S. adolescents aged 13–17 years eligible for the vaccines for children program, 2009. Publ. Health Rep. 2011;126(Suppl. 2):S124–S134. doi: 10.1177/00333549111260S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004;l159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Winer R.L., Feng Q., Hughes J.P., O׳Reilly S., Kiviat N.B., Koutsky L.A. Risk of female human papillomavirus acquisition associated with first male sex partner. J. Infect. Dis. 2008;197:279–282. doi: 10.1086/524875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavazos-Rehg P.A., Krauss M.J., Spitznagel E.L., Schootman M., Bucholz K.K., Peipert J.F. Age of sexual debut among US adolescents. Contraception. 2009;80:158–162. doi: 10.1016/j.contraception.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finer L.B., Philbin J.M. Sexual initiation, contraceptive use, and pregnancy among young adolescents. Pediatrics. 2013;131:886–891. doi: 10.1542/peds.2012-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman M., McGrath C.J., Hirth J.M., Berenson A.B. Age at HPV vaccine initiation and completion among US adolescent girls: trend from 2008 to 2012. Vaccine. 2015;33:585–587. doi: 10.1016/j.vaccine.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stokley S., Jeyarajah J., Yankey D., Cano M., Gee J., Roark J. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014 – United States. MMWR Morb. Mortal. Wkly. Rep. 2014;63:620–624. [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman M., Islam M., Berenson A.B. Differences in HPV immunization levels among young adults in various regions of the United States. J. Community Health. 2015;40:404–408. doi: 10.1007/s10900-015-9995-2. [DOI] [PubMed] [Google Scholar]
- 27.Kreimer A.R., Rodriguez A.C., Hildesheim A., Herrero R., Porras C., Schiffman M. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J. Natl. Cancer Inst. 2011;103:1444–1451. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safaeian M., Porras C., Pan Y., Kreimer A., Schiller J.T., Gonzalez P. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev. Res. (Phila.) 2013;6:1242–1250. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material