Abstract
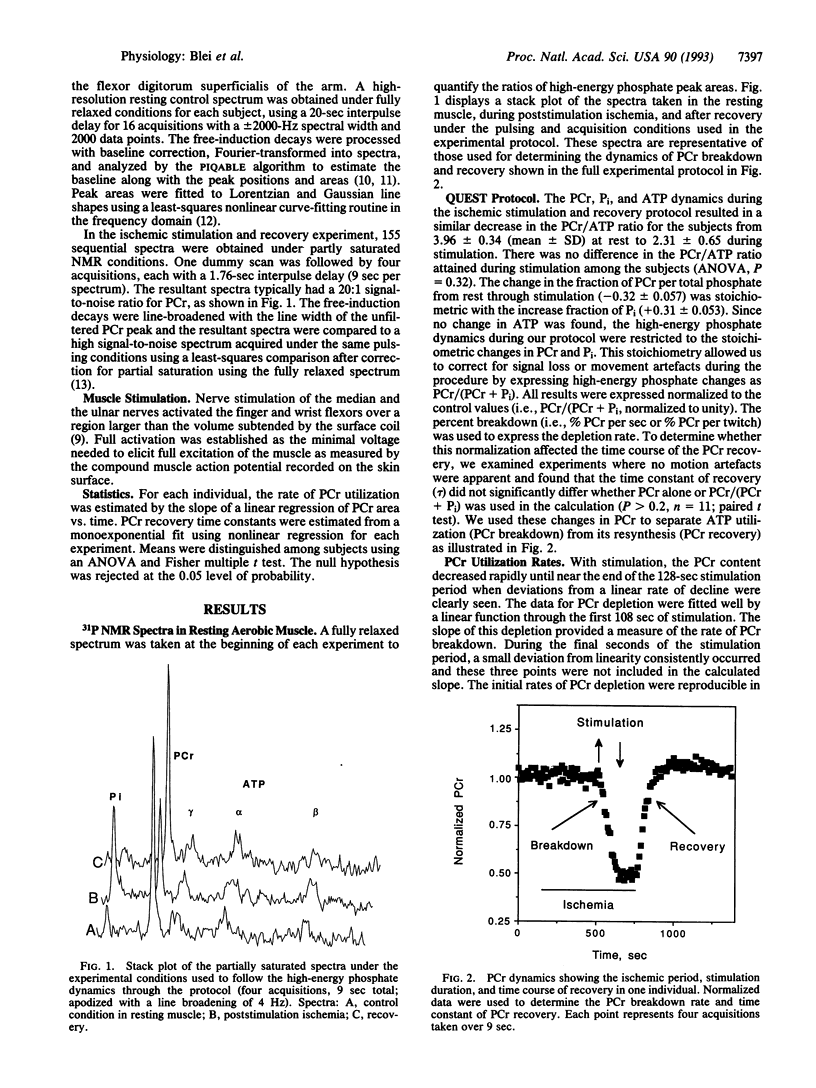
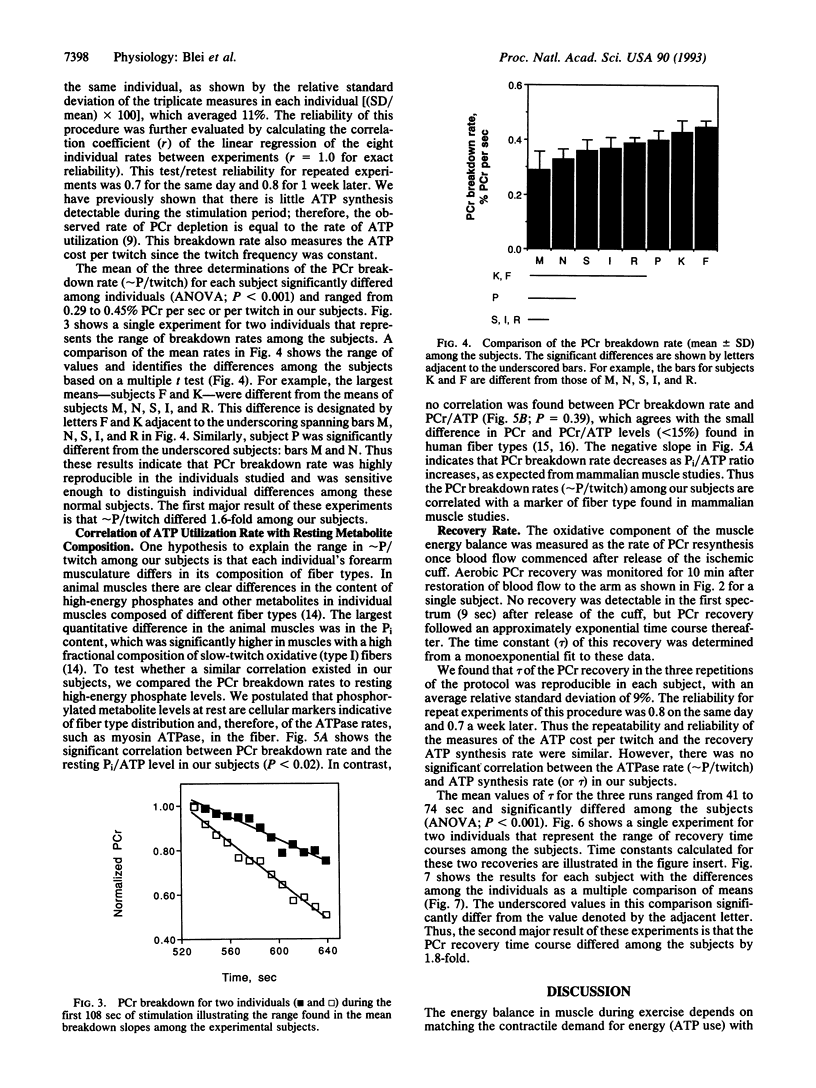
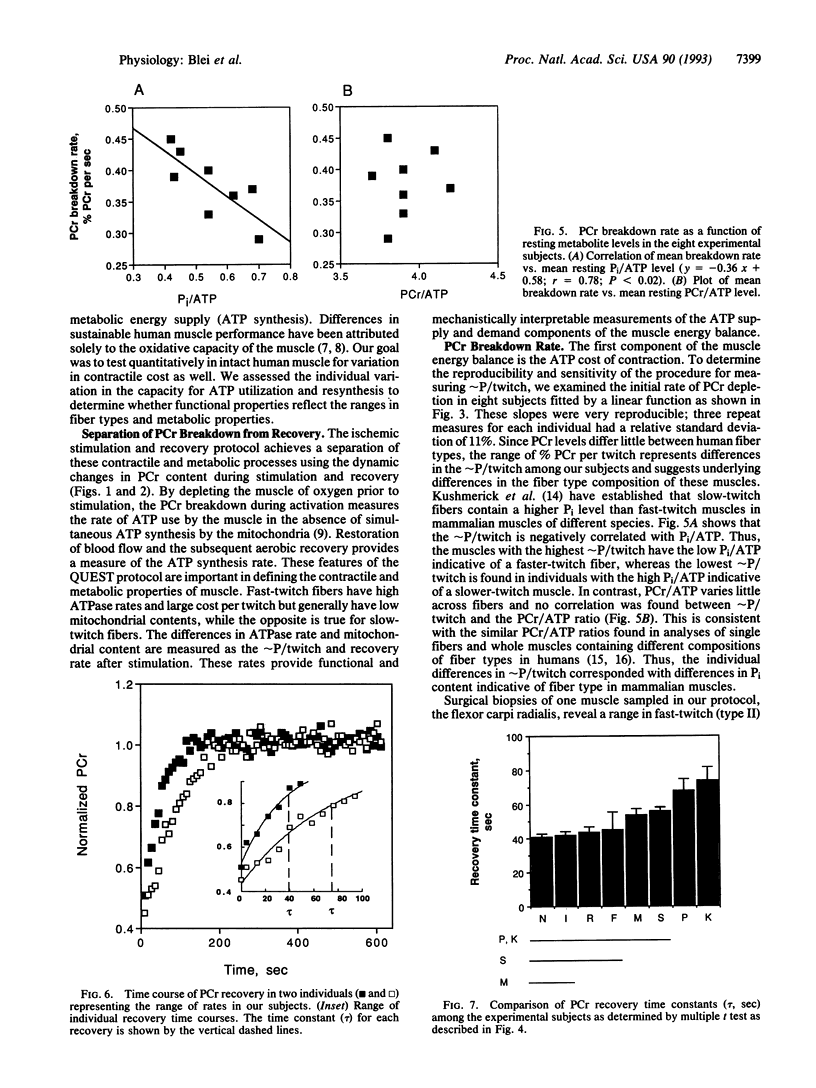
This study determined the variation among individuals in ATP use during contraction and ATP synthesis after stimulation in a human limb muscle. Muscle energetics were evaluated using a metabolic stress test that separates ATP utilization from synthesis by 31P NMR spectroscopy. Epicutaneous supramaximal twitch stimulation (1 Hz) of the median and ulnar nerves was applied in combination with ischemia of the finger and wrist flexors in eight normal subjects. The linear creatine phosphate (PCr) breakdown during ischemic stimulation defined ATP use (delta PCr per twitch or approximately P/twitch) and was highly reproducible as shown by the relative standard deviation [(standard deviation/mean) x 100] of 11% in three repeated measures. The time constant of the monoexponential PCr change during aerobic recovery represented ATP synthesis rate and also showed a low relative standard deviation (9%). Individuals were found to differ significantly in both mean approximately P/twitch (PCr breakdown rates, 0.29-0.45% PCr per sec or % PCr per twitch; ANOVA, p < 0.001) and in mean recovery time constants (41-74 sec; ANOVA, P < 0.001). This range of approximately P/twitch corresponded with the range of fiber types reported for a flexor muscle. In addition, approximately P/twitch was negatively correlated with a metabolite marker of slow-twitch fiber composition (Pi/ATP). The nearly 2-fold range of recovery time constants agreed with the range of mitochondrial volume densities found in human muscle biopsies. These results indicate that both components involved in the muscle energy balance--oxidative capacity and contractile costs--vary among individuals in human muscle and can be measured noninvasively by 31 P NMR.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achten E., Van Cauteren M., Willem R., Luypaert R., Malaisse W. J., Van Bosch G., Delanghe G., De Meirleir K., Osteaux M. 31P-NMR spectroscopy and the metabolic properties of different muscle fibers. J Appl Physiol (1985) 1990 Feb;68(2):644–649. doi: 10.1152/jappl.1990.68.2.644. [DOI] [PubMed] [Google Scholar]
- Blei M. L., Conley K. E., Kushmerick M. J. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol. 1993 Jun;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis D., Bergström M., Hultman E. ATP utilization and force during intermittent and continuous muscle contractions. J Appl Physiol (1985) 1987 Jul;63(1):167–174. doi: 10.1152/jappl.1987.63.1.167. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley G. A., Tullson P. C., Terjung R. L. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987 Jul 5;262(19):9109–9114. [PubMed] [Google Scholar]
- Edström L., Hultman E., Sahlin K., Sjöholm H. The contents of high-energy phosphates in different fibre types in skeletal muscles from rat, guinea-pig and man. J Physiol. 1982 Nov;332:47–58. doi: 10.1113/jphysiol.1982.sp014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman F. W., Eng J., Berkowitz B. A., Balaban R. S. NMR spectral analysis of kinetic data using natural lineshapes. Magn Reson Med. 1990 Mar;13(3):490–497. doi: 10.1002/mrm.1910130316. [DOI] [PubMed] [Google Scholar]
- Howald H., Hoppeler H., Claassen H., Mathieu O., Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. 1985 Apr;403(4):369–376. doi: 10.1007/BF00589248. [DOI] [PubMed] [Google Scholar]
- Kent-Braun J. A., McCully K. K., Chance B. Metabolic effects of training in humans: a 31P-MRS study. J Appl Physiol (1985) 1990 Sep;69(3):1165–1170. doi: 10.1152/jappl.1990.69.3.1165. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Meyer R. A., Brown T. R. Regulation of oxygen consumption in fast- and slow-twitch muscle. Am J Physiol. 1992 Sep;263(3 Pt 1):C598–C606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Moerland T. S., Wiseman R. W. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully K. K., Boden B. P., Tuchler M., Fountain M. R., Chance B. Wrist flexor muscles of elite rowers measured with magnetic resonance spectroscopy. J Appl Physiol (1985) 1989 Sep;67(3):926–932. doi: 10.1152/jappl.1989.67.3.926. [DOI] [PubMed] [Google Scholar]
- Meyer R. A. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol. 1989 Dec;257(6 Pt 1):C1149–C1157. doi: 10.1152/ajpcell.1989.257.6.C1149. [DOI] [PubMed] [Google Scholar]
- Park J. H., Brown R. L., Park C. R., McCully K., Cohn M., Haselgrove J., Chance B. Functional pools of oxidative and glycolytic fibers in human muscle observed by 31P magnetic resonance spectroscopy during exercise. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8976–8980. doi: 10.1073/pnas.84.24.8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D., Staron R. S. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Richards T. L., Davis C. A., Barker B. R., Beinert W. D., Genant H. K. Lipid/water ratio of bone marrow measured by phase-encoded proton nuclear magnetic resonance spectroscopy. Invest Radiol. 1987 Sep;22(9):741–746. doi: 10.1097/00004424-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Söderlund K., Hultman E. ATP and phosphocreatine changes in single human muscle fibers after intense electrical stimulation. Am J Physiol. 1991 Dec;261(6 Pt 1):E737–E741. doi: 10.1152/ajpendo.1991.261.6.E737. [DOI] [PubMed] [Google Scholar]
- Vandenborne K., McCully K., Kakihira H., Prammer M., Bolinger L., Detre J. A., De Meirlier K., Walter G., Chance B., Leigh J. S. Metabolic heterogeneity in human calf muscle during maximal exercise. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5714–5718. doi: 10.1073/pnas.88.13.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]



