Introduction
Macrophages constitute up to 7% of the HIV-1-infected cell population in vivo [1], and their contribution to HIV-1 pathogenesis is increasingly recognized [2] [3]. Multiple lines of evidence support a key role for macrophages in HIV-1 persistence: infected macrophages resist viral cytopathic effects [4], harbour replication competent virus for weeks to months [5], reside in tissues where ART penetration is reduced [6] and are capable of efficient cell-to-cell viral transmission to uninfected CD4+ T cells [7] [8].
Cell-to-cell infection increases the probability of escape from antiretroviral inhibition, and has been proposed to account for ongoing replication in the face of ART [9], a potential explanation for viral persistence [10]. However this proposal is controversial since others have suggested that ART has equivalent efficacy against both cell-free and cell-to-cell spread between T cells [11]. A related unanswered question that is central to this debate is whether resistance to antiretroviral inhibition is quantitative, resulting from a greater number of viruses transferred per cell [9], or whether qualitative differences inherent to the mechanism of cell-to-cell infection between different target cell types are involved [12]. The ability of ART to control HIV-1 replication and spread is of central importance to design of ART regimens [13], and has direct relevance to establishment and maintenance of viral reservoirs and potential HIV-1 cure [14]. For this reason, and given the potential importance of the macrophage reservoir [10] together with evidence that infected tissue macrophages persist in patients during ART [15], we sought to establish whether cell-to-cell transmission from MDM to autologous primary CD4+ T cells is amenable to antiretroviral inhibition. Our approach contrasts with previous studies that have used highly permissive reporter cell-lines and/or readout of viral Gag transfer between cells that may not accurately represent productive infection in the in vivo situation [9, 11, 16].
Methods
MDM isolation and infection
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples from healthy HIV-1-uninfected donors by density gradient centrifugation (Histopaque, Sigma) and monocytes were enriched to high purity (> 95% CD14+) by untouched magnetic selection (MACS Monocyte Isolation Kit II, Miltenyi Biotech). Monocytes were seeded in 24 or 96 well plates at 5 × 105 cells/mL and differentiated to macrophages for 7 days in X-VIVO 10 (Lonza) medium supplemented with 1% human serum as previously described [7]. MDM were subsequently infected for 7 days [7] with replication-competent infectious molecular clones which stably express Renilla reniformis luciferase and macrophage-tropic Env from the BaL or YU-2 isolates [17] in cis with all HIV-1 viral proteins in an isogenic NL4.3 backbone (known as NL-LucR.T2A [17], kindly provided by J. Kappes and C. Ochsenbauer). Viral stocks were prepared by 293T transfection with polyethyleneimine and titered on TZM-bl (JC53) cells as described [18].
CD4+ T cell isolation and infection and ART treatment
Autologous CD4+ T cells were enriched from PBMCs by untouched magnetic selection (MACS CD4+ T cell Isolation Kit, Miltenyi Biotech) to high purity (> 95% CD3+CD4+) and stimulated for 3 days with 1 μg/mL PHA and 10 IU/mL IL-2 (Centre for AIDS Reagents, NIBSC, UK) in RPMI with 10% fetal bovine serum and 1% penicillin/streptomycin (RPMI-10) [7]. Activated CD4+ T cells were then pre-incubated for 1 h with non-toxic concentrations of the nucleoside reverse transcriptase (RT) inhibitor azidothymidine (AZT), the non-nucleoside RT inhibitor nevirapine (NVP), the integrase inhibitor raltegravir (RAL, all from Centre for AIDS Reagents, NIBSC) prior to coculture with infected MDM at a donor to target cell ratio of 1:2, or cell-free infection as described [7]. Cocultures were left static or gently shaken at 75 rpm at 37°C at 5% CO2 [18, 19]. MDM viability under these conditions was assessed with the MTS viability assay (Promega) as previously described [13]. Infections were allowed to proceed for 48 h (approximating a single cycle of replication) during which time antiretroviral drugs were maintained in the medium. At 48 h CD4+ T cells were gently removed (<0.1% macrophage contamination determined by flow cytometry staining for CD3 and CD14), washed in PBS and lysed with Glo-Lysis buffer (Promega). For luciferase quantification cell lysates were mixed 1:1 with Ren-Glo assay solution (Promega) at room temperature and luminescence measured at 1000 ms−1 integration on a SpectroMax M5 plate reader. After subtracting background values, data were normalised to untreated controls to derive the transmission index [9] (Tx): infectiondrug/infectioncontrol. Antiretroviral titration data were fitted to a sigmoidal-dose response curve in GraphPad Prism 5.0 to obtain the 50% inhibitory concentration (IC50). Repeat experiments in multiple independent donors were compared using t-test, one-way ANOVA with Dunnett’s post-test or two-way ANOVA with Tukey’s post-test, and two-tailed α <0.05 was considered significant. Statistical analysis was performed in Prism 5.0.
Results
Reduced ART efficacy against cell-to-cell HIV-1 infection of CD4+ T cells
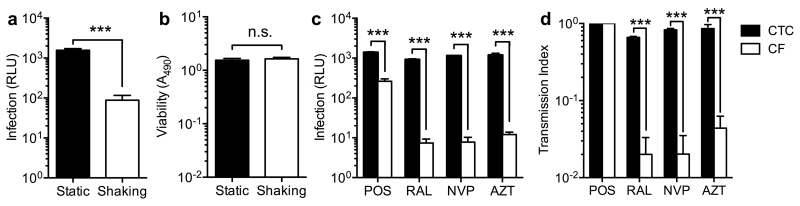
We measured luciferase activity to quantify expression of integrated virus in CD4+ T cells because this is a direct measure of productive infection not confounded by measuring non-infectious transfer of Gag by flow cytometry, or post-integration latency and/or the presence of nonintegrated viral DNA forms by PCR. To assess the relative efficiency of cell-to-cell and cell-free infection we first cocultured infected MDM and CD4+ T cells under gentle shaking conditions (75 rpm) that permit cell-free diffusion but dramatically reduce cell-to-cell transmission by preventing sustained cell-cell contacts [19], and compared the resulting infection levels to parallel co-cultures without gentle shaking. Infections were maintained for 48 h as this approximates a single cycle of infection by both cell-to-cell and cell-free routes [9, 11]. Luciferase production when cell-to-cell transmission was inhibited was approximately 20-fold lower (P<0.001 t-test, Fig. 1a), extending our previous observation that cell-to-cell viral transfer from macrophages is considerably more efficient [7]. Shaking had no effect on cell viability as measured by MTS assay (Fig. 1b) as has been reported previously [18, 19]. As expected, antiretroviral agents effectively inhibited conventional low-multiplicity cell-free infection at the approximate maximum plasma concentrations in patients (Cmax) [20-22]. By contrast, inhibition of luciferase production after cell-to-cell transmission was significantly impaired for all antiretrovirals tested (Fig. 1c), which was reflected in a significantly higher transmission index (Tx, Fig. 1d).
Figure 1. Reduced ART inhibition of CD4+ T cell infection by cell-to-cell spread from MDM compared to cell-free infection.
(a) CD4+ T cells were infected by coculture with MDM infected with HIV-1-LucR in either static (cell-to-cell spread) or gently shaking cultures (cell-free spread), T cells gently washed off and infection quantified by assay of Renilla luciferase activity (relative light units, RLU) in T cell lysates. Shaking culture resulted in productive CD4+ T cell infection above background in all donors (P=0.036 t test, not shown). Bars represent mean ± S.E.M. of experiments in n=4 independent donors, ***P<0.001 t test. (b) Gentle shaking for 48h did not affect MDM viability by MTS assay; bars represent mean ± S.E.M. of experiments in n=5 independent donors, n.s. = non-significant by t test. (c) The reverse transcriptase (RT) inhibitors azidothymidine (AZT, 10 μM) and nevirapine (NVP, 10 μM) and the integrase inhibitor raltegravir (RAL, 2 μM) reduced cell-free HIV-1 infection of CD4+ T cells robustly, but showed minimal impact on luciferase expression during cell-to-cell transmission. Bars represent mean ± S.E.M. of experiments in n=3 independent donors, ***P<0.001 two-way ANOVA plus Tukey’s post-test. (d) Antiretroviral inhibition efficiency was defined by the transmission index (Tx): infection(drug)/infection(no drug). Cell-to-cell transmission reduces ART efficacy and Tx approaches 1, whereas conventional cell-free infection results in a significantly lower Tx. Bars represent mean ± S.E.M. of data from b), ***P<0.001 two-way ANOVA plus Tukey’s post-test.
ART efficacy is dependent upon multiplicity of infection of the target cell
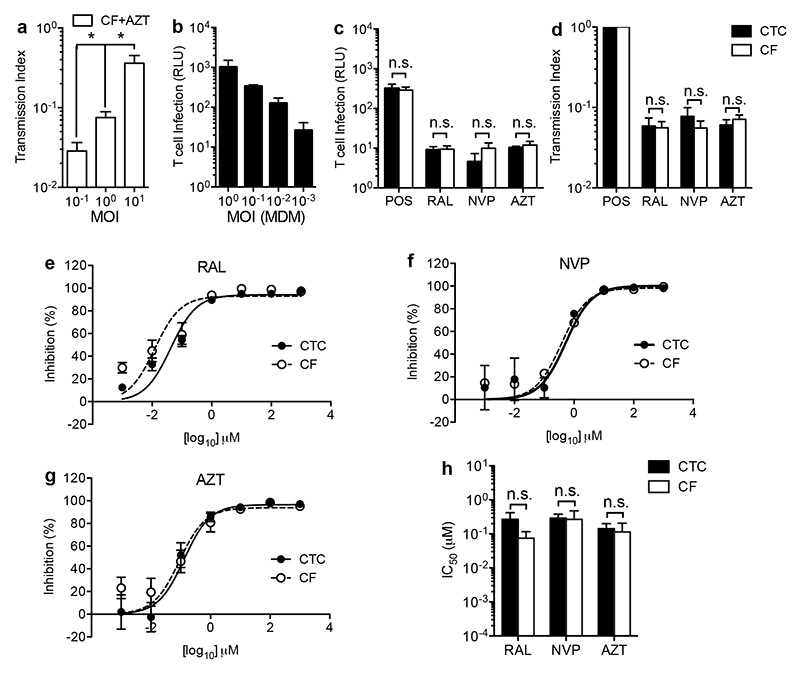
We reasoned that the impaired efficacy of ART against cell-to-cell transmission could result from qualitative differences in the mechanism of viral transfer at the macrophage-T cell interface as a result of virological synapse formation [12], or might simply reflect a higher HIV-1 MOI by direct cell-to-cell spread compared to cell-free spread [9]. To address this, we first infected CD4+ T cells with increasing MOI cell-free inocula in the presence or absence of AZT, which resulted in a stepwise increase in Tx, reflecting the reduced probability of inhibition with increased virus challenge (Fig. 2a). To directly address the effect of MOI under cell-to-cell transmission conditions, we titered initial macrophage inocula to yield equivalent T cell infection by the cell-free and cell-to-cell routes (Fig. 2b and POS control in Fig. 2c). When MOI was controlled in this way, we observed that inhibition by antiretroviral drugs was equally effective by either route (Fig. 2c-d). Under these conditions, titration of ART in both cell-to-cell and cell-free infections demonstrated that the effectiveness of inhibition is not influenced by the mode of viral transmission across a range of antiretroviral concentrations (Fig. 2e-h).
Figure 2. ART efficacy is dependent upon multiplicity of infection of the target cell.
(a) Increasing MOI of CD4+ T cell infection with cell-free HIV-1 ± 10 μM AZT results in a stepwise increase in Tx. Bars represent mean ± S.D. of experimental replicates from a representative experiment in n=1 donor, *P<0.05 one-way ANOVA plus Bonferonni post-test. (b) Titration of MDM HIV-1 inocula permitted control of CD4+ T cell infection levels in the cell-to-cell (CTC) conditions to achieve equal multiplicity to cell-free (CF) conditions (see untreated positive control, POS in (c)). Bars represent mean ± SEM of experiments in n=3 donors. (c) Titration of CTC infections in turn yielded equivalent levels of antiretroviral inhibition of luciferase expression at approximate in vivo Cmax concentrations (RAL 2 μM, AZT and NVP 10 μM,); bars represent mean ± SEM of experiments in n=5 independent donors; n.s. = non-significant by two-way ANOVA plus Tukey’s post-test. (d) Equalising MOI of cell-free and cell-to-cell infection resulted in equivalent Tx; Bars represent mean ± S.E.M. of data from (c); n.s. = non-significant by two-way ANOVA plus Tukey’s post-test. Titration of antiretroviral agents RAL (e), NVP (f) and AZT (g) resulted in equivalent IC50 values (h, inhibition % expressed as 100 × (1-[luciferasedrug/luciferasecontrol]). Lines are best-fit sigmoidal dose-response curves, bars represent mean ± SEM of experiments in n=3 (CF) and n=5 (CTC) independent donors. No significant difference in IC50 was observed by two-way ANOVA plus Tukey’s post-test (n.s. = non-significant).
Discussion
These findings have important implications for HIV-1 treatment and eradication strategies. Available evidence suggests that the enhanced efficiency of cell-to-cell transmission derives from polarized budding of HIV-1 from the donor cell towards the target cell [23], viral receptor clustering on the target cell [7, 24] and the close proximity of donor and target cells at the virological synapse [7, 12, 18]. If cell-to-cell infection were qualitatively drug-insensitive, the implication is that efforts to drive transcription from latently infected cells under ART would fail due to uninhibited expansion of the infected cell population [9]. However, our data demonstrate that cell-to-cell infection is equally susceptible to antiretroviral inhibition when the target cell viral challenge ‘dose’ is equivalent, suggesting that the increased efficiency is not qualitative, but quantitative. This agrees with recent data on productive T cell-to-T cell spread [9, 11, 25] and supports the concept that aspects of HIV-1 cell-to-cell transmission relevant to antiretroviral activity are qualitatively similar to cell-free infection [12]. However the precise mechanisms governing high multiplicity macrophage HIV-1 transmission, particularly the synaptic molecular architecture and the influence of target cell/viral phenotype, require further definition.
In this and other model systems MOI appears to be the principal in vitro determinant of antiretroviral activity in the absence of resistance mutations [9, 11]. The extremely high titer extracellular concentrations of virus needed to saturate antiretroviral activity are unlikely to be reached in vivo by cell-free spread, where virion infectivity is limited by fluid phase dilution and diffusion coupled with rapid Env degeneration [12]. By contrast, sufficiently high multiplicities might be achieved in tissues in which target cells are densely packed, cell-to-cell transmission occurs [26, 27], and antiretroviral penetrance is reduced [6].
Acknowledgements
We thank Dr John Kappes and Dr Christina Ochsenbauer for providing the luciferase reporter constructs, Sir Andrew McMichael for support and guidance, and the Centre for AIDS Reagents, National Institute for BC for providing antiretroviral agents. This work was supported by the Wellcome Trust (094449/Z/10/Z), the MRC (G0901732), Dormeur Investment Services. QJS is a James Martin Senior Fellow and a Jenner Vaccine Institute Investigator.
Footnotes
Conflicts of interest
No conflicts of interest are declared.
References
- [1].Schacker T, Little S, Connick E, Gebhard K, Zhang ZQ, Krieger J, et al. Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J Infect Dis. 2001 Feb 15;183(4):555–62. doi: 10.1086/318524. [DOI] [PubMed] [Google Scholar]
- [2].Cobos-Jimenez V, Booiman T, Hamann J, Kootstra NA. Macrophages and HIV-1. Curr Opin HIV AIDS. 2011 Sep;6(5):385–90. doi: 10.1097/COH.0b013e3283497203. [DOI] [PubMed] [Google Scholar]
- [3].Aggarwal A, McAllery S, Turville SG. Revising the Role of Myeloid cells in HIV Pathogenesis. Curr HIV/AIDS Rep. 2012 Dec 16; doi: 10.1007/s11904-012-0149-1. [DOI] [PubMed] [Google Scholar]
- [4].Swingler S, Mann AM, Zhou J, Swingler C, Stevenson M. Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog. 2007 Sep 7;3(9):1281–90. doi: 10.1371/journal.ppat.0030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005 Jul 6;24(13):2481–9. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sigal A, Baltimore D. As good as it gets? The problem of HIV persistence despite antiretroviral drugs. Cell Host Microbe. 2012 Aug 16;12(2):132–8. doi: 10.1016/j.chom.2012.07.005. [DOI] [PubMed] [Google Scholar]
- [7].Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008 May 1;111(9):4660–3. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- [8].Gousset K, Ablan SD, Coren LV, Ono A, Soheilian F, Nagashima K, et al. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS pathogens. 2008 Mar;4(3):e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011 Sep 1;477(7362):95–8. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- [10].Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012 Sep 21;37(3):377–88. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Permanyer M, Ballana E, Ruiz A, Badia R, Riveira-Munoz E, Gonzalo E, et al. Antiretroviral agents effectively block HIV replication after cell-to-cell transfer. J Virol. 2012 Aug;86(16):8773–80. doi: 10.1128/JVI.01044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sattentau QJ. Cell-to-Cell Spread of Retroviruses. Viruses. 2010 Jun;2(6):1306–21. doi: 10.3390/v2061306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carter GC, Bernstone L, Baskaran D, James W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and Pak1. Virology. 2011 Jan 20;409(2):234–50. doi: 10.1016/j.virol.2010.10.018. [DOI] [PubMed] [Google Scholar]
- [14].Durand CM, Blankson JN, Siliciano RF. Developing strategies for HIV-1 eradication. Trends Immunol. 2012 Nov;33(11):554–62. doi: 10.1016/j.it.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zalar A, Figueroa MI, Ruibal-Ares B, Bare P, Cahn P, de Bracco MM, et al. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antiviral Res. 2010 Aug;87(2):269–71. doi: 10.1016/j.antiviral.2010.05.005. [DOI] [PubMed] [Google Scholar]
- [16].Martin N, Sattentau Q. Cell-to-cell HIV-1 spread and its implications for immune evasion. Curr Opin HIV AIDS. 2009 Mar;4(2):143–9. doi: 10.1097/COH.0b013e328322f94a. [DOI] [PubMed] [Google Scholar]
- [17].Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, et al. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010 Dec 5;408(1):1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Martin N, Welsch S, Jolly C, Briggs JA, Vaux D, Sattentau QJ. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J Virol. 2010 Apr;84(7):3516–27. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007 Jan;81(2):1000–12. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Heeswijk RP, Veldkamp AI, Mulder JW, Meenhorst PL, Wit FW, Lange JM, et al. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1-infected individuals. Aids. 2000 May 26;14(8):F77–82. doi: 10.1097/00002030-200005260-00001. [DOI] [PubMed] [Google Scholar]
- [21].Wintergerst U, Rolinski B, Vocks-Hauck M, Wahn V, Debatin KM, Notheis G, et al. Pharmacokinetics of orally administered zidovudine in HIV-infected children and adults. Infection. 1995 Nov-Dec;23(6):344–8. doi: 10.1007/BF01713563. [DOI] [PubMed] [Google Scholar]
- [22].Rizk ML, Hang Y, Luo WL, Su J, Zhao J, Campbell H, et al. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob Agents Chemother. 2012 Jun;56(6):3101–6. doi: 10.1128/AAC.06417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jolly C, Welsch S, Michor S, Sattentau QJ. The regulated secretory pathway in CD4(+) T cells contributes to human immunodeficiency virus type-1 cell-to-cell spread at the virological synapse. PLoS pathogens. 2011 Sep;7(9):e1002226. doi: 10.1371/journal.ppat.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004 Jan 19;199(2):283–93. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Del Portillo A, Tripodi J, Najfeld V, Wodarz D, Levy DN, Chen BK. Multiploid inheritance of HIV-1 during cell-to-cell infection. J Virol. 2011 Jul;85(14):7169–76. doi: 10.1128/JVI.00231-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, et al. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012 Oct 11;490(7419):283–7. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sewald X, Gonzalez DG, Haberman AM, Mothes W. In vivo imaging of virological synapses. Nat Commun. 2012;3:1320. doi: 10.1038/ncomms2338. [DOI] [PMC free article] [PubMed] [Google Scholar]