Abstract
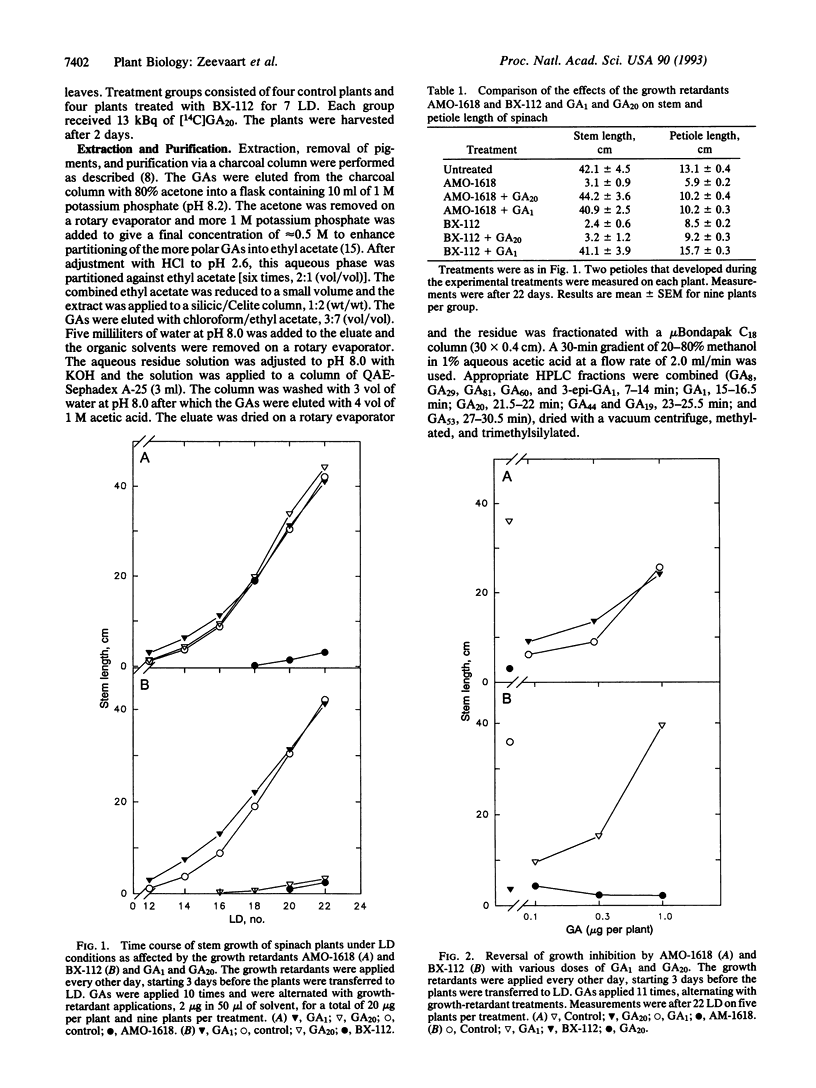
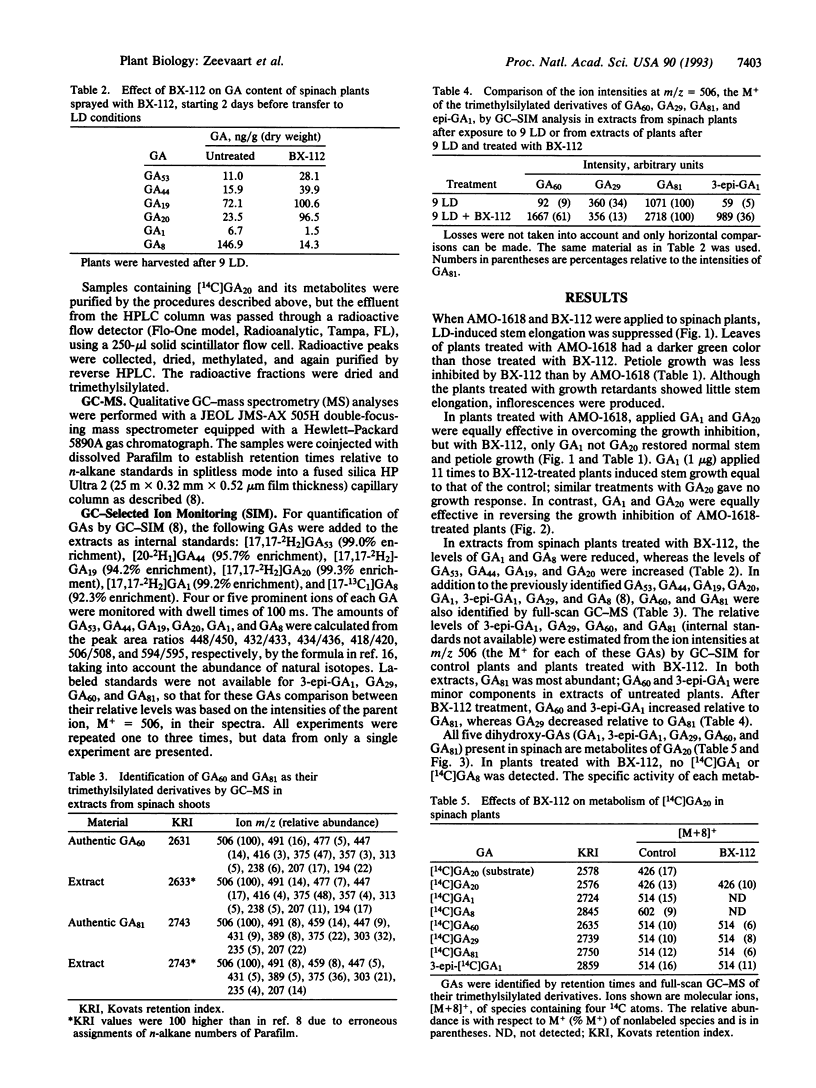
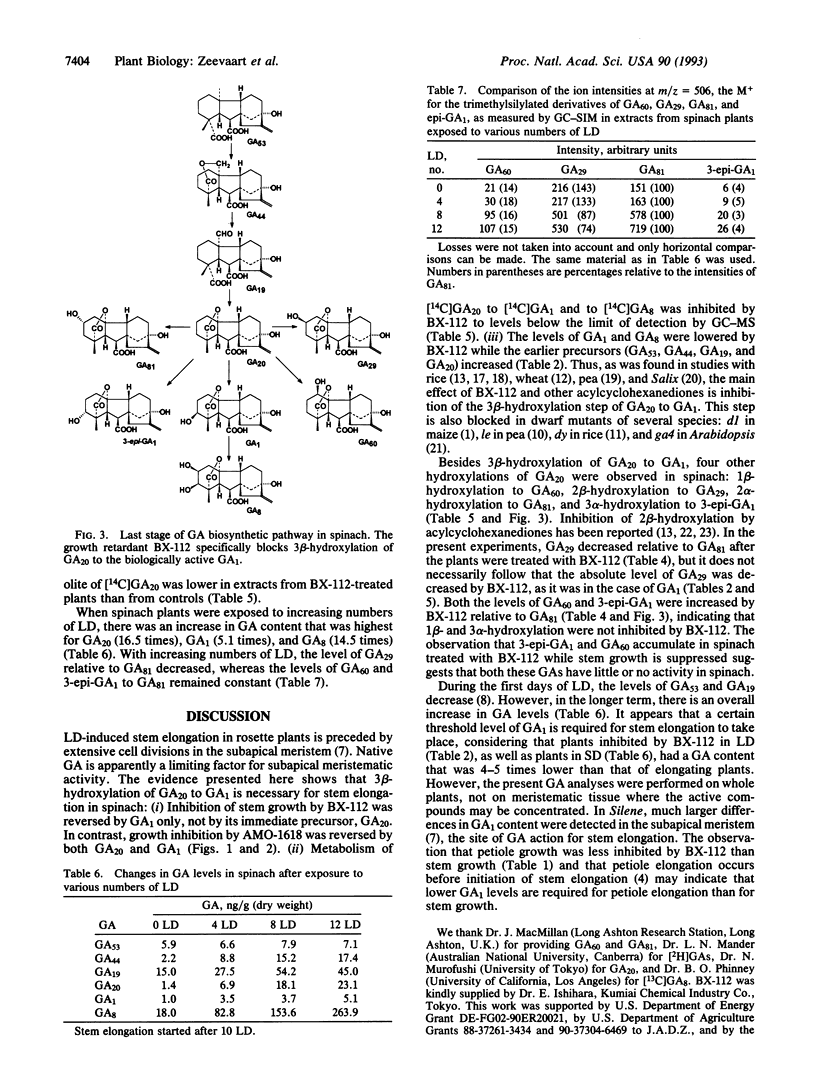
The effects of the growth retardants 2'-isopropyl-4'-(trimethylammonium chloride)-5'-methylphenyl piperidine-1-carboxylate (AMO-1618) and calcium 3,5-dioxo-4-propionylcyclohexanecarboxylate (BX-112) on stem elongation were investigated in the rosette plant spinach (Spinacia oleracea L.) under long-day (LD) conditions. Stem growth induced by a LD treatment was prevented by both retardants. The inhibition caused by AMO-1618 was reversed by gibberellin A1 (GA1) and GA20, whereas the effects of BX-112 were reversed by GA1 only. Six GAs (GA53, GA44, GA19, GA20, GA1, and GA8) were quantified by gas chromatography-selected ion monitoring using internal standards. Plants treated with BX-112 had reduced levels of GA1 and GA8 and accumulated GA53, GA44, GA19, and GA20. The relative levels of four additional GAs (3-epi-GA1, GA29, GA60, and GA81) were compared by ion intensities only. Relative to GA81, the level of GA29 was decreased by BX-112, whereas the levels of GA60 and 3-epi-GA1 were increased. Transfer of spinach from short-day conditions to LD conditions caused an increase in all identified GAs of the early 13-hydroxylation pathway with GA20, GA1, and GA8 showing the largest increases. These findings support the position that, of the GAs belonging to the early 13-hydroxylation pathway, GA1 is the primary GA active per se for stem elongation in spinach. The increase in endogenous GA1 in plants in LD conditions is most likely the primary factor for stem elongation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujioka S., Yamane H., Spray C. R., Gaskin P., Macmillan J., Phinney B. O., Takahashi N. Qualitative and Quantitative Analyses of Gibberellins in Vegetative Shoots of Normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 Seedlings of Zea mays L. Plant Physiol. 1988 Dec;88(4):1367–1372. doi: 10.1104/pp.88.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour S. J., Zeevaart J. A., Schwenen L., Graebe J. E. Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol. 1986 Sep;82(1):190–195. doi: 10.1104/pp.82.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Odriozola P., Miguel de la Villa F., Solano López D., de las Heras B., Franco Vicario R., Bilbao Goitia P., García Jiménez N., Muñoz Sánchez J. Repercusiones hemodinámicas de la ascitis a tensión y de la paracentesis evacuadora. Rev Clin Esp. 1992 Oct;191(5):245–251. [PubMed] [Google Scholar]
- Sponsel V. M., Reid J. B. Use of an Acylcyclohexanedione Growth Retardant, LAB 198 999, to Determine Whether Gibberellin A(20) Has Biological Activity per se in Dark-Grown Dwarf (le) Seedlings of Pisum sativum. Plant Physiol. 1992 Oct;100(2):651–654. doi: 10.1104/pp.100.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M., Koornneef M., Zeevaart J. A. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7983–7987. doi: 10.1073/pnas.87.20.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M., Zeevaart J. A., Gage D. A. Identification of Gibberellins in Spinach and Effects of Light and Darkness on their Levels. Plant Physiol. 1991 Dec;97(4):1521–1526. doi: 10.1104/pp.97.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M., Zeevaart J. A. Gibberellins and Stem Growth as Related to Photoperiod in Silene armeria L. Plant Physiol. 1990 Apr;92(4):1094–1100. doi: 10.1104/pp.92.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Effects of photoperiod on growth rate and endogenous gibberellins in the long-day rosette plant spinach. Plant Physiol. 1971 Jun;47(6):821–827. doi: 10.1104/pp.47.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A., Gage D. A. ent-kaurene biosynthesis is enhanced by long photoperiods in the long-day plants Spinacia oleracea L. and Agrostemma githago L. Plant Physiol. 1993 Jan;101(1):25–29. doi: 10.1104/pp.101.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]



