Abstract
Background
Objective motor performance measures, especially gait assessment, could improve assessment of surgical low back disorder procedures. However, no study has compared the relative effectiveness of gait parameters for assessing motor performance in low back disorder after surgery. The purpose of the current review was to determine the sensitive gait parameters that address physical improvements in each specific spinal disorder after surgical intervention.
Methods
Articles were searched with the following inclusion criteria: 1) population studied consisted of individuals with low back disorders requiring surgery; 2) low back disorder was measured objectively using gait assessment tests pre- and post-surgery. The quality of the selected studies was assessed using Delphi consensus, and meta-analysis was performed to compare pre- and post-surgical changes.
Findings
Thirteen articles met inclusion criteria, which, almost exclusively, addressed only two types of spinal disorders/interventions: 1) scoliosis/spinal fusion; and 2) stenosis/decompression. For patients with scoliosis, improvements in motion of hip and shoulder (effect size=0.32–1.58), energy expenditure (effect size=0.59–1.18), and activity symmetry of upper-body muscles during gait were present after spinal fusion. For patients with spinal stenosis, increases in gait speed, stride length, cadence, symmetry, smoothness of walking, and walking endurance (effect size=0.60–2.50), and decrease in gait variability (effect size=1.45) were observed after decompression surgery.
Interpretation
For patients with scoliosis, improvements can be better assessed by measuring upper-body motion and EMG rather than the lower extremities during gait. For patients with spinal stenosis, motor performance improvements can be captured by measuring walking spatio-temporal parameters, gait patterns, and walking endurance.
Keywords: Back pain, physical impairment, operation, functional disorder, outcome, evidence
Introduction
Low back disorder (LBD), defined broadly as a common disorder involving the muscles and bones of the lumbar region, is the fifth most common reason for medical treatment, and costs up to 50 billion dollars annually in the United States alone [1]. Nearly 80% of adults experience at least one episode of LBD, and up to 85% have lifetime recurrences [2]. Previous records demonstrated an increasing rate of lumbar surgery from 2.5 procedures per 1000 Medicare enrollees in 1992, to 4.0 procedures/1000 in 2003 in the United States [3]. Many patients with LBD undergo numerous treatment modalities, some of which are highly controversial [4]. Accordingly, the number of re-operations is as high as 15% for the total number of lumbar spine surgeries [5]. With the multitude of options available, it is important to have clear methods for assessing surgical outcomes in order to evaluate best practices, reduce the re-operation rates, maximize value (cost/quality), and improve quality of life and function.
The severity of LBD is commonly categorized according to the degree of subjective pain, disability, and physical impairment [6]. Disability is defined as diminished capacity for everyday activities and gainful employment, while physical impairment corresponds to the physiological abnormality that may induce loss of motor performance [6]. Pain and disability assessments are highly personal; for example, the visual analog scale (VAS) for pain and the Oswestry Disability Questionnaire are patient-reported measures [6, 7]. One potential problem with patient-reported outcome measures (PROMs) is that they incorporate psychological factors, and along with patient attitudes and beliefs, these may skew outcome measurements and results [8]. Objective methods, or functional outcome measures (FOMs), may improve assessment of individual differences following surgical procedures [9]. Therefore, it is important to utilize objective assessments, in addition to subjective questionnaires, to refine diagnoses and evaluate surgical efficacy, especially to assess improvements in motor performance.
Several studies [10–12] have demonstrated a high reliability in using gait assessment as an objective tool for detecting physical impairments in patients with LBDs. In these studies, analysis of gait demonstrated several differences in spatio-temporal parameters between patients with LBD and healthy controls; these differences were more pronounced for those with higher self-reported levels of low back pain [10]. Furthermore, participants with spinal scoliosis showed significant trunk asymmetry motion during walking, which worsened with the severity of the deformity [12]. In lieu of motion analysis, electromyography assessment of muscles during gait in participants with LBD indicated an abnormal extra muscle activation of lumbar muscles during walking [11]. Although evidence exists that gait assessment provides a robust tool for quantitative measurement of the interconnection between motor performance and musculoskeletal pain and deformity of the low back, no study, to our knowledge, has compared the relative effectiveness of different gait parameters for assessing motor performance in LBD after surgery. Therefore, the aim of the current systematic review was to summarize results from clinical studies to determine the sensitive gait parameters that address physical improvements in each specific spinal disorder after surgical intervention.
Methods
Article selection
A systematic literature review was planned and performed using methods specified in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic review [13]. Both controlled vocabulary terms (e.g., MeSH) and key words were used to search the following databases for articles focused on objective measures or instruments for assessing LBD pre- and post-surgery: PubMed/MEDLINE (1946–2014), Elsevier/Embase (1947–2014), Wiley/Cochrane Library (1898–2014), Thomson-Reuters/Web of Science (1898–2014), and EBSCO/CINAHL (Plus with Full Text)(1981–2014). Literature searches were completed in August, 2014. The complete PubMed/MEDLINE search strategy, upon which the other database searches were also based, is available in Appendix A. Reference lists of, and citations to, the articles ultimately selected from the database searches were also screened.
Inclusion criteria were as follows: 1) the population studied consisted of individuals with LBD requiring surgery; and 2) LBD was measured objectively using gait assessment tests pre- and post-surgery. Gait measurements include quantitative assessments of walking performance in front of researcher, such as spatio-temporal parameters, endurance, or muscle activation patterns. No publication date or language limits were applied. Two independent reviewers performed the study selection (TGN, TY). In case of disagreements, a third reviewer (NT) cast the deciding vote. Titles and abstracts of retrieved references were initially screened for relevance. Subsequently the full texts of the potential articles were analyzed to see if they met full inclusion criteria. Letters, case studies, and systematic reviews/meta-analyses were excluded.
Quality assessment of selected studies
The quality of the selected studies was assessed using the Delphi consensus process [14]. The Delphi quality assessment was developed from three rounds of consensus among experts about a set of generic core items for quality assessment of randomized clinical trials. The criteria for Delphi consensus include: treatment allocation (randomization and treatment concealment); similarity of groups at the baseline measurements; availability of eligibility criteria for selecting participants; blinding the patient, caregiver, and outcome assessor to the study design; presenting point estimator and variability measures; and inclusion of intention-to-treatment analysis. Four of the criteria including randomization, concealing randomization, blinding of the care provider, and blinding of the patient were not rated because it is difficult, if not impossible, to randomize or blind patients and care providers to surgical treatment assignment. However, blinding of outcome assessors was included as one of the quality criteria.
An additional score was added to the Delphi criteria to account for inclusion of a healthy participants control group when comparing pre- and post-surgery outcomes. This additional score was added because in many objective parameters, in contrast with subjective ones, the normal ranges for a healthy population may not be obvious.
Comparison of objective gait assessment: Meta-analysis
Objective parameters were compared between pre- and post-surgery sessions, and also with a control group when possible. Cohen’s effect size was calculated with either the reported mean (standard deviation - SD) values pre- and post-surgery or the P-value and sample size when mean (SD) values were not available. Meta-analysis was performed to compare changes pre-and post-surgery in parameters collected from similar samples of participants (i.e., similar LBD diagnoses) undergoing analogous surgical procedures. Only studies with similar timing of post-surgery assessments (i.e., either one year or two years follow up measurements) were used in the meta-analysis. For the purpose of meta-analysis, studies were weighted based on both the number of participants within the pre- and post-surgery measurements and the quality scores of the studies. To assess homogeneity of outcomes in the selected studies, the Q test (significant inhomogeneity for P < 0.05) was performed and the I2 index (0% –100% scale) was calculated. The overall surgery effect on selected parameters was estimated using Cohen’s Random Effects approach. All results for the meta-analysis were generated by MetaXL (version 1.32; EpiGear, Wilston, Australia).
Results
We found 13,866 articles through database searches, and 15 additional articles through citation analysis of the most relevant articles. Of the 11,097 articles that remained after duplicates were removed, 11,020 were excluded because of irrelevance to the topic (Figure 1). Strict inclusion criteria, as outlined above, were applied to the full text of 77 articles. Of these, 13 met the full set of criteria [15–27] (Tables 1 and 2). Except for one study [19], objective parameters were reported quantitatively pre- and post-surgery in the selected articles.
Figure 1.
Flowchart of the process of literature search and extraction of studies meeting the inclusion criteria
Table 1.
Changes in gait parameters pre- and post-surgery. For each study participants’ Information, diagnosis, and treatment are provided. See Table 2 for parameter definitions and measurement methods.
| Reference | Subject | Surgery | Age mean (SD or range) | Significantly altered gait parameters based on effect size (ES) |
|---|---|---|---|---|
| Adachi [15] | Stenosis: n=33 | Laminoplasty | Stenosis: 68.8 (45–83) | Pre vs one-year post (intermittent claudication): Increase in intermittent claudication score |
| Deen [16] | Stenosis: n=50 | Decompression | Stenosis: 72 | Pre vs three-month post (walking endurance): Reduction in walking time to first pain symptoms at preferred speed (ES=2.50), and at 0.54 m/sec (ES=2.34), walking time to severe pain symptoms at preferred speed (ES=1.50), and at 0.54 m/sec (ES=1.37) |
| Engsberg [17] | Scoliosis: n=20 Healthy: n=9 |
Fusion | Scoliosis: 49 (10) Healthy: 45 (9) |
Two-year post vs healthy (body motion and walking endurance): Reduction in shoulder coronal orientation with respect to pelvis (ES=2.85), increase in shoulder sagittal orientation with respect to pelvis (ES=1.81). No change in walking endurance, hip and knee motion, or vertical alignment. |
| Gottipati [26] | Stenosis: n=12 | Reconstructive | Stenosis: 59.6 (8.4) | Pre vs six-month post (spatio-temporal and body motion): Reduction in standing alignment (ES=1.77), walking alignment (ES=1.68), mid-terminal knee stance knee flexion (ES=2.12), increase in gait speed (ES=0.59), stride length (ES=0.96), step width (ES=0.30), and no change in cadence |
| Hasday [18] | Fracture: n=6 | Rod removal | Fracture: 28.0 (6.7) | Pre vs immediate post (spatio-temporal parameters): Increase in walking speed and stride length; reduction in cadence |
| Hopf [19] | Scoliosis: n=23 Health: n=8 |
Fusion | Scoliosis: 10.4 (11.3–29.3) Healthy: 10.7 (14–34) |
Pre vs four-week post (electromyography): Reduction in asymmetry in lumbar muscles of the convex side of double major scolioses, the glutaeus medius and tensor fascia lata muscles of the concave side of thoracic curvatures. No change in lower extremity muscles. |
| Khodadadeh [20] | Scoliosis: n=30 Healthy: n=20 |
Fusion | Scoliosis: 47 Healthy: 40 |
Pre vs six-month and two-year post (spatio-temporal parameters): Increase in speed (ES=0.63) only in two-year. No change in cadence and step length. |
| Lenke [21] | Scoliosis: n=30 | Fusion | Scoliosis: 14 (12–18) | Pre vs one-year and two-year post (spatio-temporal parameters and body motion): Reduction in speed (ES=0.63) and cadence (ES=0.70) in one-year and two-year. Reduction in stride length (ES=0.35) in two-year, reduction in stride width (ES=0.32) in one-year. No change in knee or ankle angle at heel contact time. |
| Mahaudens [22] | Scoliosis: n=19 | Fusion | Scoliosis: 15.0 (1.4) | Pre vs one-year post (spatio-temporal parameters, body motion, electromyography, and walking efficiency): Increase in step length (ES=0.75), and reduction in cadence (ES=0.60). Increase in range of hip motion (ES=0.83), and reduction in range of shoulder motion (ES=0.79). Increase in external work (ES=1.18) and muscle efficiency (ES=0.83). No change in walking speed, stance phase, knee and ankle range of motion, duration of muscle activity of lower extremity and postural muscles. |
| Papdakis [23] | Scoliosis: n=12 | Decompression | Scoliosis: 50 (14) | Pre vs six-month and one-year post (gait variability): Reduction in differences of gait patterns between gait cycles in one-year (ES=1.08) and two-year (ES=1.45). |
| Paul [27] | Scoliosis: n=16 | Fusion | Scoliosis: 14.4 (3.8) | Pre vs one-year post (spatio-temporal parameters and body motion): Reduction in center of mass excursion during walking (ES=1.15), reduction in inclination angle (ES=0.57). No change in gait speed. |
| Shiomi [24] | Scoliosis: n=68 Healthy: n=168 |
Fusion | Scoliosis: 14.9 (12–24) Healthy: 14.3 (9–21) |
Pre vs immediate post (spatio-temporal parameters): Increase in step width (ES=0.60). No change in walking speed, step length, and cadence. |
| Suda [25] | Stenosis: n=35 Healthy: n=50 |
Decompression | Scoliosis: 63.7 Healthy: 64.4 |
Immediate post vs pre (spatio-temporal parameters and walking patterns): Increase in walking speed (ES=0.90), stride length (ES=0.74), cadence (ES=0.70). Reduction in walking symmetry, reappearance, smoothness, sway, rhythm, impact (ES>0.99). No change in step width. |
Table 2.
Objective gait parameter definitions. The reference and measurement method for each parameter is presented.
| Gait Parameters | Definition | Reference | |
|---|---|---|---|
| Intermittent claudication score | Score based on walking distance without intermittent claudication | Adachi [15] (Walkway-eyeball) | |
| Time to first symptom at 0.54 m/sec or preferred speed | Time to first symptoms such as leg pain, back pain, generalized fatigue, etc at predefined speed of 0.54 m/sec or participant’s preferred speed on a treadmill | Deen [15] (Treadmill) | |
| Total ambulation time at 0.54 m/sec or preferred speed | Time to severe symptoms (level of discomfort that make patient stop walking) at predefined speed of 0.54 m/sec or participant’s preferred speed on a treadmill | ||
| Treadmill time | Walking time on treadmill until achieving 70–75% max heart rate | Engsberg [17] (Treadmill-Camera) | |
| Hip or knee flexion at initial contact | Hip or knee flexion angle at the heel strike; mean value across left and right sides | ||
| Shoulder sagittal, coronal, or transverse RoM wrt pelvis | Range of angular rotation of shoulders in sagittal, coronal, or transverse plane with respect to pelvis | ||
| Sagittal or coronal plane vertical alignment | Horizontal distance from S2 to a vertical line dropped from C7 in the sagittal or coronal plane at the heel strike; mean value across left and right sides | ||
| Static or dynamic alignment | Anterior-posterior distance between C7 and the posterior superior aspect of the S1 vertebrae during quiet standing or walking | Gottipati [26] (Walkway-Camera) | |
| Mid terminal stance knee flexion | The magnitude of knee flexion at mid terminal stance | ||
| Speed | Walking velocity | ||
| Step length | The distance between the point of initial contact of one foot and the point of initial contact of the opposite foot | ||
| Stride length | The distance between successive points of initial contact of the same foot | ||
| Step width | Mediolateral space between the two feet during gait | ||
| Cadence | Number of steps per unit time | ||
| Change in stride length, velocity, and cadence | Change as a percentage of values from a normal sample | Hasday [18] (Walkway-Electronic footswitch and Forceplate) | |
| knee or ankle angle at initial contact | Knee or ankle angle in the sagittal, coronal, or transverse plane at the heel strike; mean value across left and right sides | Lenke [21] (Walkway-Camera) | |
| Stance phase | Interval in which the foot is on the ground as a percentage of the gait cycle | Mahaudens [22] (Treadmill-Camera, EMG, and Ergospirometer) | |
| Pelvis, hip, shoulder, knee, or ankle motion | Range of pelvis, hip, shoulder, knee, or ankle rotation in the frontal, sagittal, or transvers plane during a gait cycle | ||
| Sagittal ankle, knee, hip, or pelvis angular velocity | Range of ankle, knee, hip, or pelvis angular velocity in the sagittal plane during a gait cycle | ||
| Quadratus lumborum, Erector spinae, Gluteus medius, Rectus femoris, Semitendinosus, Tibialis anterior, Gastrocnemius EMG duration | Duration of muscle activity as the percentage of a gait cycle duration | ||
| External and internal work | The external work performed by the muscles to accelerate and lift the center of mass of the body | ||
| Internal work | Internal work performed to move the body segments relative to the center of mass of the body | ||
| Energy Cost | Energy consumption measured with oxygen intake | ||
| Muscle efficiency | Total work over energy cost | ||
| Gait Variability -Differential entropy | Spectral differential entropy in frequency domain; a measure of differences in gait patterns between gait cycles | Papdakis [23] (Walkway-tri-axial accelerometer sensor) | |
| CoM peak lateral or vertical displacement | The peak lateral or vertical excursion of the CoM from the path of longitudinal motion | Paul [27] (Walkway-Camera and Forceplate) | |
| Coronal or Sagittal peak inclination angle | The angle between the vertical line through the CoP and the CoM-CoP line in coronal or sagittal plane | ||
| Step width | Mediolateral space between the two feet during gait | Shiomi [24] (Walkway-Forceplate) | |
| Speed | Walking velocity | Suda [25] (Walkway-Forceplate) | |
| Stride length | The distance between successive points of initial contact of the same foot | ||
| Interval | Mediolateral space between the two feet during gait | ||
| Cadence | Number of steps per unit time | ||
| Symmetry | Symmetry of the walking motion | ||
| Reappearance | Represents reproducibility of the walking motion in each gait cycle | ||
| Smoothness | Represents the smoothness of gait | ||
| Sway | Sway of the trunk (i.e., deviation of the center of gravity) | ||
| Rhythm | Rhythm index indicates the regularity of gait | ||
| Impact | Impact index represents the magnitude of the impulse occurring at the time of heel contact | ||
| Speed | Meta-analysis Parameters | Walking velocity | Engsberg [17] Khodadadeh [20] (Walkway-Forceplate) Lenke [21] Mahaudens [28] Paul [27] Shiomi [24] |
| Stride length | The distance between successive points of initial contact of the same foot | ||
| Cadence | Number of steps per unit time | ||
RoM: range of motion
EMG: electromyography
CoP: center of pressure
CoM: center of mass
Quality assessment of selected studies
Based on the Delphi criteria, the average score for the selected studies was 2.2/6 (ranged 0–4, Table 3). No evidence was found in any of the studies to confirm blinding the outcome assessor. In more than half of the studies, no healthy control group was included to compare the measured parameters pre- and post-surgery. Therefore, assessing improvement or deterioration for some objective parameters was methodologically challenging in these studies [15, 16, 18, 21–23, 26, 27].
Table 3.
Quality assessment of selected papers using Delphi criteria.
| Adachi [15] |
Deen [16] |
Engsberg [17] |
Gottipati [26] |
Hasday [18] |
Hopf [19] |
Khodadadeh [20] |
Lenke [21] |
Mahuadens [22] |
Papadakis [23] |
Paul [27] |
Shiomi [24] |
Suda [25] |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Include healthy control group | √ | √ | √ | √ | √ | ||||||||
| No significant difference at baseline | √ | √ | |||||||||||
| Indicate eligibility criteria | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Blinding of the outcome assessor | |||||||||||||
| Provide point estimates and measure of variability | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| Include an intention-to-treat analysis | √ | √ | √ | √ | |||||||||
| Score | 0 | 3 | 4 | 3 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 4 | 4 |
Participants, diagnoses, and surgical treatments
The mean (SD) sample size of the selected studies was 27 (17), ranging from 6 to 68 participants. For studies that used a healthy control group, the mean (SD) sample size was 55 (75), ranging from 8 to 186 participants (Table 1). The mean (SD) age of the selected samples was 39 (23) for the intervention group, and 35 (22) for the healthy control group. For intervention samples, three studies (23%) recruited adolescent individuals under 18 years [21, 22, 27], four (31%) recruited older adults over 40 years [15, 16, 25, 26], and the remaining six studies (46%) were comprised of samples with mixed ages [17–20, 23, 24].
Without targeting any specific surgery or disorder, the focus of the current review was on gait improvements in LBD patients following a surgical procedure. The selected papers, however, covered three specific types of LBD (scoliosis, stenosis, and fractures), and four categories of surgery (spinal fusion, decompression, rod removal, and reconstructive surgery). Most of the studies (7 articles – 54%) [17, 19–22, 24, 25], included scoliosis deformity and spinal fusion, and the meta-analysis was performed only for scoliosis patients who underwent spinal fusion.
Alterations in objective gait parameters pre- and post- surgery
An analysis of gait results revealed that shoulder and hip orientation during walking showed obvious changes at one-year post-fusion surgery in follow-up assessments for spinal deformity (i.e., scoliosis) (Tables 1 and 2). Moreover, spinal fusion improved balancing ability during walking, which was observed as a reduction in center of mass (CoM) deviation from the walking pathway [27]. Interestingly, no significant alterations in lower extremity kinematics during gait was observed at the one-year assessment for spinal deformity. Furthermore, significant improvement in muscle efficiency (i.e., total work measured by mechanical work from muscles to perform walking divided by energy cost measured by oxygen consumption during walking [28]) was observed following spinal deformity fusion surgery (Tables 1 and 2). Focusing on electromyography (EMG) data during walking, a reduction in asymmetry of upper body muscle activation was discovered following spinal fusion surgery in patients with scoliosis [19]; however, no significant differences was found in either the lower extremity or the postural muscle activity duration prior to and following surgery (Table 1 and 2).
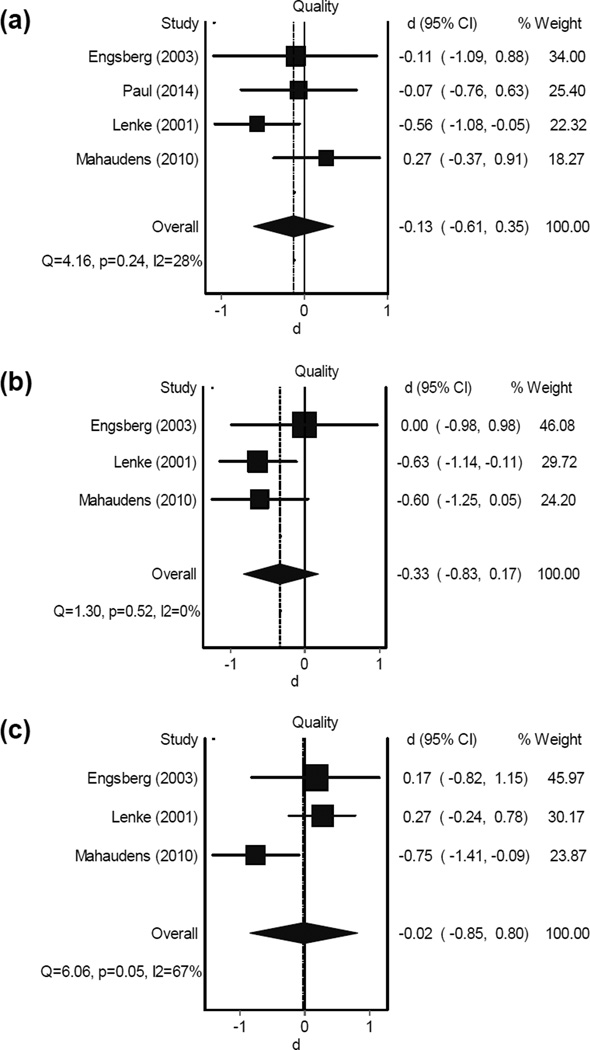
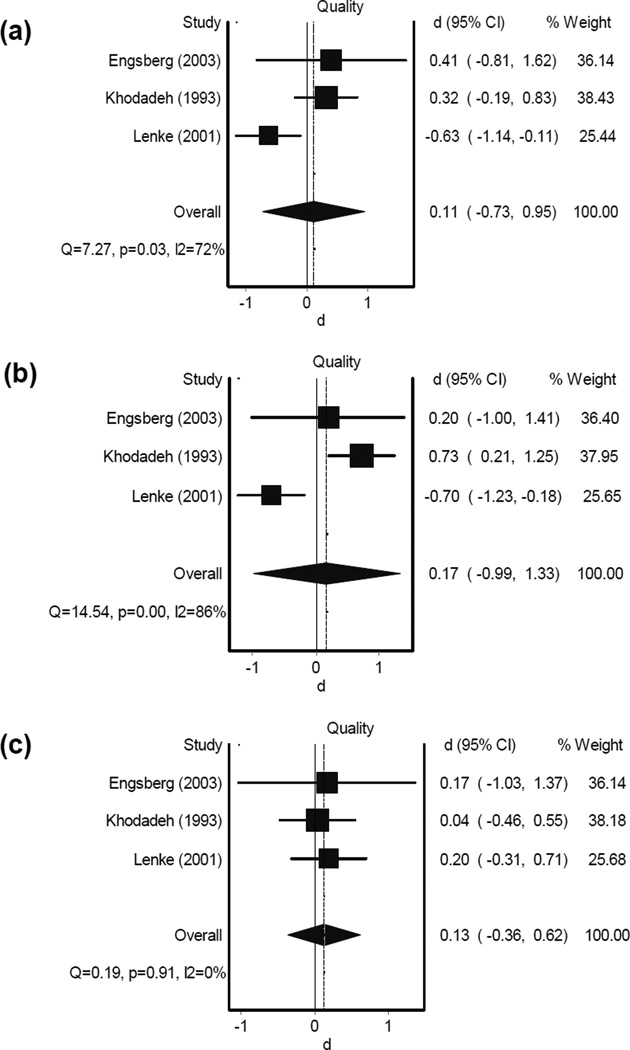
Results from the meta-analysis for scoliosis spinal fusion surgery revealed no improvements in gait speed, stride length, or cadence in one-year follow-up measurements [17, 21, 22, 27]; overall effect sizes-d (confidence intervals - CI) from four studies were −0.13 (−0.61,0.35), −0.33 (−0.83,0.17), and −0.02 (−0.80,0.85) for changes (negative d: reduction) in gait speed, cadence, and stride length, respectively (Figure 2). Changes in gait parameters were homogeneous among selected articles (P > 0.05, I2 < 60%). These gait parameters, however, tended to improve in the two-year follow-up data, as was observed with a faster walking speed (Figure 3). Although less homogeneity was observed in gait outcomes two years following scoliosis surgery (P < 0.03, I2 > 73%), results demonstrated small overall d (CI) of 0.11 (−0.73,0.95), 0.17 (−0.99,1.33), and 0.13 (−0.36,0.62) for gait speed, cadence, and stride length changes (positive d: increase) [17, 20, 21]. In two other studies [20, 24], gait speed, cadence, and stride length assessments showed no significant alterations in pre- versus post-surgery measurements at immediate and 6-month follow-up (P values were not reported). Moreover, Shiomi et al. reported an increase in step width after spinal fusion surgery in patients with scoliosis. Since pre-surgery and control group step lengths were similar, this change suggests a deterioration in walking pattern immediately after surgery [24].
Figure 2.
Forest plot for alterations of gait parameters (a: gait speed, b: cadence, and c: stride length) in scoliosis patients one year following fusion surgery. The dotted vertical line corresponds to the summary effect size of the sample. The solid vertical line correspondents to an effect size of 0 (no effect).
d: effect size
CI: confidence interval
Figure 3.
Forest plot for alterations of gait parameters (a: gait speed, b: cadence, and c: stride length) in scoliosis patients two years following fusion surgery. The dotted vertical line corresponds to the summary effect size of the sample. The solid vertical line correspondents to an effect size of 0 (no effect).
d: effect size
CI: confidence interval
Observations regarding other disorders and surgeries revealed improvements in gait variability during walking (i.e., more similarity in walking pattern among gait cycles) following decompression surgery in LBD patients with stenosis (~73% reduction in gait variability in one-year post-surgery measurement) [23]. Furthermore, results of other work suggested improvement in walking symmetry, smoothness and regularity, trunk sway, and a reduction in heel strike impact immediately after stenosis decompression treatment [25]. Also, endurance assessment at three-month follow-up showed increases as high as ~340% in walking duration [16]. Unlike fusion treatment for scoliosis, decompression surgery was found to be successful in improving gait speed, stride length, and cadence in patients with stenosis [25]. Also, a reduction in intermittent claudication was observed in patients with spinal stenosis after restorative laminoplasty [15]; reported results from this study demonstrated 99% improvement in intermittent claudication scores from 0.3 points prior to surgery to 2.9 points one-year post-surgery.
In two studies, gait performance was assessed after spine reconstructive and rod-removal surgeries. In the first study, Hasday et al. observed improvements in gait speed, stride length, and cadence after rod removal surgery in six patients with motion segment fractures [18]. In the second study, gait behaviors were investigated following multi-segmental reconstructive surgery (for at least four spinal segments) in individuals with degenerative spinal disorder who had positive spine alignment [26]. After surgery sagittal alignment was improved by 70% during standing and walking measurements, which subsequently led to resolving crouch walking, reduced knee flexion angle at mid-stance, and increases in step and stride lengths (Table 1; see Table 2 for definitions).
Discussion
Gait assessment for scoliosis and spinal fusion
Gait analysis demonstrated improvements in patients with scoliosis disorders, especially in shoulder, trunk, and hip motion; however, analysis of lower extremity motion, overall, showed no substantial change even at two-year follow-up tests (Tables 1 and 2). Similarly, muscle activation asymmetry was observed in the upper-body muscles (lumbar muscles of the convex side of double major scolioses, the gluteus medius, and tensor fascia lata muscles of the concave side of thoracic curvatures) prior to spinal fusion, which was reduced after surgery. But, no asymmetric muscle activation was reported prior to surgery in the knee extensors (vastus lateralis) and foot (peroneus longus) muscles [19].
Based on six different studies that reported gait speed, cadence, and stride length, no conclusive result was observed following scoliosis treatment [17, 20–22, 24]. One study showed a significant increase in gait speed two years post-surgery [20]. Another study, showed a significant reduction in gait speed, cadence, and stride length after one year [21]. Since the overall meta-analysis effect size is small (< 0.33), the parameters of gait speed, cadence, and stride length may not be suitable for assessing gait improvement in individuals with scoliosis. On the contrary, upper-body motion, improvements in movement efficiency (i.e., increase in external work with respect to the amount of energy expenditure), and balancing ability during walking are parameters that suggested to represent gait improvement in spinal deformity patients [17, 22, 27]. Previous studies demonstrated an asymmetry of upper trunk torsion in the transverse plane when comparing gait patterns between adolescent idiopathic scoliosis groups and normal healthy controls [12]. Other studies showed a muscular inefficiency to execute walking and higher energy expenditure (oxygen uptake) in scoliosis compared to healthy individuals [28, 29]. Interestingly, gait speed, stride length, cadence, and overall knee and ankle motion during walking in patients with scoliosis followed the physiological patterns of normal healthy samples [12, 30]. This concurs with our conclusion regarding negligible alterations in lower extremity movements, pre- and post-fusion surgery.
Gait assessment for stenosis and decompression
In contrast to results in patients with scoliosis and spinal fusion, one study detected a substantial increase in gait speed (22%) following stenosis decompression surgery, which was associated with increase in both stride length (11%), and cadence (6%) [25]. These alterations in speed were observed six months after surgery. In the same study, improvements in gait patterns were also noted (Tables 1 and 2). Deep tendon reflex changes, sensory loss, and muscle weakness are signs of spinal stenosis, which may lead to gait disturbance and buttock and proximal thigh pain [31], and reduced walking ability [32]. Specifically, gait speed within a 15-minute walk test was significantly lower in individuals with spinal stenosis compared to those without spinal symptoms [33]. Therefore, speed of walking and possibly lower extremity motion may be appropriate objective measures for improvement of stenosis condition after treatment.
Substantial improvement in stride-to-stride variability, an indicator of functional capability [38], was observed following decompression treatment [23]. Higher gait variability was observed in patients diagnosed with stenosis in contrast with healthy controls, perhaps associated with acute radicular pain and neurological claudication [23]; both conditions further limit normal walking tolerance [34]. Results of the current review paper demonstrate an overall walking tolerance enhancement in patients with stenosis after decompression [16]; this was indicated by an increased time until symptom presentation while walking on a treadmill.
Research limitations in objective assessment methodology
As specified in Table 3, the selected papers varied in quality measured by the Delphi criteria. Other than limitations related to Delphi consensus, several other limitations exist, which are highlighted here. First, lack of subjective evaluations coupled with objective assessments of motor performance was one important drawback, which further limited investigation of the association between objective and subjective evaluations. For instance, among presented studies, only two (15%) included pain assessment [17, 20], and only two (15%) included disability questionnaires (including The Japanese Orthopaedic Association (JOA), the Scoliosis Patient Questionnaire (SRS-30), and Oswestry Disability Questionnaire) [15, 17].
A second drawback was that in many studies the age of participants was not strictly controlled. At the same time, no statistical analysis was performed in these studies to account for the potentially confounding effect of age on conclusions. Since gait behaviors can fluctuate with age [35], a wide age distribution among participants can reduce the reliability of objective parameters for assessing motor performance.
Third, previous research has demonstrated that physical impairments may be highlighted only under more challenging conditions [36]. However, none of the studies that implemented objective gait assessments incorporated the more challenging fast walking or dual task walking (e.g., walking while counting) conditions to demonstrate improvement in motor performance after surgery.
Conclusions
Recommendations
Based on the compiled results extracted from the selected studies, we made the following recommendations regarding gait assessments as an indicator of motor performance following spinal surgery. (Of note, age-specific recommendations are presented here according to available evidence.) For patients with scoliosis, 1) use hip and shoulder motion analysis (rather than lower extremities) during gait, for both adolescent individuals (≤18 years) and older adults (≥40 years); 2) measure energy expenditure and efficiency in executing gait and forward movement for both adolescent individuals and older adults; and 3) assess EMG for asymmetry in the upper body muscles, rather than in the knee and foot muscles for adolescent individuals.
For older adults with degenerative spine complications, specifically with spinal stenosis, measure parameters related to agility of walking and gait pattern such as gait speed, stride length, cadence, symmetry, and smoothness of walking, walking endurance, and walking variability.
Limitations and summary
Due to disparities in patient samples and treatment types, it was not possible to perform meta-analysis for most of the outcome measures. Despite the limitation outlined above regarding the available literature on objective gait assessments in patients with LBD following surgical procedures, the available research indicates that motion analysis during gait can provide crucial information for assessing alterations in motor performance specific to spinal scoliosis or spinal stenosis. Systematic use of these shared measures would allow objective comparison of interventions. Using this information would improve surgical assessment, which would lead to improved quality-of-life of patients with LBD, and positively influence their ability to return to a higher functional status.
Highlights.
-
-
The available research indicates that gait abnormalities reflect low back disorders.
-
-
We summarized gait assessment methods for evaluating low back surgeries.
-
-
Gait improvements can be better assessed by upper-body motion and EMG in scoliosis.
-
-
Assessing efficiency in executing gait can highlight improvements in scoliosis.
-
-
Gait improvements can be captured by spatio-temporal parameters in stenosis.
Acknowledgements
This study was partially supported by an STTR-Phase II Grant (Award No. 2R42AG032748) from the National Institute on Aging, and the Arizona Center on Aging. We thank Mr. Tigran Gevorgi Nahapetian for supporting the literature search and study selection.
Appendix
The following search strategy was used in the PubMed database:
(((((((((("Back Pain"[Mesh]) OR "Sacroiliitis"[Mesh]) OR "Back Injuries"[Mesh])OR "Sciatica"[Mesh]) OR "Spinal Diseases"[Mesh])))
OR
((((((((((((spinal OR spine OR spines OR back OR backs OR vertebra* OR disc OR discs OR disk OR disks[Text Word])) AND arthrit*[Text Word])) OR sacroiliitis[Text Word]) OR (((curve OR curves OR curved OR curving[Text Word])) AND (spine OR spines OR spinal[Text Word]))) OR ((kyphosis[Text Word]) OR scoliosis[Text Word]))) OR (((vertebral OR compressure[Text Word])) AND (fracture OR fractures OR fracturing OR fractured[Text Word]))) OR sciatica[Text Word]) OR spinal stenosis[Text Word]) OR ((((herniat* OR ruptur* OR prolapse OR prolapses OR prolapsed OR prolapsing OR degenerative OR tear OR tears OR torn OR slipped OR slip[Text Word]))) AND ((disc OR discs OR disk OR disks[Text Word])))) OR "back pain"[Text Word]))
AND
((((((("Gait"[Mesh]) OR "Postural Balance"[Mesh]) OR "Range of Motion, Articular"[Mesh]) OR ("Muscle Strength"[Mesh] OR "Muscle Strength Dynamometer"[Mesh]))))
OR
((((((gait[Text Word]) OR balance[Text Word]) OR ("range of motion" OR ROM[Text Word])) OR "muscle strength"[Text Word]) OR ("timed up and go" OR "TUG"[Text Word])) OR "sit to stand"[Text Word])))
AND
(((((((((surgery[Text Word]) OR (((decompression OR fusion OR replacement OR injection[Text Word])) AND (spine OR spinal OR disc OR disk OR vertebra* OR back[Text Word]))) OR ((laser* [Text Word]))) OR ((discectomy OR diskectomy[Text Word]))) OR (((((((laminectomy[Text Word]) OR nucleoplasty[Text Word]) OR verterbroplasty[Text Word]) OR kyphoplasty[Text Word]) OR rhizotomy[Text Word]) OR cordotomy[Text Word]) OR foraminectomy[Text Word])) OR ((IntraDiscal Electrothermal Therapy OR IDET[Text Word]))) OR (((((radiofrequency lesioning[Text Word]) OR (percutaneous vertebral augmentation OR PVA[Text Word])) OR dorsal root entry[Text Word]) OR "interbody fusion" OR "interbody fusions"[Text Word]) OR (botox OR botulinum[Text Word]))))
OR
("General Surgery"[Mesh] OR "Surgical Procedures, Operative"[Mesh] OR "surgery"[Subheading] OR "lasers"[MeSH Terms] OR "diskectomy"[MeSH] OR "laminectomy"[MeSH Terms] OR "vertebroplasty"[MeSH Terms] OR "kyphoplasty"[MeSH Terms] OR "rhizotomy"[MeSH Terms] OR "cordotomy"[MeSH Terms] OR "Decompression, Surgical"[Mesh] OR "Spinal Fusion"[Mesh] OR "Total Disc Replacement"[Mesh] OR "Foraminotomy"[Mesh] OR "Pulsed Radiofrequency Treatment"[Mesh] OR "Injections, Spinal"[Mesh] OR "Botulinum Toxins, Type A"[Mesh]))
Search strategies applied in the other databases (Embase, Cochrane Library, Web of Science, CINAHL and ClinicalTrials.gov) were derived from the PubMed search. The database searches were conducted without using publication date restrictions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There is no conflict of interest for the current study.
Author contributions
Nima Toosizadeh wrote the manuscript and contributed to all aspects of the study, especially to the search, analysis, and interpretation of results; Tzu Chuan Yen contributed to the search, analysis and editing of the manuscript; Carol Howe contributed to the search, analysis and editing of the manuscript; Michael Dohm contributed to developing the systematic review questions and design, and interpreting the findings; Jane Mohler contributed to editing the manuscript; and Bijan Najafi had a substantive role in the design of the systematic review, study supervision, and interpretation of results.
References
- 1.Waterman BR, Belmont PJ, Jr, Schoenfeld AJ. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. The Spine Journal. 2012;12(1):63–70. doi: 10.1016/j.spinee.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Archives of Internal Medicine. 2009;169(3):251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein JN, Lurie JD, Olson P, Bronner KK, Fisher ES, Morgan MTS. United States trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31(23):2707. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim T, Tleyjeh I, Gabbar O. Surgical versus non-surgical treatment of chronic low back pain: a meta-analysis of randomised trials. International orthopaedics. 2008;32(1):107–113. doi: 10.1007/s00264-006-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachemson AL. Evaluation of results in lumbar spine surgery. Acta Orthopaedica. 1993;64(s251):130–133. doi: 10.3109/17453679309160143. [DOI] [PubMed] [Google Scholar]
- 6.Waddell G. Clinical assessment of lumbar impairment. Clinical orthopaedics and related research. 1987;221:110–120. [PubMed] [Google Scholar]
- 7.Katz J, Melzack R. Measurement of pain. Surgical Clinics of North America. 1999;79(2):231–252. doi: 10.1016/s0039-6109(05)70381-9. [DOI] [PubMed] [Google Scholar]
- 8.McGregor A, Dore C, McCarthy I, Hughes S. Are subjective clinical findings and objective clinical tests related to the motion characteristics of low back pain subjects? Journal of Orthopaedic & Sports Physical Therapy. 1998;28(6):370–377. doi: 10.2519/jospt.1998.28.6.370. [DOI] [PubMed] [Google Scholar]
- 9.Beurskens AJ, Henrica C, Köke AJ, van der Heijden GJ, Knipschild PG. Measuring the functional status of patients with low back pain: assessment of the quality of four diseasespecific questionnaires. Spine. 1995;20(9):1017–1028. doi: 10.1097/00007632-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, Al-Zaabie N, Nelson RM. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. International Journal of rehabilitation research. 2003;26(2):101–108. doi: 10.1097/00004356-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. 1996;64(2):231–240. doi: 10.1016/0304-3959(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 12.Kramers-de Quervain IA, Müller R, Stacoff A, Grob D, Stüssi E. Gait analysis in patients with idiopathic scoliosis. European Spine Journal. 2004;13(5):449–456. doi: 10.1007/s00586-003-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of clinical epidemiology. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 15.Adachi K, Futami T, Ebihara A, et al. Spinal canal enlargement procedure by restorative laminoplasty for the treatment of lumbar canal stenosis. Spine J. 2003;3(6):471–478. doi: 10.1016/s1529-9430(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 16.Deen HG, Zimmerman RS, Lyons MK, McPhee MC, Verheijde JL, Lemens SM. Use of the exercise treadmill to measure baseline functional status and surgical outcome in patients with severe lumbar spinal stenosis. Spine. 1998;23(2):244–248. doi: 10.1097/00007632-199801150-00019. [DOI] [PubMed] [Google Scholar]
- 17.Engsberg JR, Bridwell KH, Wagner JM, Uhrich ML, Blanke K, Lenke LG. Gait changes as the result of deformity reconstruction surgery in a group of adults with lumbar scoliosis. Spine (Phila Pa 1976) 2003;28(16):1836–1843. doi: 10.1097/00007632-200308150-00012. discussion 44. [DOI] [PubMed] [Google Scholar]
- 18.Hasday CA, Passoff TL, Perry J. Gait abnormalities arising from latrogenic loss of lumbar lordosis secondary to Harrington instrumentation in lumbar fractures. Spine (Phila Pa 1976) 1983;8(5):501–511. doi: 10.1097/00007632-198307000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Hopf C, Scheidecker M, Steffan K, Bodem F, Eysel P. Gait analysis in idiopathic scoliosis before and after surgery: a comparison of the pre- and postoperative muscle activation pattern. Eur Spine J. 1998;7(1):6–11. doi: 10.1007/s005860050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khodadadeh S, Eisenstein SM. Gait analysis of patients with low back pain before and after surgery. Spine (Phila Pa 1976) 1993;18(11):1451–1455. [PubMed] [Google Scholar]
- 21.Lenke LG, Engsberg JR, Ross SA, Reitenbach A, Blanke K, Bridwell KH. Prospective dynamic functional evaluation of gait and spinal balance following spinal fusion in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2001;26(14):E330–E337. doi: 10.1097/00007632-200107150-00020. [DOI] [PubMed] [Google Scholar]
- 22.Mahaudens P, Detrembleur C, Mousny M, Banse X. Gait in thoracolumbar/lumbar adolescent idiopathic scoliosis: effect of surgery on gait mechanisms. Eur Spine J. 2010;19(7):1179–1188. doi: 10.1007/s00586-010-1292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part B. Preoperative versus postoperative gait variability. Physiol Meas. 2009;30(11):1187–1195. doi: 10.1088/0967-3334/30/11/004. [DOI] [PubMed] [Google Scholar]
- 24.Shiomi A. [Idiopathic scoliosis by spinal fusion and brace treatment: evaluation by gait analysis] Nihon Seikeigeka Gakkai Zasshi. 1995;69(9):665–674. [PubMed] [Google Scholar]
- 25.Suda Y, Saitou M, Shibasaki K, Yamazaki N, Chiba K, Toyama Y. Gait analysis of patients with neurogenic intermittent claudication. Spine (Phila Pa 1976) 2002;27(22):2509–2513. doi: 10.1097/00007632-200211150-00016. [DOI] [PubMed] [Google Scholar]
- 26.Gottipati P, Fatone S, Koski T, Sugrue PA, Ganju A. Crouch gait in persons with positive sagittal spine alignment resolves with surgery. Gait & posture. 2014;39(1):372–377. doi: 10.1016/j.gaitpost.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Paul JC, Patel A, Bianco K, et al. Gait stability improvement after fusion surgery for adolescent idiopathic scoliosis is influenced by corrective measures in coronal and sagittal planes. Gait & posture. 2014 doi: 10.1016/j.gaitpost.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Mahaudens P, Detrembleur C, Mousny M, Banse X. Gait in adolescent idiopathic scoliosis: energy cost analysis. European Spine Journal. 2009;18(8):1160–1168. doi: 10.1007/s00586-009-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindh M. Energy expenditure during walking in patients with scoliosis: the effect of surgical correction. Spine. 1978;3(2):122–134. doi: 10.1097/00007632-197806000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Chen P-Q, Wang J-L, Tsuang Y-H, Liao T-L, Huang P-I, Hang Y-S. The postural stability control and gait pattern of idiopathic scoliosis adolescents. Clinical biomechanics. 1998;13(1):S52–S58. doi: 10.1016/s0268-0033(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 31.Arbit E, Pannullo S. Lumbar stenosis: a clinical review. Clinical orthopaedics and related research. 2001;384:137–143. [PubMed] [Google Scholar]
- 32.Winter CC, Brandes M, Müller C, et al. Walking ability during daily life in patients with osteoarthritis of the knee or the hip and lumbar spinal stenosis: a cross sectional study. BMC musculoskeletal disorders. 2010;11(1):233. doi: 10.1186/1471-2474-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong HC, Haig AJ, Geisser ME, Yamakawa KS, Miner JA. Comparing pain severity and functional status of older adults without spinal symptoms, with lumbar spinal stenosis, and with axial low back pain. Gerontology. 2006;53(2):111–115. doi: 10.1159/000096861. [DOI] [PubMed] [Google Scholar]
- 34.Amundsen T, Weber H, Lilleas F, Nordal HJ, Abdelnoor M, Magnaes B. Lumbar spinal stenosis: clinical and radiologic features. Spine. 1995;20(10):1178–1186. doi: 10.1097/00007632-199505150-00013. [DOI] [PubMed] [Google Scholar]
- 35.Wolfson L. Gait and balance dysfunction: a model of the interaction of age and disease. The Neuroscientist. 2001;7(2):178–183. doi: 10.1177/107385840100700212. [DOI] [PubMed] [Google Scholar]
- 36.Camicioli R, Bouchard T, Licis L. Dual-tasks and walking fast: Relationship to extra-pyramidal signs in advanced Alzheimer disease. Journal of the neurological sciences. 2006;248(1):205–209. doi: 10.1016/j.jns.2006.05.013. [DOI] [PubMed] [Google Scholar]